Effects of Blood Flow Restriction Training in Patients before and after Anterior Cruciate Ligament Reconstruction: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Search Strategy
2.3. Study Selection
2.4. Data Extraction
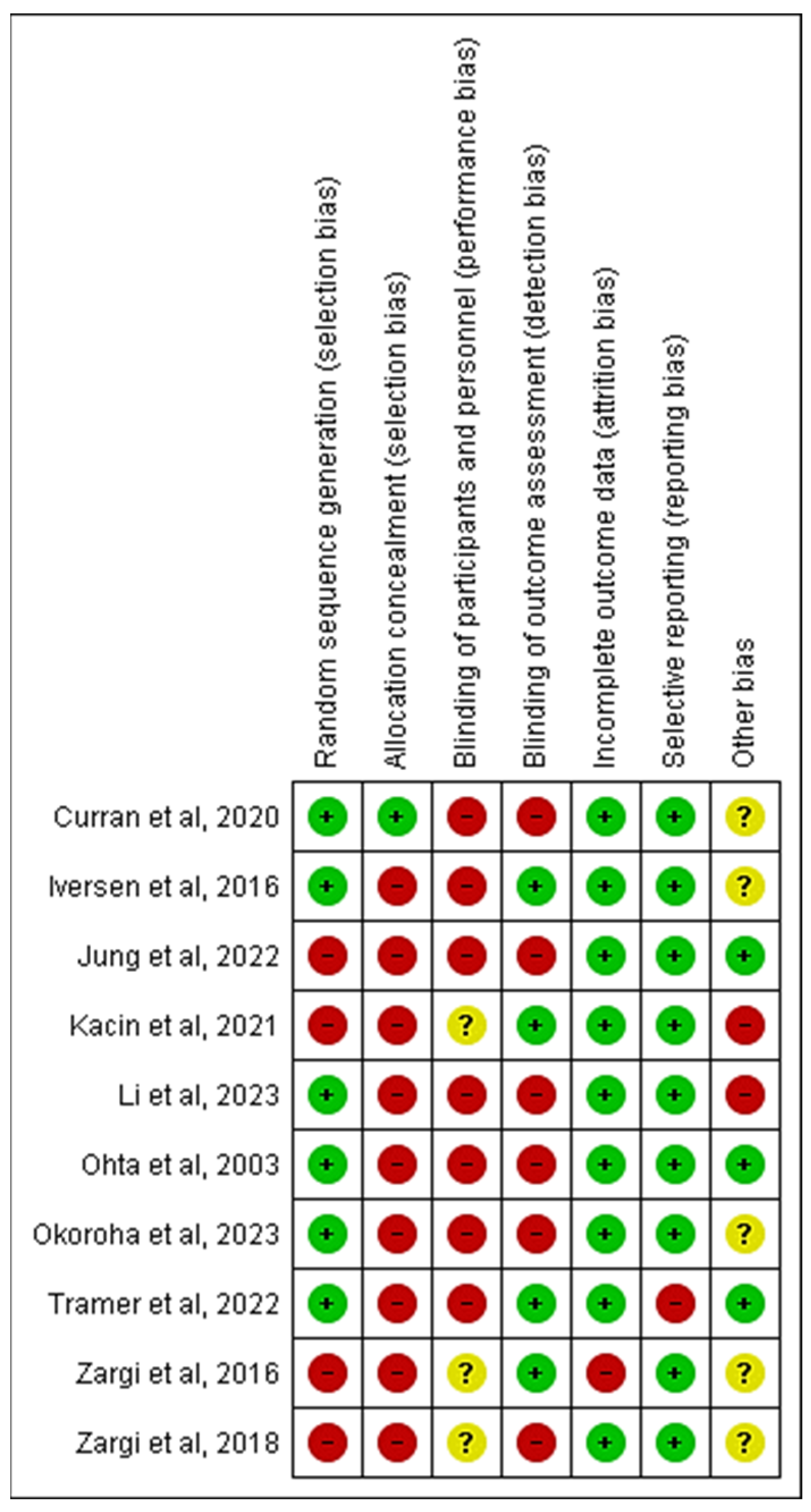
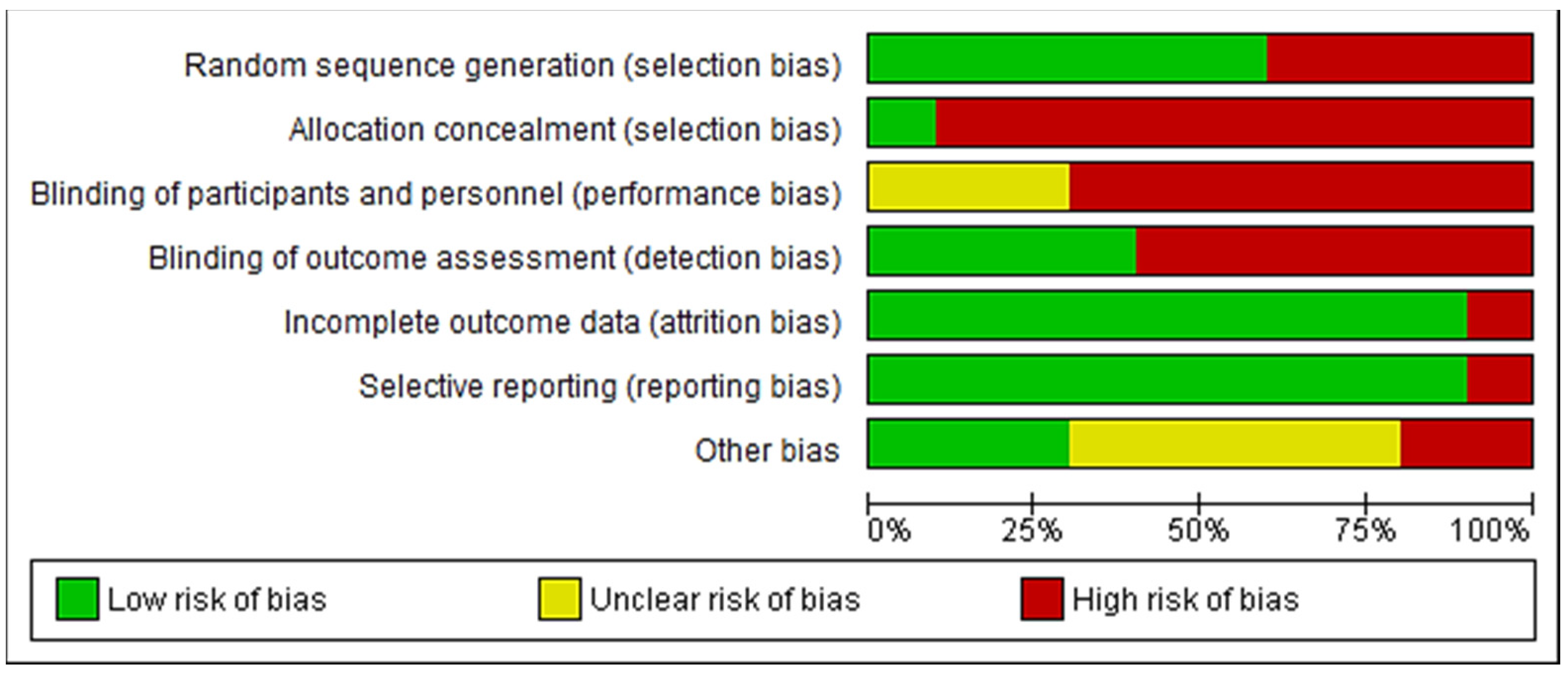
2.5. Risk of Bias and Quality Assessment
2.6. Data Synthesis and Analysis
3. Results
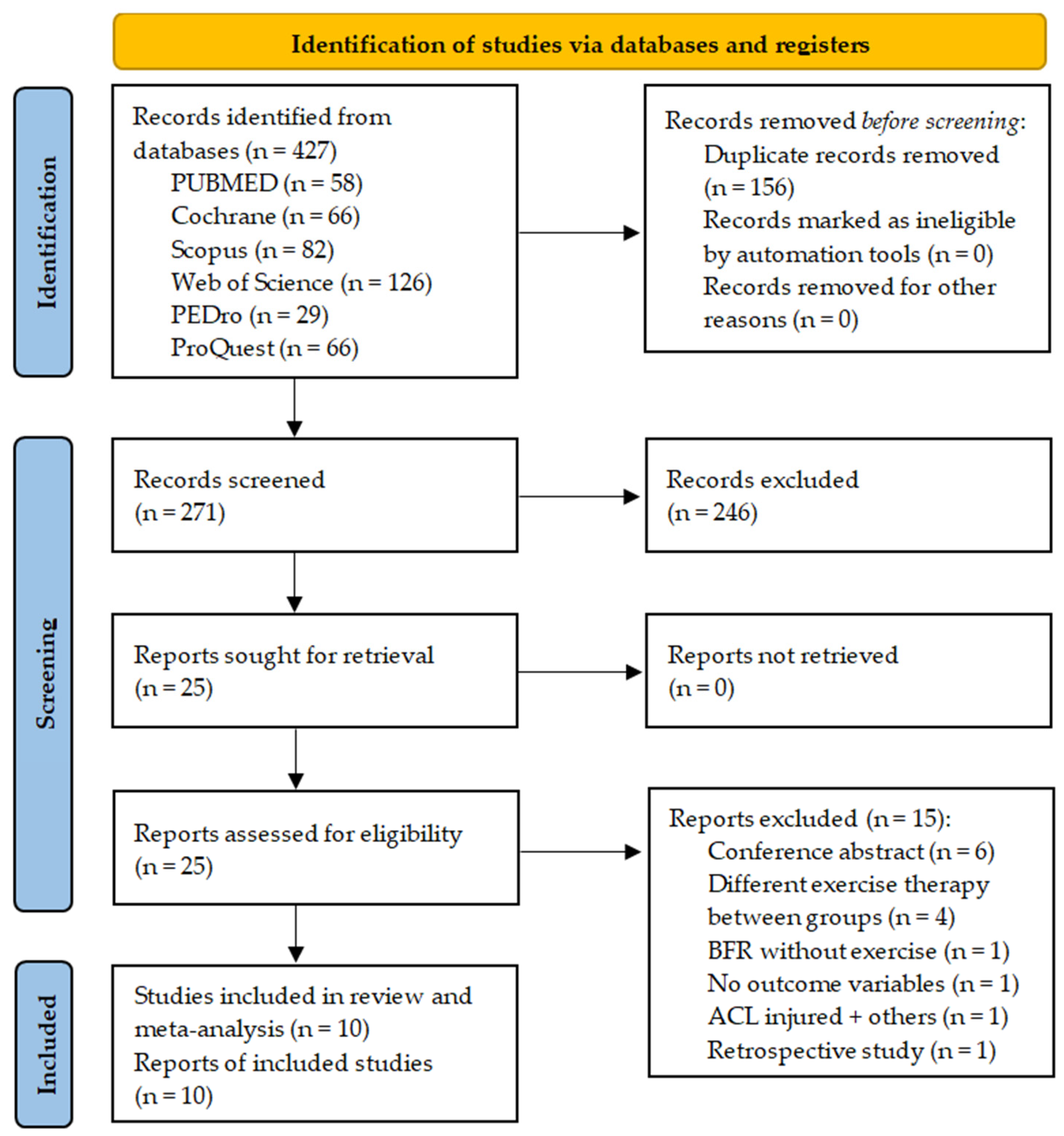
3.1. Literature Search and Screening
3.2. Characteristics of the Eligible Studies
3.3. Outcome Measures
3.4. Study Quality and Risk of Bias
3.5. Synthesis of Results
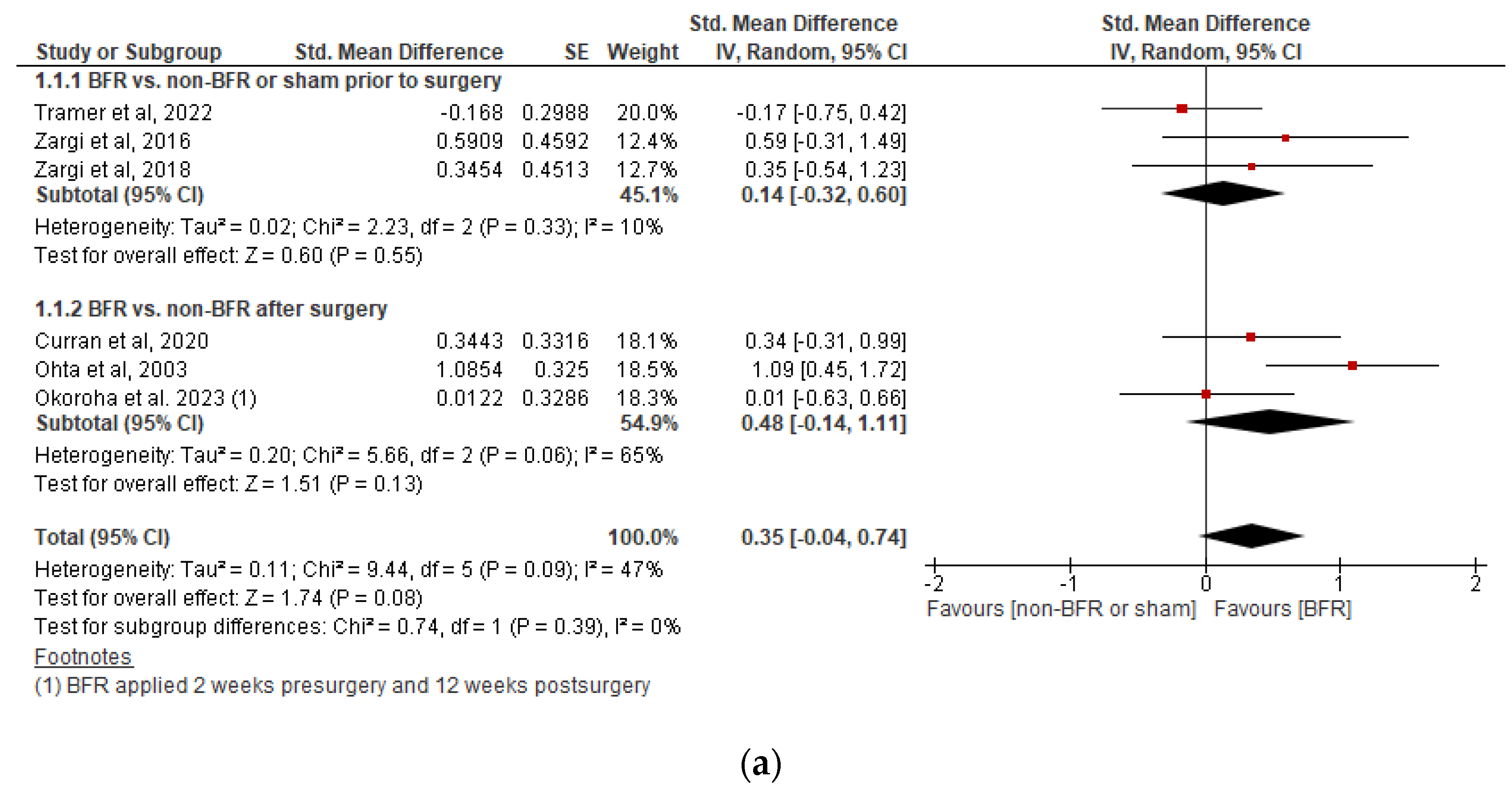
3.5.1. Effects on Knee Extensor Isometric Strength
3.5.2. Effects on Knee Extensor Isokinetic Strength
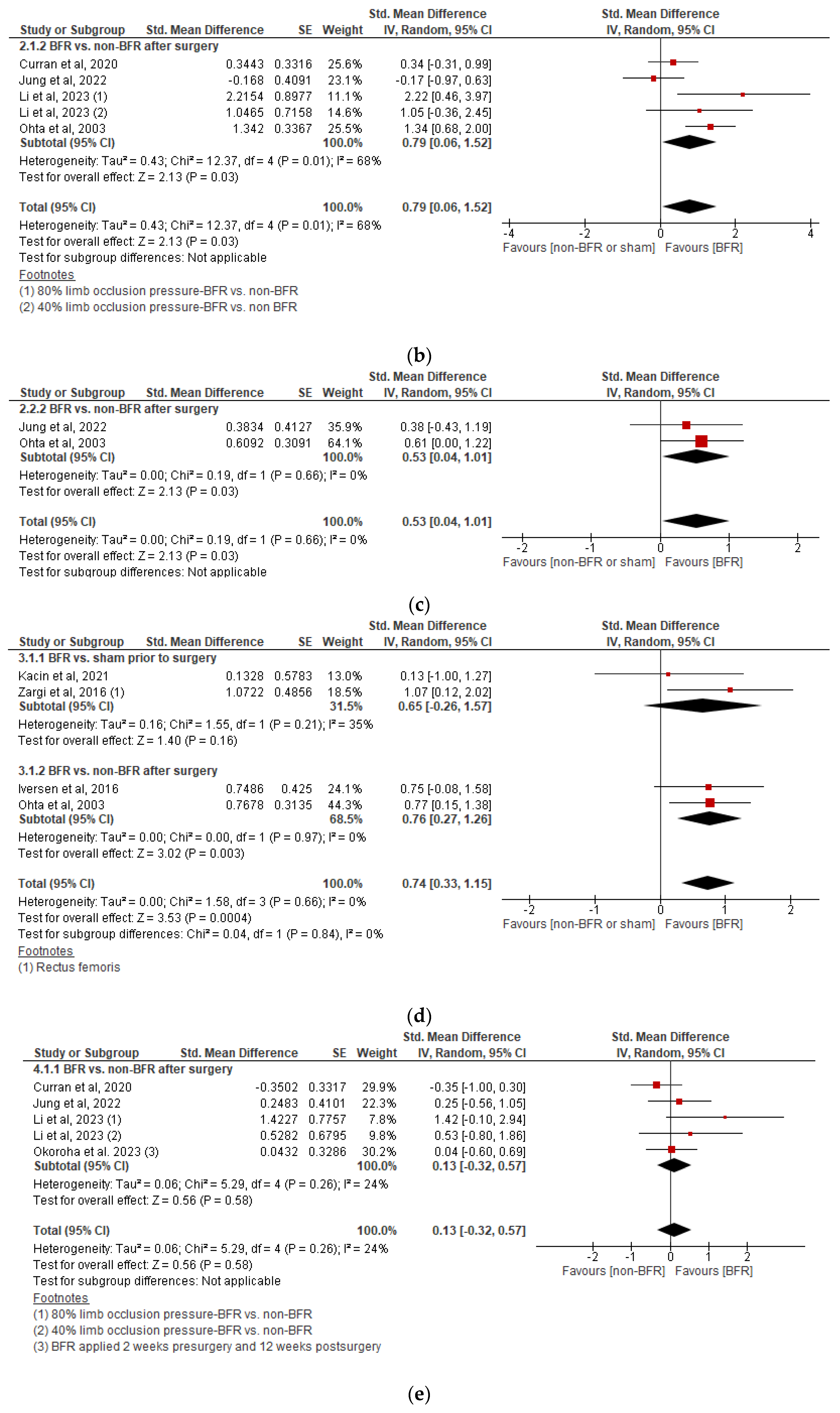
3.5.3. Effects on Knee Flexor Isokinetic Strength
3.5.4. Effects on Quadriceps Cross-Sectional Area
3.5.5. Effects on Perceived Knee Function
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| PubMed | ||
|---|---|---|
| Search | Strategy | Results |
| #7 | #3 AND #6 | 58 |
| #6 | #4 OR #5 | 18,374 |
| #5 | (“blood”[All Fields] AND “flow”[All Fields] AND “restriction”[All Fields]) OR “blood flow restriction”[All Fields] OR “kaatsu”[All Fields] OR “vascular occlusion”[All Fields] OR ((“occlusion”[All Fields] OR “occlused”[All Fields] OR “occlusions”[All Fields] OR “occlusive”[All Fields] OR “occlusives”[All Fields]) AND (“training”[All Fields] OR “train”[All Fields] OR “trained”[All Fields] OR “trainings”[All Fields] OR “trains”[All Fields])) OR ((“blood vessels”[MeSH Terms] (“blood”[All Fields] AND “vessels”[All Fields]) OR “blood vessels”[All Fields] OR (“blood”[All Fields] AND “vessel”[All Fields]) OR “blood vessel”[All Fields]) AND (“restrict”[All Fields] OR “restricted”[All Fields] OR “restricting”[All Fields] OR “restriction”[All Fields] OR “restrictions”[All Fields] OR “restrictive”[All Fields] OR “restrictiveness”[All Fields] OR “restricts”[All Fields]) AND (“therapeutics”[MeSH Terms] OR “therapeutics”[All Fields] OR “therapies”[All Fields] OR “therapy”[All Fields] OR “therapys”[All Fields])) | 18,278 |
| #4 | “blood flow restriction therapy”[MeSH Terms] | 70 |
| #3 | #1 OR #2 | 31,109 |
| #2 | (“anterior”[All Fields] AND “cruciate”[All Fields] AND “ligament”[All Fields] AND “reconstruction”[All Fields]) OR “anterior cruciate ligament reconstruction”[All Fields] OR (“anterior”[All Fields] AND “cruciate”[All Fields] AND “ligament”[All Fields] AND “injuries”[All Fields]) OR “anterior cruciate ligament injuries”[All Fields] OR (“anterior”[All Fields] AND “cruciate”[All Fields] AND “ligament”[All Fields]) OR “anterior cruciate ligament”[All Fields] OR (“acl”[All Fields] AND “injury”[All Fields]) OR “acl injury”[All Fields] OR (“acl”[All Fields] AND “tear”[All Fields]) OR “acl tear”[All Fields] OR (“acl”[All Fields] AND “rupture”[All Fields]) OR “acl rupture”[All Fields] | 31,069 |
| #1 | “anterior cruciate ligament reconstruction”[MeSH Terms] OR “anterior cruciate ligament injuries”[MeSH Terms] OR “anterior cruciate ligament”[MeSH Terms] | 20,659 |
| Web of Science | ||
| Search | Strategy | Results |
| #3 | #1 AND #2 | 126 |
| #2 | (blood flow restriction OR kaatsu OR vascular occlusion OR occlusive training OR occlusion training OR blood vessel restriction) (Topic) | 158,145 |
| #1 | (anterior cruciate ligament OR acl injury OR acl tear OR acl rupture OR acl reconstruction OR acl rehabilitation) (Topic) | 44,178 |
| Scopus | ||
| Search | Strategy | Results |
| #3 | #1 AND #2 | 82 |
| #2 | TITLE-ABS-KEY (“blood flow restriction” OR “occlusion training” OR “occlusive training” OR “kaatsu” OR “blood vessel restriction” OR “vascular occlusion”) | 12,113 |
| #1 | TITLE-ABS-KEY (“anterior cruciate ligament” OR “acl injury” OR “acl tear” OR “acl rupture” OR “acl reconstruction” OR “acl rehabilitation”) | 39,649 |
| Cochrane library | ||
| Search | Strategy | Results |
| #3 | #1 AND 2 | 66 |
| #2 | (blood flow restriction therapy OR blood flow restriction exercise OR blood flow restriction training OR blood flow restriction OR kaatsu OR occlusive training OR blood vessel restriction):ti,ab,kw | 2893 |
| #1 | (anterior cruciate ligament OR acl):ti,ab,kw | 4312 |
| PEDro | ||
| Search | Strategy | Results |
| #5 | #2 AND #3 | 18 |
| #4 | #1 AND #3 | 11 |
| #3 | “blood flow restriction” | 133 |
| #2 | “anterior cruciate ligament” | 410 |
| #1 | “ACL” | 312 |
| ProQuest | #5 | |
| Search | Strategy | Results |
| #3 | #1 OR #2 | 66 |
| #2 | abstract((“anterior cruciate ligament reconstruction” OR “anterior cruciate ligament injuries” OR “anterior cruciate ligament” OR “acl”) AND (“blood flow restriction” OR “kaatsu” OR “vascular occlusion” OR “occlusive training” OR “occlusion training” OR “ blood vessel restriction”)) | 42 |
| #1 | title((“anterior cruciate ligament reconstruction” OR “anterior cruciate ligament injuries” OR “anterior cruciate ligament” OR “acl”) AND (“blood flow restriction” OR “kaatsu” OR “vascular occlusion” OR “occlusive training” OR “occlusion training” OR “ blood vessel restriction”)) | 51 |
Appendix B
Appendix C
| Reference | Items | Total Score | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | ||
| Curran et al., 2020 [37] | Y | Y | Y | Y | N | N | N | Y | N | Y | Y | 6/10 |
| Iversen et al., 2016 [36] | Y | Y | N | Y | N | N | Y | Y | Y | Y | Y | 7/10 |
| Jung et al., 2022 [42] | Y | N | N | Y | N | N | N | Y | N | Y | Y | 4/10 |
| Kacin et al., 2021 [35] | Y | N | N | Y | Y | N | Y | Y | Y | Y | Y | 7/10 |
| Li et al., 2023 [43] | Y | Y | N | Y | N | N | N | N | N | Y | Y | 4/10 |
| Ohta et al., 2003 [38] | Y | Y | N | Y | N | N | N | Y | N | Y | Y | 5/10 |
| Okoroha et al., 2023 [44] | Y | Y | N | N | N | N | N | N | N | Y | Y | 3/10 |
| Tramer et al., 2023 [39] | Y | Y | N | Y | N | N | Y | Y | Y | Y | Y | 7/10 |
| Zargi et al., 2016 [40] | Y | N | N | Y | Y | Y | Y | N | N | Y | Y | 6/10 |
| Zargi et al., 2018 [41] | Y | N | N | Y | Y | Y | N | Y | Y | Y | Y | 7/10 |
Appendix D
References
- Musahl, V.; Karlsson, J. Anterior Cruciate Ligament Tear. N. Engl. J. Med. 2019, 380, 2341–2348. [Google Scholar] [CrossRef]
- Sanders, T.L.; Maradit Kremers, H.; Bryan, A.J.; Larson, D.R.; Dahm, D.L.; Levy, B.A.; Stuart, M.J.; Krych, A.J. Incidence of Anterior Cruciate Ligament Tears and Reconstruction: A 21-Year Population-Based Study. Am. J. Sports Med. 2016, 44, 1502–1507. [Google Scholar] [CrossRef]
- Grapar Žargi, T.; Drobnič, M.; Vauhnik, R.; Koder, J.; Kacin, A. Factors Predicting Quadriceps Femoris Muscle Atrophy during the First 12 Weeks Following Anterior Cruciate Ligament Reconstruction. Knee 2017, 24, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Piussi, R.; Broman, D.; Musslinder, E.; Beischer, S.; Thomeé, R.; Hamrin Senorski, E. Recovery of Preoperative Absolute Knee Extension and Flexion Strength after ACL Reconstruction. BMC Sports Sci. Med. Rehabil. 2020, 12, 1–7. [Google Scholar] [CrossRef]
- Krishnan, C.; Williams, G.N. Factors Explaining Chronic Knee Extensor Strength Deficits after ACL Reconstruction. J. Orthop. Res. 2011, 29, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Brinlee, A.W.; Dickenson, S.B.; Hunter-Giordano, A.; Snyder-Mackler, L. ACL Reconstruction Rehabilitation: Clinical Data, Biologic Healing, and Criterion-Based Milestones to Inform a Return-to-Sport. Sports Health 2022, 14, 770. [Google Scholar] [CrossRef]
- de Jong, S.N.; van Caspel, D.R.; van Haeff, M.J.; Saris, D.B.F. Functional Assessment and Muscle Strength before and after Reconstruction of Chronic Anterior Cruciate Ligament Lesions. Arthrosc. J. Arthrosc. Relat. Surg. 2007, 23, e1–e21. [Google Scholar] [CrossRef] [PubMed]
- Rush, J.L.; Norte, G.E.; Lepley, A.S. Limb Differences in Hamstring Muscle Function and Morphology after Anterior Cruciate Ligament Reconstruction. Phys. Ther. Sport 2020, 45, 168–175. [Google Scholar] [CrossRef]
- Lindström, M.; Strandberg, S.; Wredmark, T.; Felländer-Tsai, L.; Henriksson, M. Functional and Muscle Morphometric Effects of ACL Reconstruction. A Prospective CT Study with 1 year Follow-Up. Scand. J. Med. Sci. Sports 2013, 23, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Zwolski, C.; Schmitt, L.C.; Quatman-Yates, C.; Thomas, S.; Hewett, T.E.; Paterno, M.V. The Influence of Quadriceps Strength Asymmetry on Patient-Reported Function at Time of Return to Sport after Anterior Cruciate Ligament Reconstruction. Am. J. Sports Med. 2015, 43, 2242–2249. [Google Scholar] [CrossRef]
- Ithurburn, M.P.; Altenburger, A.R.; Thomas, S.; Hewett, T.E.; Paterno, M.V.; Schmitt, L.C. Young Athletes after ACL Reconstruction with Quadriceps Strength Asymmetry at the Time of Return-to-Sport Demonstrate Decreased Knee Function 1 Year Later. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Logerstedt, D.; Lynch, A.; Axe, M.J.; Snyder-Mackler, L. Pre-Operative Quadriceps Strength Predicts IKDC2000 Scores 6months after Anterior Cruciate Ligament Reconstruction. Knee 2013, 20, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Shultz, S.J.; Schmitz, R.J.; Kulas, A.S.; Labban, J.D.; Wang, H.M. Quadriceps Muscle Volume Positively Contributes to ACL Volume. J. Orthop. Res. 2022, 40, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.M.; Nieman, D.C.; Swain, D.P. Quantity and Quality of Exercise for Developing and Maintaining Cardiorespiratory, Musculoskeletal, and Neuromotor Fitness in Apparently Healthy Adults: Guidance for Prescribing Exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef] [PubMed]
- van Grinsven, S.; van Cingel, R.E.H.; Holla, C.J.M.; van Loon, C.J.M. Evidence-Based Rehabilitation Following Anterior Cruciate Ligament Reconstruction. Knee Surg. Sports Traumatol. Arthrosc. 2010, 18, 1128–1144. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, E.; Sumen, Y.; Urabe, Y.; Deie, M.; Murakami, Y.; Adachi, N.; Ochi, M. An Early Return to Vigorous Activity May Destabilize Anterior Cruciate Ligaments Reconstructed with Hamstring Grafts. Arch. Phys. Med. Rehabil. 2004, 85, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Patterson, S.D.; Hughes, L.; Warmington, S.; Burr, J.; Scott, B.R.; Owens, J.; Abe, T.; Nielsen, J.L.; Libardi, C.A.; Laurentino, G.; et al. Blood Flow Restriction Exercise Position Stand: Considerations of Methodology, Application, and Safety. Front. Physiol. 2019, 10, 533. [Google Scholar] [CrossRef]
- Lorenz, D.S.; Bailey, L.; Wilk, K.E.; Mangine, R.E.; Head, P.; Grindstaff, T.L.; Morrison, S. Blood Flow Restriction Training. J. Athl. Train. 2021, 56, 937–944. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, L.P.; do Espírito Santo, R.C.; Ramis, T.R.; Portes, J.K.S.; da Silva Chakr, R.M.; Xavier, R.M. The Effects of Resistance Training with Blood Flow Restriction on Muscle Strength, Muscle Hypertrophy and Functionality in Patients with Osteoarthritis and Rheumatoid Arthritis: A Systematic Review with Meta-Analysis. PLoS ONE 2021, 16, e0259574. [Google Scholar] [CrossRef] [PubMed]
- Centner, C.; Wiegel, P.; Gollhofer, A.; König, D. Effects of Blood Flow Restriction Training on Muscular Strength and Hypertrophy in Older Individuals: A Systematic Review and Meta-Analysis. Sports Med. 2019, 49, 95–108. [Google Scholar] [CrossRef]
- Pearson, S.J.; Hussain, S.R. A Review on the Mechanisms of Blood-Flow Restriction Resistance Training-Induced Muscle Hypertrophy. Sports Med. 2015, 45, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Wengle, L.; Migliorini, F.; Leroux, T.; Chahal, J.; Theodoropoulos, J.; Betsch, M. The Effects of Blood Flow Restriction in Patients Undergoing Knee Surgery: A Systematic Review and Meta-Analysis. Am. J. Sports Med. 2022, 50, 2824–2833. [Google Scholar] [CrossRef]
- Hughes, L.; Paton, B.; Rosenblatt, B.; Gissane, C.; Patterson, S.D. Blood Flow Restriction Training in Clinical Musculoskeletal Rehabilitation: A Systematic Review and Meta-Analysis. Br. J. Sports Med. 2017, 51, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Altman, D.G.; Booth, A.; et al. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015: Elaboration and Explanation. BMJ 2015, 350, g7647. [Google Scholar] [CrossRef] [PubMed]
- Verhagen, A.P.; De-Vet, H.C.W.; De-Bie, R.A.; Kessels, A.G.H.; Boers, M.; Bouter, L.M.; Knipschild, P.G. The Delphi List: A Criteria List for Quality Assessment of Randomized Clinical Trials for Conducting Systematic Reviews Developed by Delphi Consensus. J. Clin. Epidemiol. 1998, 51, 1235–1241. [Google Scholar] [CrossRef] [PubMed]
- Umehara, T.; Tanaka, R. Effective Exercise Intervention Period for Improving Body Function or Activity in Patients with Knee Osteoarthritis Undergoing Total Knee Arthroplasty: A Systematic Review and Meta-Analysis. Braz. J. Phys. Ther. 2018, 22, 265. [Google Scholar] [CrossRef] [PubMed]
- Hahne, A.J.; Ford, J.J.; McMeeken, J.M. Conservative Management of Lumbar Disc Herniation with Associated Radiculopathy: A Systematic Review. Spine 2010, 35, E488–E504. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Maher, C.G.; Sherrington, C.; Herbert, R.D.; Moseley, A.M.; Elkins, M. Reliability of the PEDro Scale for Rating Quality of Randomized Controlled Trials. Phys. Ther. 2003, 83, 713–721. [Google Scholar] [CrossRef]
- de Morton, N.A. The PEDro Scale Is a Valid Measure of the Methodological Quality of Clinical Trials: A Demographic Study. Aust. J. Physiother. 2009, 55, 129–133. [Google Scholar] [CrossRef]
- Xie, C.X.; Machado, G.C. Clinimetrics: Grading of Recommendations, Assessment, Development and Evaluation (GRADE). J. Physiother. 2021, 67, 66. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge: New York, NY, USA, 1988; ISBN 9780203771587. [Google Scholar]
- The Cochrane Colaboration. Cochrane Handbook for Systematic Reviews of Interventions; Higgins, J.P.T., Green, S., Eds.; Wiley & Sons: Chichester, UK, 2019; ISBN 9781119536604. [Google Scholar]
- Cohen, J. Quantitative Methods in Psychology: A Power Primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Kacin, A.; Drobnič, M.; Marš, T.; Miš, K.; Petrič, M.; Weber, D.; Tomc Žargi, T.; Martinčič, D.; Pirkmajer, S. Functional and Molecular Adaptations of Quadriceps and Hamstring Muscles to Blood Flow Restricted Training in Patients with ACL Rupture. Scand. J. Med. Sci. Sports 2021, 31, 1636–1646. [Google Scholar] [CrossRef] [PubMed]
- Iversen, E.; Røstad, V.; Larmo, A. Intermittent Blood Flow Restriction Does Not Reduce Atrophy Following Anterior Cruciate Ligament Reconstruction. J. Sport Health Sci. 2016, 5, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Curran, M.T.; Bedi, A.; Mendias, C.L.; Wojtys, E.M.; Kujawa, M.V.; Palmieri-Smith, R.M. Blood Flow Restriction Training Applied with High-Intensity Exercise Does Not Improve Quadriceps Muscle Function after Anterior Cruciate Ligament Reconstruction: A Randomized Controlled Trial. Am. J. Sports Med. 2020, 48, 825–837. [Google Scholar] [CrossRef] [PubMed]
- Ohta, H.; Kurosawa, H.; Ikeda, H.; Iwase, Y.; Satou, N.; Nakamura, S. Low-Load Resistance Muscular Training with Moderate Restriction of Blood Flow after Anterior Cruciate Ligament Reconstruction. Acta Orthop. Scand. 2003, 74, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Tramer, J.S.; Khalil, L.S.; Jildeh, T.R.; Abbas, M.J.; McGee, A.; Lau, M.J.; Moutzouros, V.; Okoroha, K.R. Blood Flow Restriction Therapy for Two Weeks Prior to Anterior Cruciate Ligament Reconstruction Did Not Impact Quadriceps Strength Compared to Standard Therapy. Arthroscopy 2023, 39, 373–381. [Google Scholar] [CrossRef]
- Grapar Zargi, T.; Drobnic, M.; Koder, J.; Strazar, K.; Kacin, A. The Effects of Preconditioning with Ischemic Exercise on Quadriceps Femoris Muscle Atrophy Following Anterior Cruciate Ligament Reconstruction: A Quasi-Randomized Controlled Trial. Eur. J. Phys. Rehabil. Med. 2016, 52, 310–320. [Google Scholar] [PubMed]
- Žargi, T.; Drobnič, M.; Stražar, K.; Kacin, A. Short-Term Preconditioning With Blood Flow Restricted Exercise Preserves Quadriceps Muscle Endurance in Patients After Anterior Cruciate Ligament Reconstruction. Front. Physiol. 2018, 9, 1150. [Google Scholar] [CrossRef]
- Jung, W.S.; Kim, S.H.; Nam, S.S.; Kim, J.W.; Moon, H.W. Effects of Rehabilitation Exercise with Blood Flow Restriction after Anterior Cruciate Ligament Reconstruction. Appl. Sci. 2022, 12, 12058. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Qing, L.; Wang, H.; Ma, H.; Huang, P. Effect of Quadriceps Training at Different Levels of Blood Flow Restriction on Quadriceps Strength and Thickness in the Mid-Term Postoperative Period after Anterior Cruciate Ligament Reconstruction: A Randomized Controlled External Pilot Study. BMC Musculoskelet. Disord. 2023, 24, 360. [Google Scholar] [CrossRef] [PubMed]
- Okoroha, K.R.; Tramer, J.S.; Khalil, L.S.; Jildeh, T.R.; Abbas, M.J.; Buckley, P.J.; Lindell, C.; Moutzouros, V. Effects of a Perioperative Blood Flow Restriction Therapy Program on Early Quadriceps Strength and Patient-Reported Outcomes After Anterior Cruciate Ligament Reconstruction. Orthop. J. Sports Med. 2023, 11, 23259671231209696. [Google Scholar] [CrossRef] [PubMed]
- Koc, B.B.; Truyens, A.; Heymans, M.J.L.F.; Jansen, E.J.P.; Schotanus, M.G.M. Effect of Low-Load Blood Flow Restriction Training After Anterior Cruciate Ligament Reconstruction: A Systematic Review. Int. J. Sports Phys. Ther. 2022, 17, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Barber-Westin, S.; Noyes, F.R. Blood Flow-Restricted Training for Lower Extremity Muscle Weakness Due to Knee Pathology: A Systematic Review. Sports Health 2019, 11, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Suchomel, T.J.; Nimphius, S.; Bellon, C.R.; Stone, M.H. The Importance of Muscular Strength: Training Considerations. Sports Med. 2018, 48, 765–785. [Google Scholar] [CrossRef] [PubMed]
- Chmielewski, T.L.; Wilk, K.E.; Snyder-Mackler, L. Changes in Weight-Bearing Following Injury or Surgical Reconstruction of the ACL: Relationship to Quadriceps Strength and Function. Gait Posture 2002, 16, 87–95. [Google Scholar] [CrossRef]
| Domains of Evidence Quality | Downgrading of Evidence Quality | |
|---|---|---|
| One Level | Two Levels | |
| Risk of bias | >25% of participants from studies with low methodological quality, lack of randomization or allocation concealment, no sample size estimation, or no participants or assessors blinding. | >50% of participants from studies with low methodological quality, lack of randomization or allocation concealment, no sample size estimation, or no participants or assessors blinding. |
| Inconsistency of results | Significant heterogeneity in outcome measurement or intervention | |
| I2 value ≥ 50% | I2 value ≥ 75% | |
| Indirectness of evidence | Strict selection criteria established to circumvent indirectness evidence domain reassessment. | |
| Imprecision | 95% CI of an SMD > 0.2 points | 95% CI of an SMD > 0.5 points |
| Sample sizes < 50 individuals | Sample sizes < 30 individuals | |
| 95% CI of the risk ratio crossing the null value | ||
| Author, Year, Country | Study Design | BFR Group: N Participants, Age ± SD | Comparison Group: N Participants, Age ± SD | N Women/Men | Graft Origin | Concomitant Surgical Procedures | Outcome (Tool) |
|---|---|---|---|---|---|---|---|
| Curran et al., 2020 USA [37] | RCT | n = 19 17.4 ± 3.5 years | n = 18 15.7 ± 1.3 years | 19/15 | 25 BPTB 6 STG 3 QT | 10 MR 3 MX 4 OMI | Knee extensor MVIC and IKS at 60°/s (Biodex 3 dynamometer). Knee-related function (IKDC). |
| Iversen et al., 2016 Norway [36] | RCT | n = 12 29 ± 7.4 years | n = 12 29.8 ± 8.3 years | 10/14 | STG | - | Quadriceps CSA (Toshiba Excelart Vantage Atlas 1.5T MRI). |
| Jung et al., 2022 Korea [42] | Controlled trial | n = 12 30.8 ± 7.6 years | n = 12 27.8 ± 8.4 years | 6/18 | - | - | Knee extensor and flexor IKS at 60°/s (Biodex 4 dynamometer). Knee-related function (IKDC). |
| Kacin et al., 2021 Slovenia [35] | Controlled trial | n = 6 38 ± 6 years | n = 6 38 ± 8 years | 6/6 | STG | - | Knee extensor and flexor IKS at 60°/s (HUMAC NORM dynamometer). Quadriceps and hamstrings CSA (Siemens Magnetom Trio Tim 3T MRI). |
| Li et al., 2023 China [43] | RCT | G1: n = 8 30.5 ± 5.3 G2: n = 9 29.7 ± 4 | n = 6 28.3 ± 5.2 | -/- | - | - | Knee extensor IKS to body weight at 60°/s (Biodex 3 dynamometer). Knee-related function (IKDC). |
| Ohta et al., 2003 Japan [38] | RCT | n = 22 28 ± 9.7 years | n = 22 30 ± 9.7 years | 19/25 | STG | - | Knee extensor and flexor IKS at 60°/s and IMS (Biodex 3 dynamometer). Quadriceps and hamstrings + adductor CSA (Toshiba Visart 1.5T MRI). |
| Okohora et al., 2023 USA [44] | RCT | n = 16 25.4 ± 10.6 years | n = 22 27.5 ± 12 years | 18/28 | 30 BPTB 13 STG 3 QT | 9 MR 18 MX | Knee extensor IMS (Lafayette hand-held dynamometer). Knee-related function (IKDC). |
| Tramer et al., 2023 USA [39] | RCT | n = 23 26.5 ± 12 years | n = 22 27 ± 11 years | 20/25 | 34 BPTB 13 STG 3 QT | - | Knee extensor IMS (Lafayette hand-held dynamometer). |
| Zargi et al., 2016 Slovenia [40] | Controlled trial | n = 10 33 ± 7 years | n = 10 34 ± 10 years | 4/16 | STG | 12 MX | Rectus femoris CSA (Siemens 3T MRI). Knee extensor MVIC (S2P Isometric Knee Dynamometer). |
| Zargi et al., 2018 Slovenia [41] | Controlled trial | n = 10 34 ± 6 years | n = 10 35 ± 5 years | 4/16 | STG | 12 MX | Knee extensor MVIC (S2P Isometric Knee Dynamometer). |
| Author, Year | Occlusion Tool (Cuff Width) | Occlusion Area | Pressure Level | Comparison Group | Intervention Duration | Common Intervention in Both Groups |
|---|---|---|---|---|---|---|
| Curran et al., 2020 [37] | Delfi Easi-Fit Tourniquet Cuff | Proximal thigh | 80% LOP | Non-BFR | 8 weeks Start: 10 weeks after surgery | 16 sessions, 2/week. Single-leg press. 5 × 10 reps, 2 to 5 sets, 70% 1RM concentric-20% 1RM eccentric, or 20% 1RM concentric-70% 1RM eccentric. |
| Iversen et al., 2016 [36] | Delfi low pressure cuff (14 cm width) | Proximal thigh | 130 to 180 mmHg | Non-BFR | 12 days Start: 2 days after surgery | 24 sessions, 2/day. Isometric quadriceps contractions, leg mobility, straight leg raise. 5 × 20 reps. |
| Jung et al., 2022 [42] | Smart Tool Plus single-chamber pneumatic cuff | Proximal thigh | 40% systolic blood pressure | Non-BFR | 12 weeks Start: 3 days after surgery | 36 sessions, 3/week, 60 min/session. ROM, weight bearing, closed and open kinetic chain exercises. 1 × 30 reps, 3 × 15 reps, 10–30% 1RM. |
| Kacin et al., 2021 [35] | Ischemic Trainer double-chamber cuff (13.5 cm width) | Proximal thigh | 150 mmHg | Sham-BFR at 20 mmHg | 3 weeks Start: Before surgery | 9 sessions, 3/week. Knee flexion and extension of the injured leg. 4 × 40 reps, 40RM. |
| Li et al., 2023 [43] | AirBands | Proximal thigh | G1: 80% LOP G2: 40% LOP | Non-BFR | 8 weeks Start: >8 weeks after surgery | 16 sessions, 2/week, 60 min/session. Isometric quadriceps contractions, squats, lunges, walking, cycling. 1 × 30 reps, 3 × 15 reps, 10–20 kg and green or red Therabands for weight-bearing exercises. |
| Ohta et al., 2003 [38] | Hand-pump tourniquet | Proximal thigh | 180 mmHg | Non-BFR | 14 weeks Start: 2 weeks after surgery | 84 sessions, 6/week. Straight leg raise, hip abduction and adduction, half squat and step-up weight-bearing exercises (6–14 kg), knee-bending and walking exercises. 1–3 × 20 reps. |
| Okoroha et al., 2023 [44] | Smart Tool Plus single-chamber pneumatic cuff | Proximal thigh | 80% LOP | Non-BFR | 14 weeks Start: 2 weeks before surgery | 10 sessions, 5/week. Knee extension, straight leg raise, long arc quads, quarter squats. 1 × 30 reps, 3 × 15 reps. |
| Tramer et al., 2023 [39] | Smart Tool Plus single-chamber pneumatic cuff | Proximal thigh | 80% LOP | Non-BFR | 2 weeks Start: 2 weeks before surgery | 10 sessions, 5/week. Knee extension, straight leg raise, long arc quads, quarter squats. 1 × 30 reps, 3 × 15 reps. |
| Zargi et al., 2016 [40] | VariFit Conture Thigh Cuff (14 cm width) | Proximal thigh | 150 mmHg | Sham-BFR at 20 mmHg | 9 days Start: 10 days before surgery | 5 sessions, 3/week. Unilateral resisted knee extension with SL. 6 × 40, 40RM. |
| Zargi et al., 2018 [41] | VariFit Conture Thigh Cuff (14 cm width) | Proximal thigh | 150 mmHg | Sham-BFR at 20 mmHg | 1 week Start: 8 days before surgery | 5 sessions, 3/week. Unilateral resisted knee extension with SL. 6 × 40, 40RM. |
| Certainty Assessment | N of Patients | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | [Blood Flow Restriction] | [Control] | Relative (95% CI) | Absolute (95% CI) | ||
| Knee extensor isometric strength (Nm) | ||||||||||||
| 6 | Controlled trials | Very serious a,b,c | Serious e | Not serious | Very serious h,j,k | None | 100 | 104 | - | SMD 0.35 SD higher (0.04 lower to 0.74 higher) | ⨁◯◯◯ Very low | CRITICAL |
| Knee extensor isokinetic strength (Nm at 60°/s) | ||||||||||||
| 4 | Controlled trials | Very serious a,b,c | Serious e,f | Not serious | Very serious i,l | None | 70 | 58 | - | SMD 0.79 SD higher (0.06 higher to 1.52 higher) | ⨁◯◯◯ Very low | CRITICAL |
| Knee flexor isokinetic strength (Nm at 60°/s) | ||||||||||||
| 2 | Controlled trials | Very serious a,b,c,d | Serious e | Not serious | Very serious h,l | None | 34 | 34 | - | SMD 0.53 SD higher (0.04 higher to 1.01 higher) | ⨁◯◯◯ Very low | CRITICAL |
| Quadriceps cross-sectional area (cm2) | ||||||||||||
| 4 | Controlled trials | Very serious a,b,c,d | Not serious | Not serious | Very serious h,l | None | 50 | 50 | - | SMD 0.74 SD higher (0.33 higher to 1.15 higher) | ⨁◯◯◯ Very low | IMPORTANT |
| Perceived knee function (IKDC) | ||||||||||||
| 4 | Controlled trials | Very serious a,c | Not serious | Not serious | Very serious h,j,k | None | 64 | 58 | - | SMD 0.13 SD higher (0.32 lower to 0.57 higher) | ⨁◯◯◯ Very low | CRITICAL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fraca-Fernández, E.; Ceballos-Laita, L.; Hernández-Lázaro, H.; Jiménez-del-Barrio, S.; Mingo-Gómez, M.T.; Medrano-de-la-Fuente, R.; Hernando-Garijo, I. Effects of Blood Flow Restriction Training in Patients before and after Anterior Cruciate Ligament Reconstruction: A Systematic Review and Meta-Analysis. Healthcare 2024, 12, 1231. https://doi.org/10.3390/healthcare12121231
Fraca-Fernández E, Ceballos-Laita L, Hernández-Lázaro H, Jiménez-del-Barrio S, Mingo-Gómez MT, Medrano-de-la-Fuente R, Hernando-Garijo I. Effects of Blood Flow Restriction Training in Patients before and after Anterior Cruciate Ligament Reconstruction: A Systematic Review and Meta-Analysis. Healthcare. 2024; 12(12):1231. https://doi.org/10.3390/healthcare12121231
Chicago/Turabian StyleFraca-Fernández, Eduardo, Luis Ceballos-Laita, Héctor Hernández-Lázaro, Sandra Jiménez-del-Barrio, María Teresa Mingo-Gómez, Ricardo Medrano-de-la-Fuente, and Ignacio Hernando-Garijo. 2024. "Effects of Blood Flow Restriction Training in Patients before and after Anterior Cruciate Ligament Reconstruction: A Systematic Review and Meta-Analysis" Healthcare 12, no. 12: 1231. https://doi.org/10.3390/healthcare12121231
APA StyleFraca-Fernández, E., Ceballos-Laita, L., Hernández-Lázaro, H., Jiménez-del-Barrio, S., Mingo-Gómez, M. T., Medrano-de-la-Fuente, R., & Hernando-Garijo, I. (2024). Effects of Blood Flow Restriction Training in Patients before and after Anterior Cruciate Ligament Reconstruction: A Systematic Review and Meta-Analysis. Healthcare, 12(12), 1231. https://doi.org/10.3390/healthcare12121231