Machine Learning Approach for Cardiovascular Death Prediction among Nonalcoholic Steatohepatitis (NASH) Liver Transplant Recipients
Abstract
1. Introduction
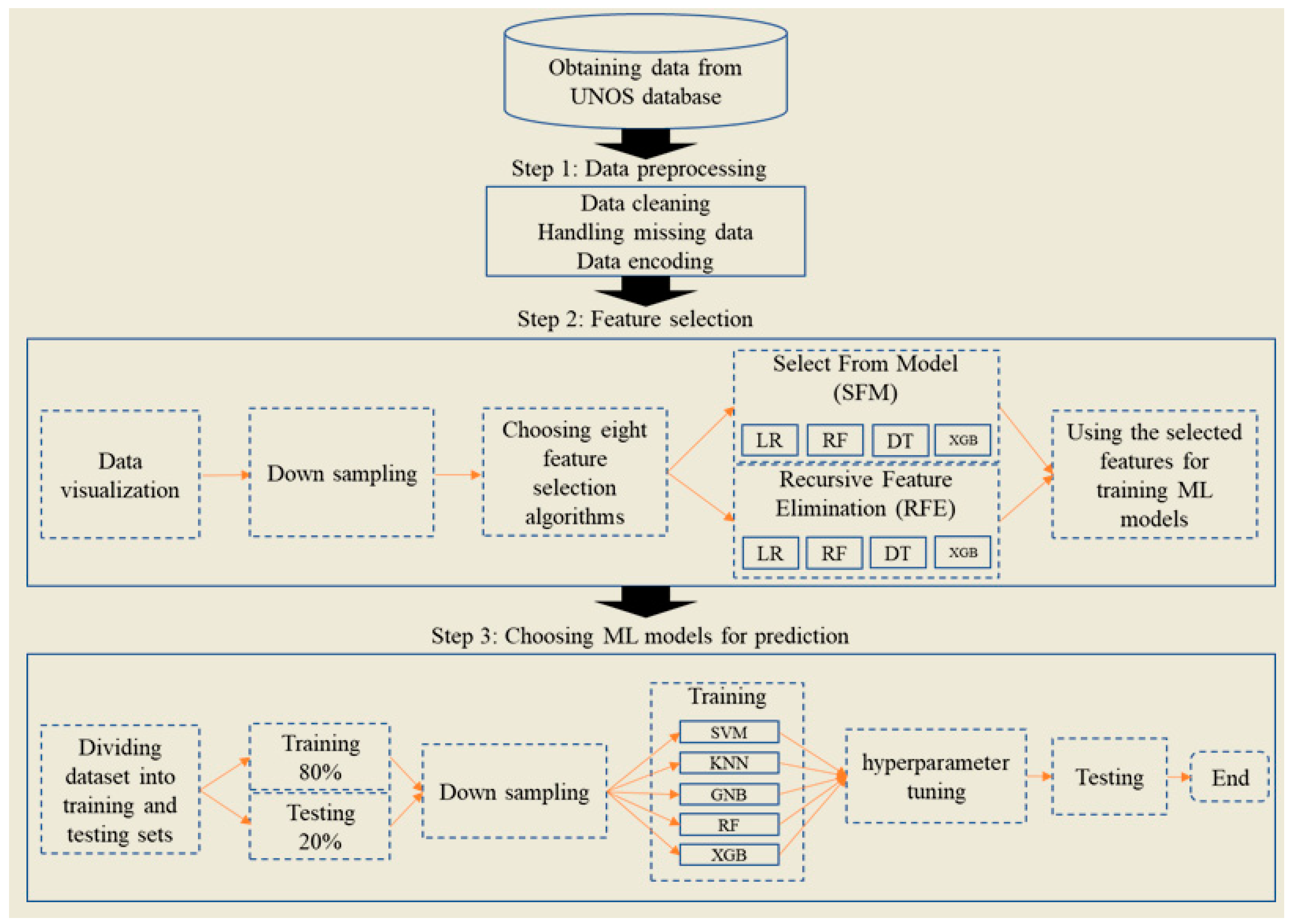
2. Materials and Methods
2.1. Study Framework
2.2. Study Population and Data Collection
2.3. Outcomes
2.4. Model Development
2.5. Statistical Analysis
2.6. ML Model Interpretation
2.7. Performance Evaluation
3. Results
3.1. Feature Importance Outputs
3.2. ML Methods’ Performance
3.3. SHAP Values
4. Discussion
4.1. Comparison with Previous Studies
4.2. Important Features
4.2.1. Blood Type
4.2.2. Body Mass Index
4.2.3. Serum Creatinine
4.2.4. State of Residency
4.3. Strengths and Limitations
4.4. Future Research Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Glossary
| ALF | Acute Liver Failure |
| CVD | Cardiovascular Death |
| DT | Decision Tree |
| GNB | Gaussian Naïve Bayes |
| GFR | Glomerular Filtration Rate |
| KNN | K-Nearest Neighbors |
| LT | Liver Transplant |
| ML | Machine Learning |
| NAFLD | Nonalcoholic Fatty Liver Disease |
| OPTN | Organ Procurement and Transplant Network |
| RF | Random Forest |
| RFE | Recursive Feature Elimination |
| RCRI | Revised Cardiac Risk Index |
| SFM | Select From Model |
| SHAP | Shapley Additive Explanations |
| SVM | Support Vector Machine |
| UNOS | United Network for Organ Sharing |
| VWF | Von Willebrand Factor |
| XGB | XGBoost |
References
- Khuroo, M.S.; Kamili, S. Aetiology and Prognostic Factors in Acute Liver Failure in India. J. Viral. Hepat. 2003, 10, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Wasley, A.; Fiore, A.; Bell, B.P. Hepatitis A in the Era of Vaccination. Epidemiol. Rev. 2006, 28, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Purcell, R.H.; Emerson, S.U. Hepatitis E: An Emerging Awareness of an Old Disease. J. Hepatol. 2008, 48, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Reuben, A.; Koch, D.G.; Lee, W.M.; Acute Liver Failure Study Group. Drug-Induced Acute Liver Failure: Results of a U.S. Multicenter, Prospective Study. Hepatology 2010, 52, 2065–2076. [Google Scholar] [CrossRef] [PubMed]
- Henrion, J. Hypoxic Hepatitis. Liver. Int. 2012, 32, 1039–1052. [Google Scholar] [CrossRef] [PubMed]
- Wijdicks, E.F.M. Hepatic Encephalopathy. N. Engl. J. Med. 2016, 375, 1660–1670. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. FastStats. Available online: https://www.cdc.gov/nchs/fastats/liver-disease.htm (accessed on 29 May 2023).
- Kwong, A.J.; Ebel, N.H.; Kim, W.R.; Lake, J.R.; Smith, J.M.; Schladt, D.P.; Schnellinger, E.M.; Handarova, D.; Weiss, S.; Cafarella, M. OPTN/SRTR 2021 Annual Data Report: Liver. Am. J. Transplant. 2023, 23, S178–S263. [Google Scholar] [CrossRef] [PubMed]
- Sheka, A.C.; Adeyi, O.; Thompson, J.; Hameed, B.; Crawford, P.A.; Ikramuddin, S. Nonalcoholic Steatohepatitis: A Review. JAMA 2020, 323, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.; Bansal, A.; Radakovich, N.; Sharma, V.; Khan, M.Z.; Harris, K.; Bachour, S.; Kleb, C.; Cywinski, J.; Argalious, M.; et al. Machine Learning Models to Predict Major Adverse Cardiovascular Events After Orthotopic Liver Transplantation: A Cohort Study. J. Cardiothorac. Vasc. Anesth. 2021, 35, 2063–2069. [Google Scholar] [CrossRef]
- Sharma, D.; Gotlieb, N.; Farkouh, M.E.; Patel, K.; Xu, W.; Bhat, M. Machine Learning Approach to Classify Cardiovascular Disease in Patients with Nonalcoholic Fatty Liver Disease in the UK Biobank Cohort. J. Am. Heart. Assoc. 2022, 11, e022576. [Google Scholar] [CrossRef]
- Dag, A.; Topuz, K.; Oztekin, A.; Bulur, S.; Megahed, F.M. A Probabilistic Data-Driven Framework for Scoring the Preoperative Recipient-Donor Heart Transplant Survival. Decis. Support Syst. 2016, 86, 1–12. [Google Scholar] [CrossRef]
- Penone, C.; Davidson, A.D.; Shoemaker, K.T.; Di Marco, M.; Rondinini, C.; Brooks, T.M.; Young, B.E.; Graham, C.H.; Costa, G.C. Imputation of Missing Data in Life-History Trait Datasets: Which Approach Performs the Best? Methods Ecol. Evol. 2014, 5, 961–970. [Google Scholar] [CrossRef]
- Giustino, G.; Pinney, S.P.; Lala, A.; Reddy, V.Y.; Johnston-Cox, H.A.; Mechanick, J.I.; Halperin, J.L.; Fuster, V. Coronavirus and Cardiovascular Disease, Myocardial Injury, and Arrhythmia: JACC Focus Seminar. J. Am. Coll. Cardiol. 2020, 76, 2011–2023. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.C.C.; Chung, T.; Yong, A.S.C.; Wong, H.S.P.; Chow, V.; Celermajer, D.S.; Kritharides, L. Long-Term Cardiovascular and Noncardiovascular Mortality of 1023 Patients with Confirmed Acute Pulmonary Embolism. Circ. Cardiovasc. Qual. Outcomes 2011, 4, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Nesto, R.W.; Bell, D.; Bonow, R.O.; Fonseca, V.; Grundy, S.M.; Horton, E.S.; Le Winter, M.; Porte, D.; Semenkovich, C.F.; Smith, S.; et al. Thiazolidinedione Use, Fluid Retention, and Congestive Heart Failure: A Consensus Statement from the American Heart Association and American Diabetes Association. Diabetes Care 2004, 27, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.M.; Provost, F. The Effect of Class Distribution on Classifier Learning; Rutgers University: New Brunswick, NJ, USA, 2001. [Google Scholar]
- Estabrooks, A.; Jo, T.; Japkowicz, N. A Multiple Resampling Method for Learning from Imbalanced Data Sets. Comput. Intell. 2004, 20, 18–36. [Google Scholar] [CrossRef]
- He, H.; Garcia, E.A. Learning from Imbalanced Data. IEEE Trans. Knowl. Data Eng. 2009, 21, 1263–1284. [Google Scholar] [CrossRef]
- Cortes, C.; Vapnik, V. Support-Vector Networks. Mach. Learn. 1995, 20, 273–297. [Google Scholar] [CrossRef]
- Fix, E. Discriminatory Analysis: Nonparametric Discrimination, Consistency Properties; USAF School of Aviation Medicine: Dayton, OH, USA, 1985. [Google Scholar]
- James, G.; Witten, D.; Hastie, T.; Tibshirani, R. An Introduction to Statistical Learning: With Applications in R; Springer: New York, NY, USA, 2021; ISBN 978-1-07-161417-4. [Google Scholar]
- Ho, T.K. Random Decision Forests. In Proceedings of the 3rd International Conference on Document Analysis and Recognition, Montreal, QC, Canada, 14–16 August 1995; Volume 1, pp. 278–282. [Google Scholar]
- Chen, T.; Guestrin, C. XGBoost: A Scalable Tree Boosting System. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13 August 2016; Association for Computing Machinery: New York, NY, USA; pp. 785–794. [Google Scholar]
- John, G.H.; Langley, P. Estimating Continuous Distributions in Bayesian Classifiers. arXiv 2013, arXiv:1302.4964. [Google Scholar]
- Rasmussen, C.E. Gaussian Processes in Machine Learning. In Advanced Lectures on Machine Learning: ML Summer Schools 2003, Canberra, Australia, February 2–14, 2003, Tübingen, Germany, August 4–16, 2003, Revised Lectures; Bousquet, O., von Luxburg, U., Rätsch, G., Eds.; Lecture Notes in Computer Science; Springer: Berlin/Heidelberg, Germany, 2004; pp. 63–71. ISBN 978-3-540-28650-9. [Google Scholar]
- Chen, X.; Jeong, J.C. Enhanced Recursive Feature Elimination. In Proceedings of the Sixth International Conference on Machine Learning and Applications (ICMLA 2007), Cincinnati, OH, USA, 13–15 December 2007; pp. 429–435. [Google Scholar]
- Huljanah, M.; Rustam, Z.; Utama, S.; Siswantining, T. Feature Selection Using Random Forest Classifier for Predicting Prostate Cancer. In IOP Conference Series: Materials Science and Engineering; IOP Publishing Ltd.: Bristol, UK, 2019; Volume 546, p. 052031. [Google Scholar] [CrossRef]
- Mangalathu, S.; Hwang, S.-H.; Jeon, J.-S. Failure Mode and Effects Analysis of RC Members Based on Machine-Learning-Based SHapley Additive exPlanations (SHAP) Approach. Eng. Struct. 2020, 219, 110927. [Google Scholar] [CrossRef]
- Bradley, A.P. The Use of the Area under the ROC Curve in the Evaluation of Machine Learning Algorithms. Pattern Recognit. 1997, 30, 1145–1159. [Google Scholar] [CrossRef]
- Spann, A.; Yasodhara, A.; Kang, J.; Watt, K.; Wang, B.; Goldenberg, A.; Bhat, M. Applying Machine Learning in Liver Disease and Transplantation: A Comprehensive Review. Hepatology 2020, 71, 1093–1105. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-L.; Soong, R.-S.; Lee, W.-C.; Jiang, G.-W.; Lin, Y.-C. Predicting Short-Term Survival after Liver Transplantation Using Machine Learning. Sci. Rep. 2020, 10, 5654. [Google Scholar] [CrossRef] [PubMed]
- Albeldawi, M.; Aggarwal, A.; Madhwal, S.; Cywinski, J.; Lopez, R.; Eghtesad, B.; Zein, N.N. Cumulative Risk of Cardiovascular Events after Orthotopic Liver Transplantation. Liver. Transpl. 2012, 18, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Dec, G.W.; Kondo, N.; Farrell, M.L.; Dienstag, J.; Cosimi, A.B.; Semigran, M.J. Cardiovascular Complications Following Liver Transplantation. Clin. Transplant. 1995, 9, 463–471. [Google Scholar] [PubMed]
- Fussner, L.A.; Heimbach, J.K.; Fan, C.; Dierkhising, R.; Coss, E.; Leise, M.D.; Watt, K.D. Cardiovascular Disease after Liver Transplantation: When, What, and Who Is at Risk. Liver Transplant. 2015, 21, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yang, S.-H.; Xu, H.; Li, J.-J. ABO Blood Group System and the Coronary Artery Disease: An Updated Systematic Review and Meta-Analysis. Sci. Rep. 2016, 6, 23250. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, B.; Radmard, N.; Faghani-Makrani, A.; Rasouli, M. Serum Creatinine and Occurrence and Severity of Coronary Artery Disease. Med. Arch. 2019, 73, 154–156. [Google Scholar] [CrossRef]
- Wannamethee, S.G.; Shaper, A.G.; Perry, I.J. Serum Creatinine Concentration and Risk of Cardiovascular Disease. Stroke 1997, 28, 557–563. [Google Scholar] [CrossRef]
- Jenkins, P.V.; O’Donnell, J.S. ABO Blood Group Determines Plasma von Willebrand Factor Levels: A Biologic Function after All? Transfusion 2006, 46, 1836–1844. [Google Scholar] [CrossRef]
- Song, J.; Chen, F.; Campos, M.; Bolgiano, D.; Houck, K.; Chambless, L.E.; Wu, K.K.; Folsom, A.R.; Couper, D.; Boerwinkle, E.; et al. Quantitative Influence of ABO Blood Groups on Factor VIII and Its Ratio to von Willebrand Factor, Novel Observations from an ARIC Study of 11,673 Subjects. PLoS ONE 2015, 10, e0132626. [Google Scholar] [CrossRef] [PubMed]
- Franchini, M.; Crestani, S.; Frattini, F.; Sissa, C.; Bonfanti, C. ABO Blood Group and von Willebrand Factor: Biological Implications. Clin. Chem. Lab. Med. 2014, 52, 1273–1276. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, C.; Ke, X.; Xiong, L.; Shi, Y.; Li, J.; Tan, X.; Ye, S. Analysis of Circulating Cholesterol Levels as a Mediator of an Association between ABO Blood Group and Coronary Heart Disease. Circ. Cardiovasc. Genet. 2014, 7, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Karakas, M.; Baumert, J.; Kleber, M.E.; Thorand, B.; Dallmeier, D.; Silbernagel, G.; Grammer, T.B.; Rottbauer, W.; Meisinger, C.; Illig, T.; et al. A Variant in the Abo Gene Explains the Variation in Soluble E-Selectin Levels—Results from Dense Genotyping in Two Independent Populations. PLoS ONE 2012, 7, e51441. [Google Scholar] [CrossRef] [PubMed]
- Paré, G.; Chasman, D.I.; Kellogg, M.; Zee, R.Y.L.; Rifai, N.; Badola, S.; Miletich, J.P.; Ridker, P.M. Novel Association of ABO Histo-Blood Group Antigen with Soluble ICAM-1: Results of a Genome-Wide Association Study of 6,578 Women. PLoS Genet. 2008, 4, e1000118. [Google Scholar] [CrossRef] [PubMed]
- Paterson, A.D.; Lopes-Virella, M.F.; Waggott, D.; Boright, A.P.; Hosseini, S.M.; Carter, R.E.; Shen, E.; Mirea, L.; Bharaj, B.; Sun, L.; et al. Genome-Wide Association Identifies the ABO Blood Group as a Major Locus Associated with Serum Levels of Soluble E-Selectin. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1958–1967. [Google Scholar] [CrossRef] [PubMed]
- Dell’Anna, P.; Adorni, D.; Bernuzzi, G.; Cantarelli, S.; Cepparulo, A.; Cocchi, T.; Dell’Anna, L.; Formentini, A.; Sassi, M.; Scognamiglio, F.; et al. Evaluation of Cardiovascular Risk in Blood Donors: Results of the CARDIORISK Study in the Parma Transfusion Service. Blood. Transfus. 2010, 8, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Longo, M.; Palmieri, L.; Marconi, M.; Giampaoli, S.; Cremonesi, G.; Rebulla, P.; Sirchia, G. A New Setting of Opportunistic Cardiovascular Screening: From Blood Donation to Preventive Cardiology. Preliminary Results of the Cardiorisk Program. G. Ital. Cardiol. 2010, 11, 578–583. [Google Scholar]
- Longo, M.; Lucci, C.; Marconi, M.; Cremonesi, G. Cross-Sectional Epidemiological Study to Evaluate the Cardiovascular Profile of a Cohort of Blood Donors. Blood. Transfus. 2007, 5, 153–157. [Google Scholar] [CrossRef]
- Vasan, S.K.; Rostgaard, K.; Majeed, A.; Ullum, H.; Titlestad, K.-E.; Pedersen, O.B.V.; Erikstrup, C.; Nielsen, K.R.; Melbye, M.; Nyrén, O.; et al. ABO Blood Group and Risk of Thromboembolic and Arterial Disease. Circulation 2016, 133, 1449–1457. [Google Scholar] [CrossRef]
- Canbay, A.; Sowa, J.-P.; Syn, W.-K.; Treckmann, J. NASH Cirrhosis—The New Burden in Liver Transplantation: How Should It Be Managed? Viszeralmedizin 2016, 32, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, N.S.; Duseja, A. Screening of Cardiovascular Disease in Nonalcoholic Fatty Liver Disease: Whom and How? J. Clin. Exp. Hepatol. 2019, 9, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Conzen, K.D.; Vachharajani, N.; Collins, K.M.; Anderson, C.D.; Lin, Y.; Wellen, J.R.; Shenoy, S.; Lowell, J.A.; Doyle, M.B.M.; Chapman, W.C. Morbid Obesity in Liver Transplant Recipients Adversely Affects Longterm Graft and Patient Survival in a Single-Institution Analysis. HPB 2015, 17, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Fracanzani, A.L.; Petta, S.; Lombardi, R.; Pisano, G.; Russello, M.; Consonni, D.; Di Marco, V.; Cammà, C.; Mensi, L.; Dongiovanni, P.; et al. Liver and Cardiovascular Damage in Patients with Lean Nonalcoholic Fatty Liver Disease, and Association with Visceral Obesity. Clin. Gastroenterol. Hepatol. 2017, 15, 1604–1611.e1. [Google Scholar] [CrossRef] [PubMed]
- Harrer, S.; Shah, P.; Antony, B.; Hu, J. Artificial Intelligence for Clinical Trial Design. Trends Pharmacol. Sci. 2019, 40, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Ye, J.; Li, F.; Lin, Y.; Wu, T.; Wang, W.; Feng, S.; Zhong, B. Early Predictors of Cardiovascular Disease Risk in Nonalcoholic Fatty Liver Disease: Non-Obese Versus Obese Patients. Dig. Dis. Sci. 2020, 65, 1850–1860. [Google Scholar] [CrossRef] [PubMed]
- Styczynski, G.; Kalinowski, P.; Michałowski, Ł.; Paluszkiewicz, R.; Ziarkiewicz-Wróblewska, B.; Zieniewicz, K.; Tataj, E.; Rabczenko, D.; Szmigielski, C.A.; Sinski, M. Cardiac Morphology, Function, and Hemodynamics in Patients with Morbid Obesity and Nonalcoholic Steatohepatitis. J. Am. Heart Assoc. 2021, 10, e017371. [Google Scholar] [CrossRef] [PubMed]
- Casanueva, F.F.; Moreno, B.; Rodríguez-Azeredo, R.; Massien, C.; Conthe, P.; Formiguera, X.; Barrios, V.; Balkau, B. Relationship of Abdominal Obesity with Cardiovascular Disease, Diabetes and Hyperlipidaemia in Spain. Clin. Endocrinol. 2010, 73, 35–40. [Google Scholar] [CrossRef]
- Gutiérrez-Cuevas, J.; Santos, A.; Armendariz-Borunda, J. Pathophysiological Molecular Mechanisms of Obesity: A Link between MAFLD and NASH with Cardiovascular Diseases. Int. J. Mol. Sci. 2021, 22, 11629. [Google Scholar] [CrossRef]
- Kjeldsen, S.E. Hypertension and Cardiovascular Risk: General Aspects. Pharmacol. Res. 2018, 129, 95–99. [Google Scholar] [CrossRef]
- Ritchie, S.A.; Connell, J.M.C. The Link between Abdominal Obesity, Metabolic Syndrome and Cardiovascular Disease. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 319–326. [Google Scholar] [CrossRef]
- World Health Organization. Obesity: Preventing and Managing the Global Epidemic: Report of a WHO Consultation; World Health Organization: Geneva, Switzerland, 2000; ISBN 978-92-4-120894-9. [Google Scholar]
- Hariharan, R.; Odjidja, E.N.; Scott, D.; Shivappa, N.; Hébert, J.R.; Hodge, A.; de Courten, B. The Dietary Inflammatory Index, Obesity, Type 2 Diabetes, and Cardiovascular Risk Factors and Diseases. Obes. Rev. 2022, 23, e13349. [Google Scholar] [CrossRef]
- Pamuk, F.; Kantarci, A. Inflammation as a Link between Periodontal Disease and Obesity. Periodontology 2022, 90, 186–196. [Google Scholar] [CrossRef]
- Gurunathan, U.; Myles, P.S. Limitations of Body Mass Index as an Obesity Measure of Perioperative Risk. BJA Br. J. Anaesth. 2016, 116, 319–321. [Google Scholar] [CrossRef]
- Rothman, K.J. BMI-Related Errors in the Measurement of Obesity. Int. J. Obes. 2008, 32, S56–S59. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.B.; Adams, J.B. Practical Applications and Limitations of Tracking Body Mass Index in Schools. J. Phys. Educ. Recreat. Danc. 2009, 80, 14–54. [Google Scholar] [CrossRef]
- Must, A.; Anderson, S.E. Body Mass Index in Children and Adolescents: Considerations for Population-Based Applications. Int. J. Obes. 2006, 30, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Pitt, B.; Filippatos, G.; Agarwal, R.; Anker, S.D.; Bakris, G.L.; Rossing, P.; Joseph, A.; Kolkhof, P.; Nowack, C.; Schloemer, P.; et al. Cardiovascular Events with Finerenone in Kidney Disease and Type 2 Diabetes. N. Engl. J. Med. 2021, 385, 2252–2263. [Google Scholar] [CrossRef]
- Tanaka, K.; Watanabe, T.; Takeuchi, A.; Ohashi, Y.; Nitta, K.; Akizawa, T.; Matsuo, S.; Imai, E.; Makino, H.; Hishida, A. Cardiovascular Events and Death in Japanese Patients with Chronic Kidney Disease. Kidney Int. 2017, 91, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, M.; Wiebe, N.; Culleton, B.; House, A.; Rabbat, C.; Fok, M.; McAlister, F.; Garg, A.X. Chronic Kidney Disease and Mortality Risk: A Systematic Review. J. Am. Soc. Nephrol. 2006, 17, 2034. [Google Scholar] [CrossRef]
- Josefsson, A.; Fu, M.; Björnsson, E.; Castedal, M.; Kalaitzakis, E. Pre-Transplant Renal Impairment Predicts Posttransplant Cardiac Events in Patients with Liver Cirrhosis. Transplantation 2014, 98, 107. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Jin, H.; Wang, D.; Liu, J.; Qin, Y.; Zhang, Y.; Zhang, Y.; Xiang, Q. Serum Creatinine Levels, Traditional Cardiovascular Risk Factors and 10-Year Cardiovascular Risk in Chinese Patients with Hypertension. Front. Endocrinol. 2023, 14, 1140093. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Tian, X.; Wu, S.; Zuo, Y.; Chen, S.; Mo, D.; Luo, Y.; Wang, Y. Metabolic Factors Mediate the Association Between Serum Uric Acid to Serum Creatinine Ratio and Cardiovascular Disease. J. Am. Heart Assoc. 2021, 10, e023054. [Google Scholar] [CrossRef] [PubMed]
- Diez-Roux, A.V.; Link, B.G.; Northridge, M.E. A Multilevel Analysis of Income Inequality and Cardiovascular Disease Risk Factors. Soc. Sci. Med. 2000, 50, 673–687. [Google Scholar] [CrossRef] [PubMed]
- Fowler-Brown, A.; Corbie-Smith, G.; Garrett, J.; Lurie, N. Risk of Cardiovascular Events and Death—Does Insurance Matter? J. Gen. Intern. Med. 2007, 22, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Kunkel, S.R. The Association between Heart Disease Mortality and Geographic Access to Hospitals: County Level Comparisons in Ohio, USA. Soc. Sci. Med. 2010, 70, 1211–1218. [Google Scholar] [CrossRef]
- Ahsan, M.M.; Siddique, Z. Machine Learning-Based Heart Disease Diagnosis: A Systematic Literature Review. Artif. Intell. Med. 2022, 128, 102289. [Google Scholar] [CrossRef]




| Characteristics | All Recipients (n = 10,871) | Alive (n = 8261) | CVD (n = 449) | Non-CVD Death (n = 2161) | p-Value | Adjusted p-Value |
|---|---|---|---|---|---|---|
| Recipient characteristics | ||||||
| Age (years) | 58.91 | 58.50 | 60.85 | 60.07 | < 0.001 | <0.001 * |
| BMI (kg/m) | 32.70 | 32.80 | 32.91 | 32.25 | 0.002 | 0.004 * |
| Ethnicity | <0.001 | <0.001 * | ||||
| Non-Hispanic | 9334 (85.86%) | 7010 (84.85%) | 390 (86.86%) | 1934 (89.50%) | ||
| Hispanic | 1537 (14.14%) | 1251 (15.14%) | 59 (13.14%) | 227 (10.50%) | ||
| Gender | 0.470278 | 0.470278 | ||||
| Female | 5160 (47.46%) | 3939 (47.68%) | 219 (48.77%) | 1002 | ||
| Male | 5711 (52.54%) | 4322 (52.31%) | 230 (51.22%) | 1159 | ||
| Blood Type | 0.257287 | 0.257287 | ||||
| O | 4780 (43.97%) | 3619 (43.80%) | 202 (45.00%) | 959 (44.38%) | ||
| A | 4037 (37.14%) | 3033 (36.71%) | 179 (40.00%) | 825 (38.18%) | ||
| B | 1436 (13.21%) | 1108 (13.41%) | 57 (12.50%) | 271 (12.55%) | ||
| AB | 600 (5.50%) | 486 (5.88%) | 11 (2.50%) | 103 (4.76%) | ||
| A1 | 14 (1.28%) | 11 (0.14%) | 0 | 3 (0.13%) | ||
| A1B | 2 (0.018%) | 2 (0.03%) | 0 | 0 | ||
| A2B | 1 (0.009%) | 1 (0.015%) | 0 | 0 | ||
| A2 | 1 (0.009%) | 1 (0.015%) | 0 | 0 | ||
| Diabetes | <0.001 * | <0.00 * | ||||
| No | 4836 (44.48%) | 3829 (46.35%) | 161 (35.85%) | 846 (39.14%) | ||
| Type I | 234 (2.15%) | 133 (1.60%) | 16 (3.56%) | 85 (3.93%) | ||
| Type II | 5462 (50.24%) | 4082 (49.42%) | 252 (56.12%) | 1128 (52.19%) | ||
| Other type | 105 (0.96%) | 82 (0.99%) | 4 (0.89%) | 19 (0.87%) | ||
| Unknown type | 190 (1.74%) | 109 (1.32%) | 14 (3.14%) | 67 (3.10%) | ||
| Diabetes status unknown | 44 (40%) | 26 (0.32%) | 2 (0.44%) | 16 (0.74%) | ||
| Albumin (g/dL) | 3.11 | 3.13 | 3.13 | 3.05 | <0.001 * | <0.001 * |
| BILIRUBIN (μmol/L) | 7.67 | 7.63 | 8.57 | 7.63 | 0.03 * | 0.05828 |
| INR | 1.97 | 1.99 | 1.98 | 1.89 | <0.001 * | <0.001 * |
| MELD score | 25.21 | 25.21 | 26.19 | 25.00 | 0.04 | 0.06 |
| SERUM CREAT (mg/dL) | 1.83 | 1.77 | 2.10 | 2.00 | <0.001 * | <0.001 * |
| SERUM SODIUM (mmol/L) | 135.12 | 134.99 | 135.22 | 135.60 | <0.001 * | <0.001 * |
| Donor characteristics | ||||||
| Age (years) | 42.61 | 42.48 | 42.27 | 43.15 | 0.27 | 0.36 |
| Gender | 0.47 | 0.47 | ||||
| Female | 4363 (40.13%) | 3294 (39.87%) | 177 (39.42%) | 892 (41.28%) | ||
| Male | 6508 (59.86%) | 4967 (60.13%) | 272 (60.58%) | 1269 (58.72%) | ||
| Diabetes | 0.43 | 0.43 | ||||
| No | 9399 (86.46%) | 7135 (86.64%) | 389 (86.67%) | 1875 (86.77%) | ||
| Yes | 105 (0.96%) | 87 (0.010%) | 5 (1.11%) | 13 (0.60%) | ||
| Unknown | 1367 (12.58%) | 1039 (12.57%) | 55 (12.24%) | 273 (12.63%) | ||
| Blood Type | 0.06 | 0.06 | ||||
| O | 1588 (14.60%) | 1193 (14.44%) | 72 (16.03%) | 323 (14.96%) | ||
| A | 2210 (20.32%) | 1636 (19.80%) | 98 (21.82%) | 476 (22.02%) | ||
| B | 156 (1.44%) | 123 (1.49%) | 4 (0.89%) | 29 (1.34%) | ||
| AB | 364 (3.35%) | 293 (3.54%) | 143 (3.11%) | 57 (2.63%) | ||
| A1 | 54 (0.49%) | 47 (0.56%) | 1 (0.22%) | 6 (0.27%) | ||
| A1B | 165 (1.51%) | 134 (1.62%) | 2 (0.44%) | 29 (1.35%) | ||
| A2B | 1307 (12.02%) | 1020 (12.35%) | 46 (10.24%) | 241 (11.15%) | ||
| A2 | 5027 (46.24%) | 3815 (46.18%) | 212 (47.21%) | 1000 (46.27%) | ||
| Feature Number | Feature | RFE_LR | SFM_LR | SFM_RF | RFE_RF | RFE_DT | SFM_DT | SFM_XGB | RFE_XGB | Number of Time Selected |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Recipient blood type | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 8 |
| 2 | Recipient height | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 6 | ||
| 3 | Actual year the registrant | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 6 | ||
| 4 | Donor blood type | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 6 | ||
| 5 | Donor home state | ✓ | ✓ | ✓ | ✓ | ✓ | 5 | |||
| 6 | Recipient bilirubin | ✓ | ✓ | ✓ | 3 | |||||
| 7 | Age | ✓ | ✓ | ✓ | 3 | |||||
| 8 | Recipient weight (kg) | ✓ | ✓ | ✓ | 3 | |||||
| 9 | Recipient dialysis | ✓ | ✓ | ✓ | 3 | |||||
| 10 | Donor hematocrit level | ✓ | ✓ | 2 | ||||||
| 11 | Recipient serum creatinine | ✓ | ✓ | 2 | ||||||
| 12 | Recipient state of residency | ✓ | ✓ | 2 | ||||||
| 13 | MELD score | ✓ | ✓ | 2 | ||||||
| 14 | Deceased donor cardiac arrest | ✓ | ✓ | 2 | ||||||
| 15 | BMI | ✓ | ✓ | 2 | ||||||
| 16 | Recipient serum sodium level | ✓ | 1 | |||||||
| 17 | Recipient work for income | ✓ | 1 | |||||||
| 18 | Recipient INR | ✓ | 1 | |||||||
| 19 | Recipient albumin level | ✓ | 1 | |||||||
| 20 | Total cold ischemic time | ✓ | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fatemi, Y.; Nikfar, M.; Oladazimi, A.; Zheng, J.; Hoy, H.; Ali, H. Machine Learning Approach for Cardiovascular Death Prediction among Nonalcoholic Steatohepatitis (NASH) Liver Transplant Recipients. Healthcare 2024, 12, 1165. https://doi.org/10.3390/healthcare12121165
Fatemi Y, Nikfar M, Oladazimi A, Zheng J, Hoy H, Ali H. Machine Learning Approach for Cardiovascular Death Prediction among Nonalcoholic Steatohepatitis (NASH) Liver Transplant Recipients. Healthcare. 2024; 12(12):1165. https://doi.org/10.3390/healthcare12121165
Chicago/Turabian StyleFatemi, Yasin, Mohsen Nikfar, Amir Oladazimi, Jingyi Zheng, Haley Hoy, and Haneen Ali. 2024. "Machine Learning Approach for Cardiovascular Death Prediction among Nonalcoholic Steatohepatitis (NASH) Liver Transplant Recipients" Healthcare 12, no. 12: 1165. https://doi.org/10.3390/healthcare12121165
APA StyleFatemi, Y., Nikfar, M., Oladazimi, A., Zheng, J., Hoy, H., & Ali, H. (2024). Machine Learning Approach for Cardiovascular Death Prediction among Nonalcoholic Steatohepatitis (NASH) Liver Transplant Recipients. Healthcare, 12(12), 1165. https://doi.org/10.3390/healthcare12121165







