Dyslipidemia Is Positively Associated with Chronic Low Back Pain in Korean Women: Korean National Health and Nutrition Examination Survey 2010–2012
Abstract
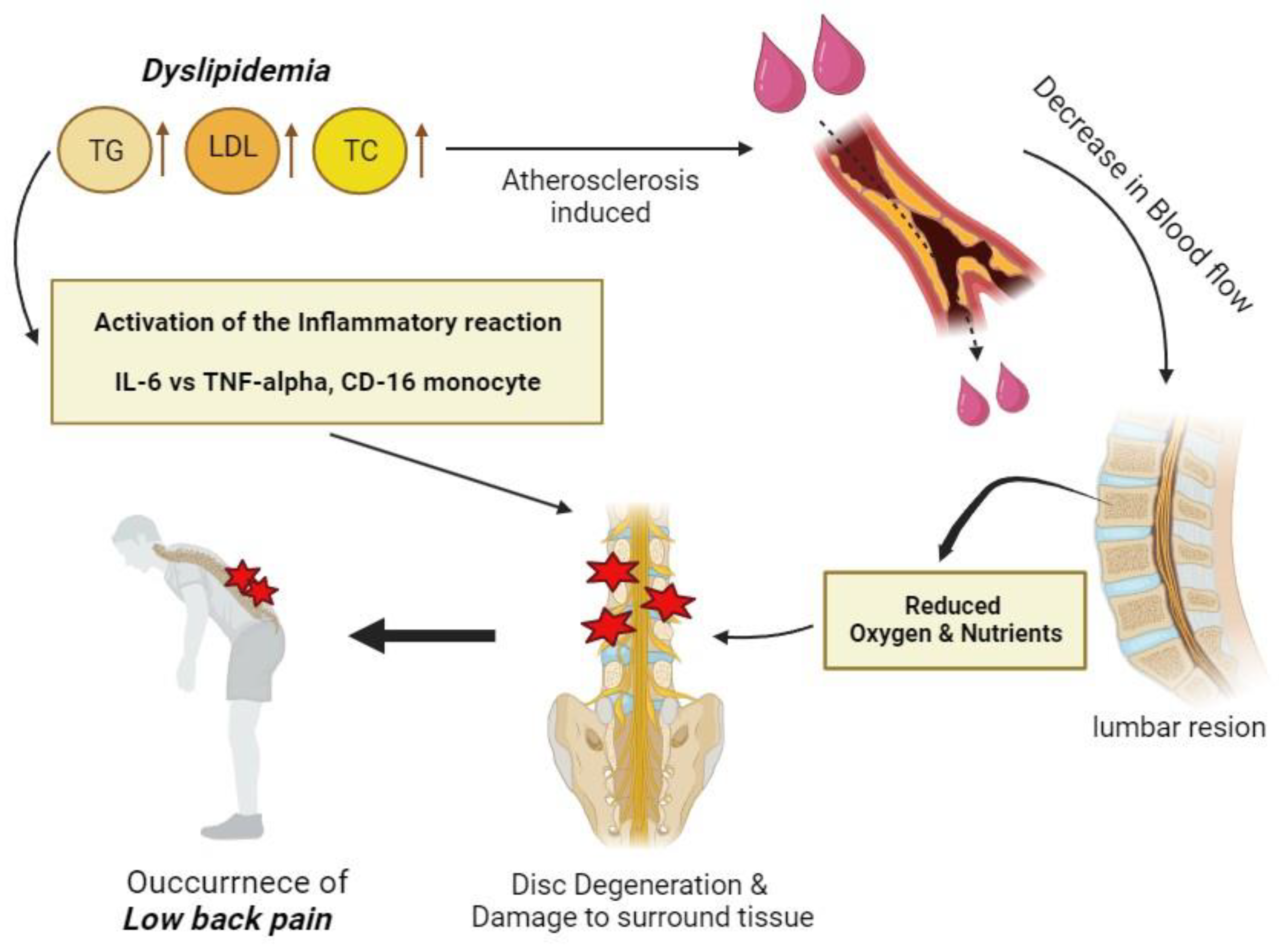
1. Introduction
2. Materials and Methods
2.1. Study Participants and Design
2.2. Study Variables
2.3. Statistical Analysis
2.4. Ethics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Low Back Pain. Available online: https://www.who.int/news-room/fact-sheets/detail/low-back-pain (accessed on 1 September 2023).
- Park, S.C.; Kang, M.S.; Yang, J.H.; Kim, T.H. Assessment and nonsurgical management of low back pain: A narrative review. Korean J. Intern. Med. 2023, 38, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Choi, J.Y.; Lee, W.M.; Park, S.M. Prevalence of chronic low back pain and its associated factors in the general population of South Korea: A cross-sectional study using the National Health and Nutrition Examination Surveys. J. Orthop. Surg. Res. 2023, 18, 29. [Google Scholar] [CrossRef] [PubMed]
- Wagn, Y.X.J.; Wang, J.Q.; Kaplar, Z. Increased low back pain prevalence in females than in males after menopause age: Evidences based on synthetic literature review. Quant. Imaging Med. Surg. 2016, 6, 199–206. [Google Scholar]
- Branch NIAMS Science Communications and Outreach. Back Pain. National Institute of Arthritis and Musculoskeletal and Skin Diseases. 2017. Available online: https://www.niams.nih.gov/health-topics/back-pain (accessed on 25 September 2023).
- Lower Back Pain Causes: 8 Reasons for Sudden & Chronic Pain. HOUSTON Mehtodist Leading Medicine. Available online: https://www.houstonmethodist.org/blog/articles/2021/may/lower-back-pain-causes-8-reasons-for-sudden-and-chronic-pain/ (accessed on 15 October 2023).
- Mehta, J.S. Does High Blood Pressure Cause Back Pain? | London Spine Care | Mehta Spine. Mehta Spine—Children and Adult Spinal Surgeon, UK. 2021. Available online: https://www.mehtaspine.co.uk/blog/does-high-blood-pressure-cause-back-pain/ (accessed on 9 October 2023).
- Teeter. How Sugar Affects Back Pain | Healthy Living. Teeter.com. 2017. Available online: https://teeter.com/blog/sugar-causes-back-pain/ (accessed on 9 October 2023).
- Shahid, S.; Akhter, Z.; Sukaina, M.; Sohail, F.; Nasir, F. Association of Diabetes with Lower Back Pain: A Narrative Review. Cureus 2021, 13, 6. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, T.; Ochiai, H.; Shirasawa, T.; Nagahama, S.; Kobayashi, M.; Minoura, A.; Miki, A.; Chen, Y.; Hoshino, H.; Kokaze, A. Association between serum lipids and low back pain among a middle-aged Japanese population: A large-scale cross-sectional study. Lipids Health Dis. 2018, 17, 266. [Google Scholar] [CrossRef]
- Suri, P.; Elgaeva, E.E.; Williams, F.M.K.; Freidin, M.B.; Zaytseva, O.O.; Aulchenko, Y.S.; Tsepilov, Y.A. Evidence of causal effects of blood pressure on back pain and back pain on type II diabetes provided by a bidirectional Mendelian randomization study. Spine J. 2023, 23, 1161–1171. [Google Scholar] [CrossRef]
- Kakihana, H.; Jinnouchi, H.; Kitamura, A.; Matsudaira, K.; Kiyama, M.; Hayama-Terada, M.; Muraki, I.; Kubota, Y.; Yamagishi, K.; Okada, T.; et al. Overweight and Hypertension in Relation to Chronic Musculoskeletal Pain Among Community-Dwelling Adults: The Circulatory Risk in Communities Study (CIRCS). J. Epidemiol. 2021, 31, 566–572. [Google Scholar] [CrossRef]
- Kerkhoff, A.C.; Moreira, L.B.; Fuchs, F.D.; Fuchs, S.C. Association between hypertension and musculoskeletal complaints: A population-based study. J. Hypertens. 2012, 30, 2112. [Google Scholar] [CrossRef]
- Bae, Y.-H.; Shin, J.-S.; Lee, J.; Kim, M.; Park, K.B.; Cho, J.-H.; Ha, I.H. Association between Hypertension and the Prevalence of Low Back Pain and Osteoarthritis in Koreans: A Cross-Sectional Study. PLoS ONE 2015, 10, e0138790. [Google Scholar] [CrossRef]
- Heuch, I.; Heuch, I.; Hagen, K.; Zwart, J.A. Does high blood pressure reduce the risk of chronic low back pain? The Nord-Trøndelag Health Study. Eur. J. Pain 2014, 18, 590–598. [Google Scholar] [CrossRef]
- Hagen, K.; Zwart, J.-A.; Holmen, J.; Svebak, S.; Bovim, G.; Stovner, L.J. Does Hypertension Protect Against Chronic Musculoskeletal Complaints?: The Nord-Trøndelag Health Study. Arch. Intern. Med. 2005, 165, 916. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jacob, L.; Rathmann, W.; Koyanagi, A.; Haro, J.M.; Kostev, K. Association between type 2 diabetes and chronic low back pain in general practices in Germany. BMJ Open Diab Res. Care 2021, 9, e002426. [Google Scholar] [CrossRef] [PubMed]
- Pozzobon, D.; Ferreira, P.H.; Dario, A.B.; Almeida, L.; Vesentini, G.; Harmer, A.R.; Ferreira, M.L. Is there an association between diabetes and neck and back pain? A systematic review with meta-analyses. PLoS ONE 2019, 14, e0212030. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Pan, F.; Ba, Z.; Wang, S.; Wu, D. The potential effect of type 2 diabetes mellitus on lumbar disc degeneration: A retrospective single-center study. J. Orthop. Surg. Res. 2018, 13, 52. [Google Scholar] [CrossRef]
- Dario, A.; Ferreira, M.; Refshauge, K.; Harmer, A.; Sánchez-Romera, J.; Pérez-Riquelme, F.; Cisneros, L.; Ordonana, J.; Ferreira, P. Mapping the association between back pain and type 2 diabetes: A cross-sectional and longitudinal study of adult Spanish twins. PLoS ONE 2017, 12, e0174757. [Google Scholar] [CrossRef]
- Fabiane, S.M.; Ward, K.J.; Iatridis, J.C.; Williams, F.M.K. Does type 2 diabetes mellitus promote intervertebral disc degeneration? Eur. Spine J. 2016, 25, 2716–2720. [Google Scholar] [CrossRef]
- Yuan, L.; Huang, Z.; Han, W.; Chang, R.; Sun, B.; Zhu, M.; Li, C.; Yan, J.; Liu, B.; Yin, H.; et al. The impact of dyslipidemia on lumbar intervertebral disc degeneration and vertebral endplate modic changes: A cross-sectional study of 1035 citizens in China. BMC Public Health 2023, 23, 1302. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, J.; Su, Y.; Guo, M.; Chen, Y.; Zhu, Y.; Nie, G.; Ke, R.; Chen, H.; Hu, J. Impact of dyslipidemia on the severity of symptomatic lumbar spine degeneration: A retrospective clinical study. Front. Nutr. 2022, 9, 1033375. [Google Scholar] [CrossRef]
- Eo, H.S.; Chung, E.J.; Lee, S.H.; Park, K.H.; Kim, J.A. Relationship between Degenerative Disc Disease and Lipid Profiles in Older Adults. Korean J. Fam. Pract. 2019, 9, 212–215. [Google Scholar] [CrossRef]
- Tan, B.; Xiang, S.-L.; Hu, Z.-M.; Zheng, Y.-H.; Ouyang, J. Association of dyslipidemia with lumbar disc herniation: A case-control study. Res. Sq. 2023. in review. [Google Scholar] [CrossRef]
- Heuch, I.; Heuch, I.; Hagen, K.; Zwart, J.-A. Do Abnormal Serum Lipid Levels Increase the Risk of Chronic Low Back Pain? The Nord-Trøndelag Health Study. PLoS ONE 2014, 9, e108227. [Google Scholar] [CrossRef] [PubMed]
- Alhowimel, A.S.; Alodaibi, F.; Alshehri, M.M.; Alqahtani, B.A.; Alotaibi, M.; Alenazi, A.M. Prevalence and Risk Factors Associated with Low Back Pain in the Saudi Adult Community: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2021, 18, 13288. [Google Scholar] [CrossRef] [PubMed]
- Hemingway, H.; Shipley, M.; Stansfeld, S.; Shannon, H.; Frank, J.; Brunner, E.; Marmot, M. Are risk factors for atherothrombotic disease associated with back pain sickness absence? The Whitehall II Study. J. Epidemiol. Community Health 1999, 53, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Narayan, K.M.V.; Boyle, J.P.; Thompson, T.J.; Gregg, E.W.; Williamson, D.F. Effect of BMI on Lifetime Risk for Diabetes in the U.S. Diabetes Care 2007, 30, 1562–1566. [Google Scholar] [CrossRef]
- Heuch, I.; Heuch, I.; Hagen, K.; Zwart, J.A. Body mass index as a risk factor for developing chronic low back pain: A follow-up in the Nord-Trøndelag Health Study. Spine 2013, 38, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Iwahashi, S.; Hashida, R.; Matsuse, H.; Higashi, E.; Bekki, M.; Iwanage, S.; Hara, K.; Higuchi, T.; Hirakawa, Y.; Kubota, A.; et al. The impact of sarcopenia on low back pain and quality of life in patients with osteoporosis. BMC Musculoskelet. Disord. 2022, 23, 142. [Google Scholar] [CrossRef]
- Tanishima, S.; Hagino, H.; Matsumoto, H.; Tanimura, C.; Nagashima, H. Association between sarcopenia and low back pain in local residents prospective cohort study from the GAINA study. BMC Musculoskelet. Disord. 2017, 18, 452. [Google Scholar] [CrossRef]
- Sakai, Y.; Matsui, H.; Ito, S.; Hida, T.; Ito, K.; Koshimizu, H.; Harada, A. Sarcopenia in elderly patients with chronic low back pain. Osteoporos. Sarcopenia 2017, 3, 195–200. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Liu, Z.; Duan, D. Inflammation in low back pain may be detected from the peripheral blood: Suggestions for biomarker. Biosci. Rep. 2016, 36, e00361. [Google Scholar] [CrossRef]
- Wang, H.; Schiltenwolf, M.; Buchner, M. The Role of TNF-α in Patients with Chronic Low Back Pain—A Prospective Comparative Longitudinal Study. Clin. J. Pain 2008, 24, 273. [Google Scholar] [CrossRef]

| Total (N = 5961) | Non-CLBP (N = 4098) | CLBP (N = 1863) | p-Value | |
|---|---|---|---|---|
| Age (years), M ± SD | 61.81 ± 10.53 | 59.78 ± 10.17 | 66.26 ± 9.91 | <0.001 |
| Weight (kg), M ± SD | 57.26 ± 8.82 | 57.29 ± 8.55 | 56.76 ± 9.35 | 0.004 |
| Height (cm), M ± SD | 153.91 ± 6.07 | 154.60 ± 5.84 | 152.38 ± 6.30 | <0.001 |
| BMI, N (%) | 0.016 | |||
| BMI < 18.5 | 147 (2.5%) | 98 (2.4%) | 49 (2.6%) | |
| 18.5 ≤ BMI < 23 | 2154 (36.1%) | 1509 (36.8%) | 645 (34.6%) | |
| 23 ≤ BMI < 25 | 1519 (25.5%) | 1073 (26.2%) | 446 (24.1%) | |
| BMI ≥ 25 | 2141 (35.6%) | 1418 (34.6%) | 723 (38.7%) | |
| Waist circumference (cm), M ± SD | 81.58 ± 9.34 | 80.00 ± 9.11 | 83.12 ± 9.65 | <0.001 |
| Income, N (%) | <0.001 | |||
| Q1 | 1474 (24.6%) | 908 (22.1%) | 566 (31.3%) | |
| Q2 | 1503 (25.3%) | 1004 (24.5%) | 499 (26.4%) | |
| Q3 | 1526 (25.5%) | 1084 (26.5%) | 442 (22.7%) | |
| Q4 | 1458 (25.1%) | 1102 (26.9%) | 356 (19.6%) | |
| Education, N (%) | <0.001 | |||
| Less than elementary school | 3229 (54.0%) | 1880 (45.5%) | 1349 (72.6%) | |
| Middle school | 900 (15.1%) | 669 (16.4%) | 231 (12.4%) | |
| High school | 1293 (21.9%) | 1075 (26.5%) | 218 (11.6%) | |
| College or higher | 539 (9.0%) | 474 (11.6%) | 65 (3.4%) | |
| Alcohol consumption, | <0.001 | |||
| N (%) | ||||
| Not drinking at all | 3838 (61.0%) | 2312 (59.0%) | 1526 (66.4%) | |
| Less than once a month | 1242 (20.3%) | 1055 (20.5%) | 187 (19.8%) | |
| About once a month | 530 (10.3%) | 448 (11.2%) | 82 (7.8%) | |
| About once a week | 275 (7.0%) | 230 (7.8%) | 45 (4.4%) | |
| Almost every day | 76 (1.4%) | 53 (1.5%) | 23 (1.6%) | |
| Smoking, N (%) | 0.187 | |||
| Nonsmoker | 5502 (92.5%) | 3794 (92.8%) | 1708 (91.8%) | |
| Smoker | 459 (7.5%) | 304 (7.2%) | 155 (8.2%) | |
| Oral contraceptive use, | <0.001 | |||
| N (%) | ||||
| No | 4707 (79.1%) | 3301 (80.7%) | 1406 (75.5%) | |
| Yes | 1254 (20.9%) | 797 (19.3%) | 457 (24.5%) | |
| Number of days of walking exercise per week | <0.001 | |||
| 0 day/week | 1365 (22.9%) | 808 (19.7%) | 557 (29.9%) | |
| 1–3 days/week | 1706 (28.6%) | 1260 (30.8%) | 446 (23.9%) | |
| ≥4 days/week | 2890 (45.5%) | 2030 (49.5%) | 860 (46.2%) | |
| Number of days of strength exercise per week | <0.001 | |||
| 0 day/week | 4998 (84.0%) | 3347 (81.8%) | 1651 (89.0%) | |
| 1–3 days/week | 629 (10.4%) | 506 (12.3%) | 123 (6.4%) | |
| ≥4 days/week | 334 (5.6%) | 245 (5.9%) | 89 (4.6%) | |
| Dietary circumstances, | <0.001 | |||
| N (%) | ||||
| Eating enough and | ||||
| varied food | 2348 (39.4%) | 1755 (43.3%) | 593 (30.9%) | |
| Eating enough but | ||||
| not diverse food | 3261 (55.7%) | 2142 (53.4%) | 1119 (60.8%) | |
| Eating poorly | ||||
| Sometimes | 304 (4.1%) | 180 (2.9%) | 124 (6.8%) | |
| Eating poorly | ||||
| frequently | 48 (0.8%) | 21 (0.4%) | 27 (1.5%) | |
| Hypertension status, | <0.001 | |||
| N (%) | ||||
| Normal | 3663 (61.4%) | 2709 (66.1%) | 954 (51.2%) | |
| Hypertension | 2298 (38.6%) | 1389 (33.9%) | 909 (48.8%) | |
| Diabetes status, N (%) | <0.001 | |||
| Normal | 5235 (87.8%) | 3657 (89.2%) | 1578 (84.7%) | |
| Diabetes | 726 (12.2%) | 441 (10.8%) | 285 (15.3%) | |
| Dyslipidemia status, | <0.001 | |||
| N (%) | ||||
| Normal | 4774 (80.1%) | 3354 (81.8%) | 1420 (76.2%) | |
| Dyslipidemia | 1187 (19.9%) | 744 (18.2%) | 443 (23.8%) |
| Risk for Chronic Low Back Pain Compared to That of Non-Chronic Low Back Pain | |||
|---|---|---|---|
| OR | 95% CI | p-Value | |
| Age (years) | 1.07 | (1.059, 1.071) | <0.001 |
| Weight (kg) | 0.99 | (0.984, 0.997) | 0.003 |
| Height (cm) | 0.94 | (0.932, 0.949) | <0.001 |
| BMI | |||
| 18.5 ≤ BMI < 23 | 1.00 | Reference | |
| BMI < 18.5 | 1.17 | (0.820, 1.668) | 0.388 |
| 23 ≤ BMI < 25 | 0.98 | (0.848, 1.131) | 0.774 |
| BMI ≥ 25 | 1.19 | (1.048, 1.355) | 0.008 |
| Waist circumference (cm) | 1.03 | (1.020, 1.032) | <0.001 |
| Income | |||
| Q1 | 1.00 | Reference | |
| Q2 | 0.76 | (0.655, 0.886) | <0.001 |
| Q3 | 0.61 | (0.521, 0.709) | <0.001 |
| Q4 | 0.52 | (0.442, 0.606) | <0.001 |
| Education | |||
| Less than elementary school | 1.00 | Reference | |
| Middle school | 0.47 | (0.401, 0.558) | <0.001 |
| High school | 0.28 | (0.234, 0.323) | <0.001 |
| College or higher | 0.18 | (0.140, 0.241) | <0.001 |
| Alcohol consumption | |||
| Not drinking at all | 1.00 | Reference | |
| Less than once a month | 0.86 | (0.699, 1.059) | 0.157 |
| About once a month | 0.62 | (0.464, 0.838) | 0.002 |
| About once a week | 0.50 | (0.342, 0.727) | <0.001 |
| Almost every day | 0.91 | (0.465, 1.770) | 0.775 |
| Smoking | |||
| Nonsmoker | 1.00 | Reference | |
| Smoker | 1.15 | (0.935, 1.406) | 0.188 |
| Oral contraceptive use | |||
| No | 1.00 | Reference | |
| Yes | 1.36 | (1.188, 1.546) | <0.001 |
| Number of days of walking exercise per week | |||
| 0 day/week | 1.00 | Reference | |
| 1–3 days/week | 0.51 | (0.439, 0.596) | <0.001 |
| ≥4 days/week | 0.62 | (0.539. 0.705) | <0.001 |
| Number of days of strength exercise per week | |||
| 0 day/week | 1.00 | Reference | |
| 1–3 days/week | 0.48 | (0.388, 0.590) | <0.001 |
| ≥4 days/week | 0.72 | (0.559, 0.928) | 0.011 |
| Dietary circumferences | |||
| Eating enough and varied food | 1.00 | Reference | |
| Eating enough but not diverse food | 1.60 | (1.412, 1.803) | <0.001 |
| Eating poorly sometimes | 3.33 | (2.522, 4.383) | <0.001 |
| Eating poorly frequently | 4.48 | (2.402, 8.363) | <0.001 |
| Hypertension status | |||
| Normal | 1.00 | Reference | |
| Hypertension | 1.86 | (1.664, 2.080) | <0.001 |
| Diabetes status | |||
| Normal | 1.00 | Reference | |
| Diabetes | 1.50 | (1.276, 1.758) | <0.001 |
| Dyslipidemia status | |||
| Normal | 1.00 | Reference | |
| Dyslipidemia | 1.41 | (1.232, 1.608) | <0.001 |
| Risk for Chronic Low Back Pain Compared to That of Non-Chronic Low Back Pain | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |||||||
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| Hypertension status | |||||||||
| Normal | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | |||
| Hypertension | 1.11 | (0.976, 1.259) | 0.112 | 1.11 | (0.977, 1.264) | 0.107 | 1.11 | (0.970, 1.265) | 0.132 |
| Diabetes status | |||||||||
| Normal | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | |||
| Diabetes | 0.99 | (0.835, 1.177) | 0.921 | 0.97 | (0.818, 1.157) | 0.756 | 0.96 | (0.802, 1.148) | 0.653 |
| Dyslipidemia status | |||||||||
| Normal | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | |||
| Dyslipidemia | 1.25 | (1.088, 1.439) | 0.002 | 1.32 | (1.144, 1.522) | <0.001 | 1.32 | (1.140, 1.530) | <0.001 |
| Risk for Chronic Low Back Pain Compared to That of Non-Chronic Low Back Pain | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Underweight (BMI < 18.5) | Normal (18.5 ≤ BMI < 23) | Overweight (23 ≤ BMI < 25) | Obesity (BMI ≥ 25) | |||||||||
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| Hypertension status | ||||||||||||
| Normal | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | ||||
| Hypertension | 2.03 | (0.732, 5.641) | 0.174 | 0.95 | (0.750, 1.205) | 0.676 | 1.10 | (0.840, 1.430) | 0.500 | 1.20 | (0.970, 1.482) | 0.093 |
| Diabetes status | ||||||||||||
| Normal | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | ||||
| Diabetes | 1.01 | (0.296, 3.463) | 0.984 | 0.71 | (0.500, 1.006) | 0.054 | 1.10 | (0.744, 1.615) | 0.642 | 1.06 | (0.820, 1.380) | 0.639 |
| Dyslipidemia status | ||||||||||||
| Normal | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | ||||
| Dyslipidemia | 0.20 | (0.020, 2.064) | 0.178 | 1.21 | (0.908, 1.602) | 0.196 | 1.12 | (0.833, 1.501) | 0.457 | 1.64 | (1.310, 2.040) | <0.001 |
| Risk for Chronic Low Back Pain Compared to That of Non-Chronic Low Back Pain | ||||||
|---|---|---|---|---|---|---|
| Age < 60 Years | Age ≥ 60 Years | |||||
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| Hypertension status | ||||||
| Normal | 1.00 | Reference | 1.00 | Reference | ||
| Hypertension | 1.11 | (0.855, 1.430) | 0.444 | 1.11 | (0.946, 1.296) | 0.206 |
| Diabetes status | ||||||
| Normal | 1.00 | Reference | 1.00 | Reference | ||
| Diabetes | 0.81 | (0.510, 1.271) | 0.351 | 0.99 | (0.819, 1.219) | 0.993 |
| Dyslipidemia status | ||||||
| Normal | 1.00 | Reference | 1.00 | Reference | ||
| Dyslipidemia | 1.61 | (1.235, 2.090) | <0.001 | 1.31 | (1.090, 1.565) | 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Lee, S.-M. Dyslipidemia Is Positively Associated with Chronic Low Back Pain in Korean Women: Korean National Health and Nutrition Examination Survey 2010–2012. Healthcare 2024, 12, 102. https://doi.org/10.3390/healthcare12010102
Kim S, Lee S-M. Dyslipidemia Is Positively Associated with Chronic Low Back Pain in Korean Women: Korean National Health and Nutrition Examination Survey 2010–2012. Healthcare. 2024; 12(1):102. https://doi.org/10.3390/healthcare12010102
Chicago/Turabian StyleKim, Sunmin, and Seon-Mi Lee. 2024. "Dyslipidemia Is Positively Associated with Chronic Low Back Pain in Korean Women: Korean National Health and Nutrition Examination Survey 2010–2012" Healthcare 12, no. 1: 102. https://doi.org/10.3390/healthcare12010102
APA StyleKim, S., & Lee, S.-M. (2024). Dyslipidemia Is Positively Associated with Chronic Low Back Pain in Korean Women: Korean National Health and Nutrition Examination Survey 2010–2012. Healthcare, 12(1), 102. https://doi.org/10.3390/healthcare12010102







