Exercise-Induced Bronchoconstriction in Children: A Comparison between Athletes and Non-Athletes
Abstract
1. Introduction
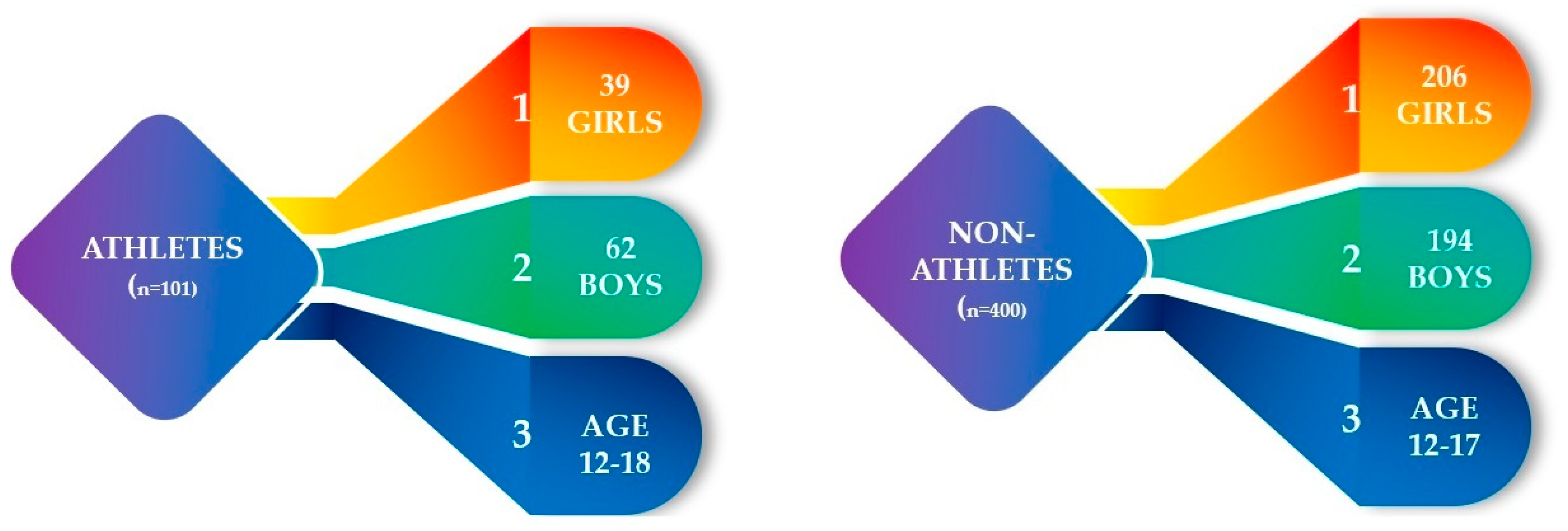
2. Materials and Methods
2.1. Spirometry
2.2. Exercise Challenge
2.3. Statistical Analysis
3. Results
3.1. Spirometry Characteristics
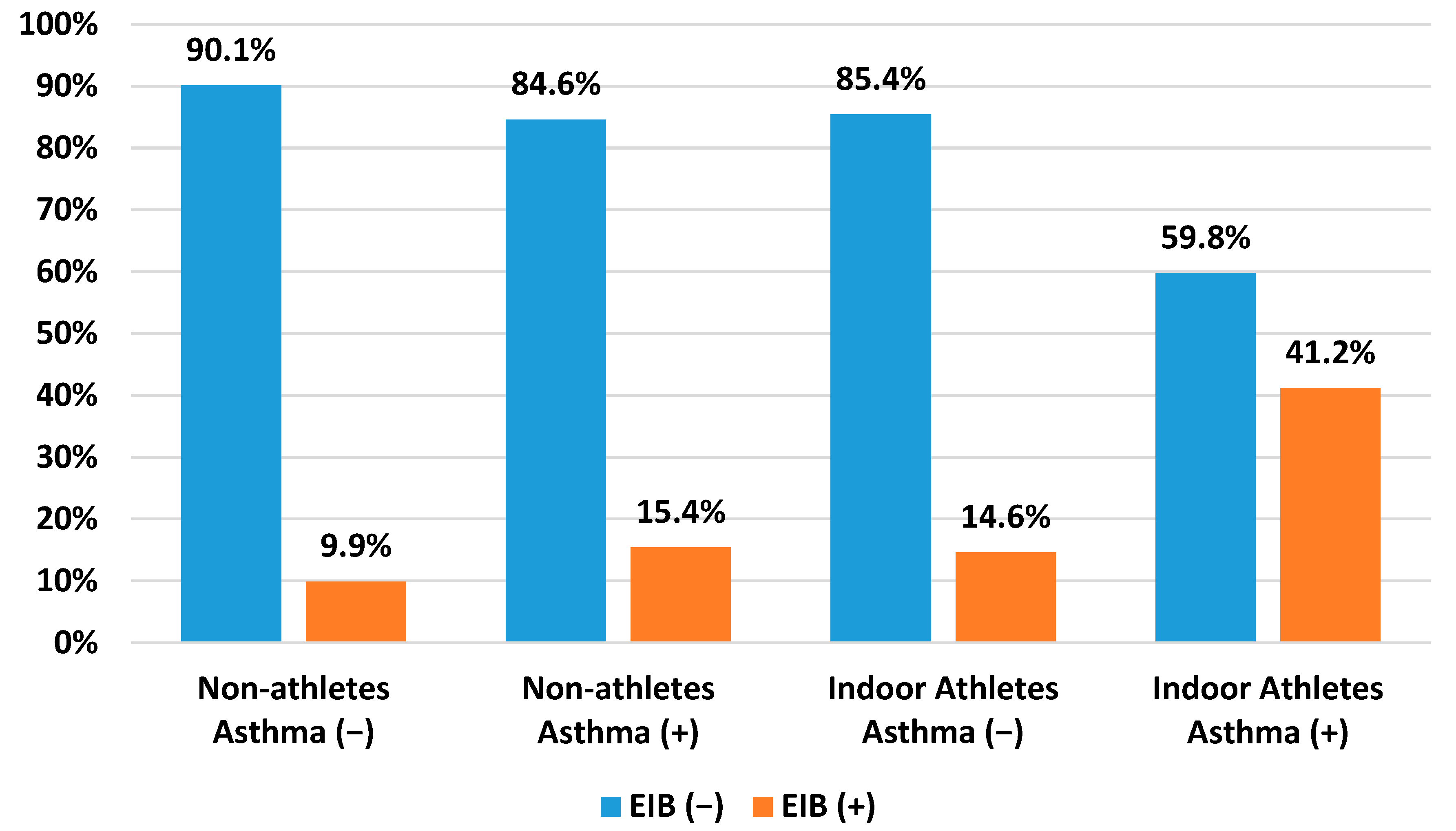
3.2. Indoor Athletes and Non-Athletes
4. Discussion
4.1. Comparison between Indoor Athletes and Non-Athletes
4.2. Asthma
4.3. Pre-Existing Chronic Cough
4.4. Spirometry in Indoor Athletes and Non-Athletes
4.5. Limitations and Study Strengths
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bonini, M.; Silvers, W. Exercise-Induced Bronchoconstriction. Immunol. Allergy Clin. N. Am. 2018, 38, 205–214. [Google Scholar] [CrossRef]
- Minic, P.B. Exercise Intolerance and Exercise-Induced Bronchoconstriction in Children. Front. Biosci. 2017, 9, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Klain, A.; Indolfi, C.; Dinardo, G.; Contieri, M.; Decimo, F.; Del Miraglia Giudice, M. Exercise-Induced Bronchoconstriction in Children. Front. Med. 2022, 8, 814976. [Google Scholar] [CrossRef]
- Goossens, J.; Decaesteker, T.; Jonckheere, A.-C.; Seys, S.; Verelst, S.; Dupont, L.; Bullens, D.M. How to detect young athletes at risk of exercise-induced bronchoconstriction? Paediatr. Respir. Rev. 2022, 44, 40–46. [Google Scholar] [CrossRef]
- Malewska-Kaczmarek, K.; Bobeff, K.; Mańkowski, T.; Podlecka, D.; Jerzyńska, J.; Stelmach, I. Adolescent Athletes at Risk of Exercise-Induced Bronchoconstriction: A Result of Training or Pre-Existing Asthma? IJERPH 2022, 19, 9119. [Google Scholar] [CrossRef]
- Atchley, T.J.; Smith, D.M. Exercise-Induced Bronchoconstriction in Elite or Endurance Athletes. Ann. Allergy Asthma Immunol. 2020, 125, 47–54. [Google Scholar] [CrossRef]
- Aggarwal, B.; Mulgirigama, A.; Berend, N. Exercise-Induced Bronchoconstriction: Prevalence, Pathophysiology, Patient Impact, Diagnosis and Management. NPJ Prim. Care Resp. Med. 2018, 28, 31. [Google Scholar] [CrossRef] [PubMed]
- Couto, M.; Kurowski, M.; Moreira, A.; Bullens, D.M.A.; Carlsen, K.-H.; Delgado, L.; Kowalski, M.L.; Seys, S.F. Mechanisms of Exercise-induced Bronchoconstriction in Athletes: Current Perspectives and Future Challenges. Allergy 2018, 73, 8–16. [Google Scholar] [CrossRef]
- Lu, K.; Sidell, M.; Li, X.; Rozema, E.; Cooper, D.M.; Radom-Aizik, S.; Crawford, W.W.; Koebnick, C. Self-Reported Physical Activity and Asthma Risk in Children. J. Allergy Clin. Immunol. Pract. 2022, 10, 231–239.e3. [Google Scholar] [CrossRef]
- Cichalewski, Ł.; Majak, P.; Jerzyńska, J.; Stelmach, W.; Kaczmarek, A.; Malewska, K.; Smejda, K.; Stelmach, I. Prevalence of Exercise-Induced Cough in Schoolchildren: A Pilot Study. Allergy Asthma Proc. 2015, 36, 65–69. [Google Scholar] [CrossRef]
- Graham, B.L.; Steenbruggen, I.; Miller, M.R.; Barjaktarevic, I.Z.; Cooper, B.G.; Hall, G.L.; Hallstrand, T.S.; Kaminsky, D.A.; McCarthy, K.; McCormack, M.C.; et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am. J. Respir. Crit. Care Med. 2019, 200, e70–e88. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.R. Standardisation of Spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.-S.; Choi, S.-H.; Koh, I.-S. The Effect of Increasing Control-to-Case Ratio on Statistical Power in a Simulated Case-Control SNP Association Study. Genom. Inform. 2009, 7, 148–151. [Google Scholar] [CrossRef]
- de Aguiar, K.B.; Anzolin, M.; Zhang, L. Global Prevalence of Exercise-Induced Bronchoconstriction in Childhood: A Meta-Analysis. Pediatr. Pulmonol. 2018, 53, 412–425. [Google Scholar] [CrossRef]
- Jonckheere, A.-C.; Seys, S.; Dilissen, E.; Schelpe, A.-S.; Van der Eycken, S.; Corthout, S.; Verhalle, T.; Goossens, J.; Vanbelle, V.; Aertgeerts, S.; et al. Early-Onset Airway Damage in Early-Career Elite Athletes: A Risk Factor for Exercise-Induced Bronchoconstriction. J. Allergy Clin. Immunol. 2019, 144, 1423–1425.e9. [Google Scholar] [CrossRef] [PubMed]
- Hildebrand, K. Powysiłkowy Skurcz Oskrzeli. Adv. Respir. Med. 2010, 79, 39–47. [Google Scholar] [CrossRef]
- Stelmach, I.; Cichalewski, Ł.; Majak, P.; Smejda, K.; Podlecka, D.; Jerzyńska, J.; Stelmach, W. School Environmental Factors Are Predictive for Exercise-Induced Symptoms in Children. Respir. Med. 2016, 112, 25–30. [Google Scholar] [CrossRef]
- Zeiger, J.S.; Weiler, J.M. Special Considerations and Perspectives for Exercise-Induced Bronchoconstriction (EIB) in Olympic and Other Elite Athletes. J. Allergy Clin. Immunol. Pract. 2020, 8, 2194–2201. [Google Scholar] [CrossRef]
- del Giacco, S.; Couto, M.; Firinu, D.; Garcia-Larsen, V. Management of Intermittent and Persistent Asthma in Adolescent and High School Athletes. J. Allergy Clin. Immunol. Pract. 2020, 8, 2166–2181. [Google Scholar] [CrossRef]
- Wolfarth, B.; Wuestenfeld, J. Special Considerations for Adolescent Athletic and Asthmatic Patients. OAJSM 2013, 4, 1–7. [Google Scholar] [CrossRef]
- Carlsen, K.-H. The Breathless Adolescent Asthmatic Athlete. Eur. Respir. J. 2011, 38, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, H.; Sawiec, P. Dolegliwości ze strony układu oddechowego związane z wysiłkiem. Med. Prakt.-Pediatr. 2019, 1, 61–71. [Google Scholar]
- Durmic, T.; Lazovic, B.; Djelic, M.; Lazic, J.S.; Zikic, D.; Zugic, V.; Dekleva, M.; Mazic, S. Sport-Specific Influences on Respiratory Patterns in Elite Athletes. J. Bras. Pneumol. 2015, 41, 516–522. [Google Scholar] [CrossRef]
- Rong, C.; Bei, H.; Yun, M.; Yuzhu, W.; Mingwu, Z. Lung Function and Cytokine Levels in Professional Athletes. J. Asthma 2008, 45, 343–348. [Google Scholar] [CrossRef]
- Lipworth, B.; Manoharan, A.; Anderson, W. Unlocking the Quiet Zone: The Small Airway Asthma Phenotype. Lancet Respir. Med. 2014, 2, 497–506. [Google Scholar] [CrossRef]
- Gawlik, R.; Kurowski, M.; Kowalski, M.; Ziętkowski, Z.; Pokrywka, A.; Krysztofiak, H.; Krzywański, J.; Bugajski, A.; Bartuzi, Z. Asthma and Exercise-Induced Respiratory Disordersin Athletes. The Position Paper of the Polish Societyof Allergology and Polish Society of Sports Medicine. Pdia 2019, 36, 1–10. [Google Scholar] [CrossRef] [PubMed]

| Non-Athletes (n = 400) | Indoor Athletes (n = 58) | p | |
|---|---|---|---|
| History of asthma (n = 43) | 26 (6.5%) | 17 (29.3%) | <0.001 |
| Nonasthma (n = 415) | 374 (93.5%) | 41 (70.7%) | |
| EIB prevalence (n = 54) | 41 (10.2%) | 13 (22.4%) | =0.007 |
| EIB symptoms during exercise challenge (n = 33) | 23 (5.7%) | 10 (17.2%) | =0.011 |
| Children reporting pre-existing cough (n = 84) | 70 (17.5%) | 14 (24.13%) | =0.485 |
| Variables | Statistical Parameter | p | |||
|---|---|---|---|---|---|
| Non-Athletes (n = 400) | Indoor Athletes (n = 58) | ||||
| M | SD | M | SD | ||
| FEV1 * (%) | 99.03 | 13.23 | 106.05 | 15.91 | <0.001 |
| FVC ** (%) | 93.74 | 13.72 | 105.37 | 13.82 | <0.001 |
| FEV1%VC *** (%) | 106.31 | 10.06 | 100.91 | 7.52 | =0.004 |
| PEF **** (%) | 85.65 | 17.78 | 94.71 | 17.45 | <0.001 |
| MEF25 ***** (%) | 84.73 | 17.81 | 90.40 | 29.29 | <0.001 |
| MEF50 (%) | 100.82 | 23.32 | 93.16 | 23.40 | =0.348 |
| MEF75 (%) | 113.69 | 34.32 | 97.14 | 20.42 | =0.011 |
| Variables | ||
|---|---|---|
| Tennis, n (%) | 2 (3.4) | |
| Dance, n (%) | 4 (6.9) | |
| Athletics, n (%) | 4 (6.9) | |
| Cycling, n (%) | 4 (6.9) | |
| Indoor athletes | Martial arts, n (%) | 8 (13.8) |
| Floorball, n (%) | 1 (1.7) | |
| Basketball, n (%) | 10 (17.2) | |
| Volleyball, n (%) | 2 (3.4) | |
| Handball, n (%) | 2 (3.4) | |
| Swimming, n (%) | 21 (36.2) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malewska-Kaczmarek, K.; Podlecka, D.; Mańkowski, T.; Jerzyńska, J.; Stelmach, I. Exercise-Induced Bronchoconstriction in Children: A Comparison between Athletes and Non-Athletes. Healthcare 2023, 11, 1349. https://doi.org/10.3390/healthcare11091349
Malewska-Kaczmarek K, Podlecka D, Mańkowski T, Jerzyńska J, Stelmach I. Exercise-Induced Bronchoconstriction in Children: A Comparison between Athletes and Non-Athletes. Healthcare. 2023; 11(9):1349. https://doi.org/10.3390/healthcare11091349
Chicago/Turabian StyleMalewska-Kaczmarek, Kamila, Daniela Podlecka, Tymoteusz Mańkowski, Joanna Jerzyńska, and Iwona Stelmach. 2023. "Exercise-Induced Bronchoconstriction in Children: A Comparison between Athletes and Non-Athletes" Healthcare 11, no. 9: 1349. https://doi.org/10.3390/healthcare11091349
APA StyleMalewska-Kaczmarek, K., Podlecka, D., Mańkowski, T., Jerzyńska, J., & Stelmach, I. (2023). Exercise-Induced Bronchoconstriction in Children: A Comparison between Athletes and Non-Athletes. Healthcare, 11(9), 1349. https://doi.org/10.3390/healthcare11091349






