Pharmacological Modulation of Temporal Discounting: A Systematic Review
Abstract
1. Introduction
2. Methods
2.1. Eligibility Criteria and Search Strategy
2.2. Data Extraction
2.3. Data Analysis
3. Results
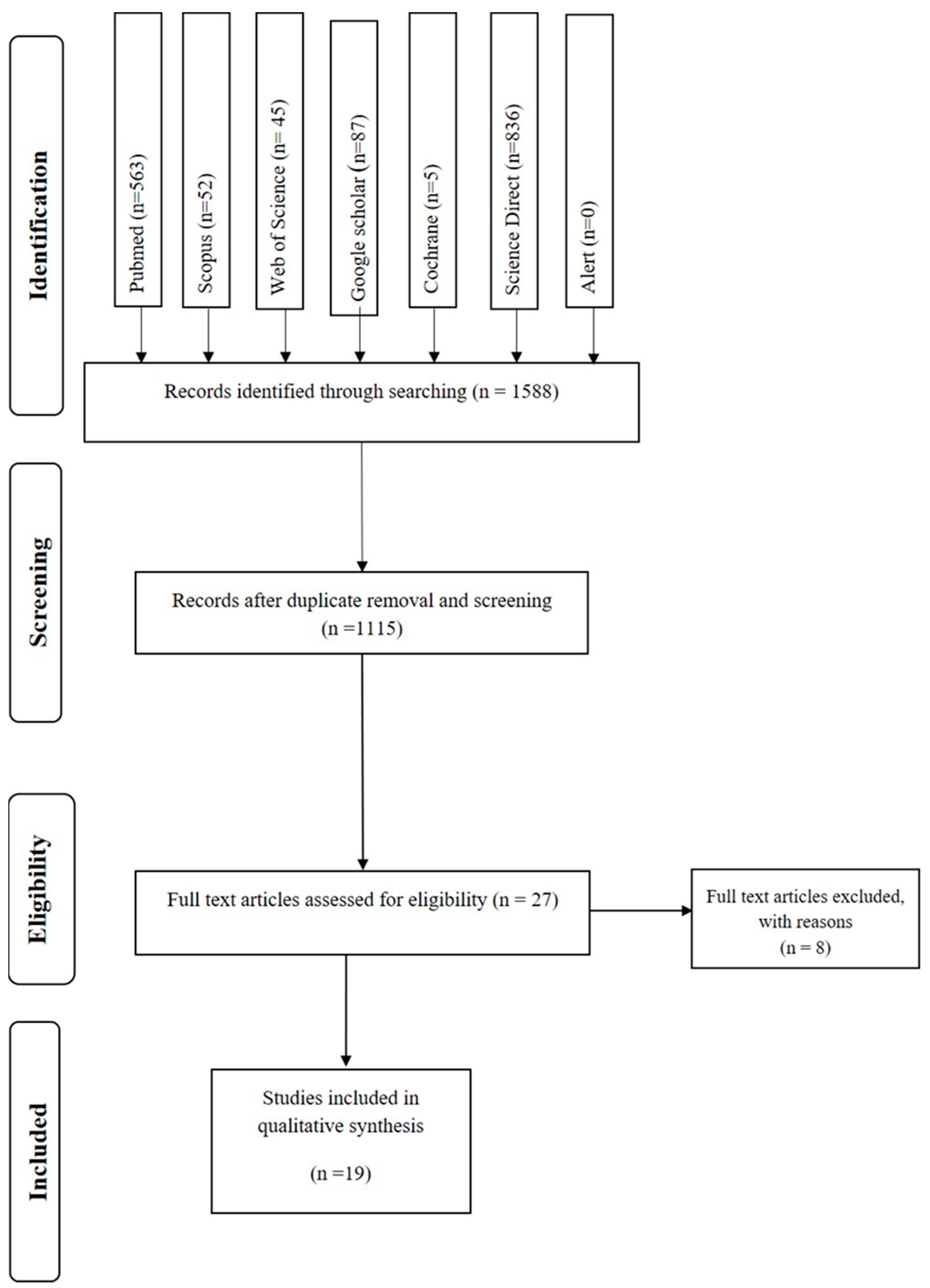
3.1. Selection of the Studies
3.2. Characteristics of the Studies
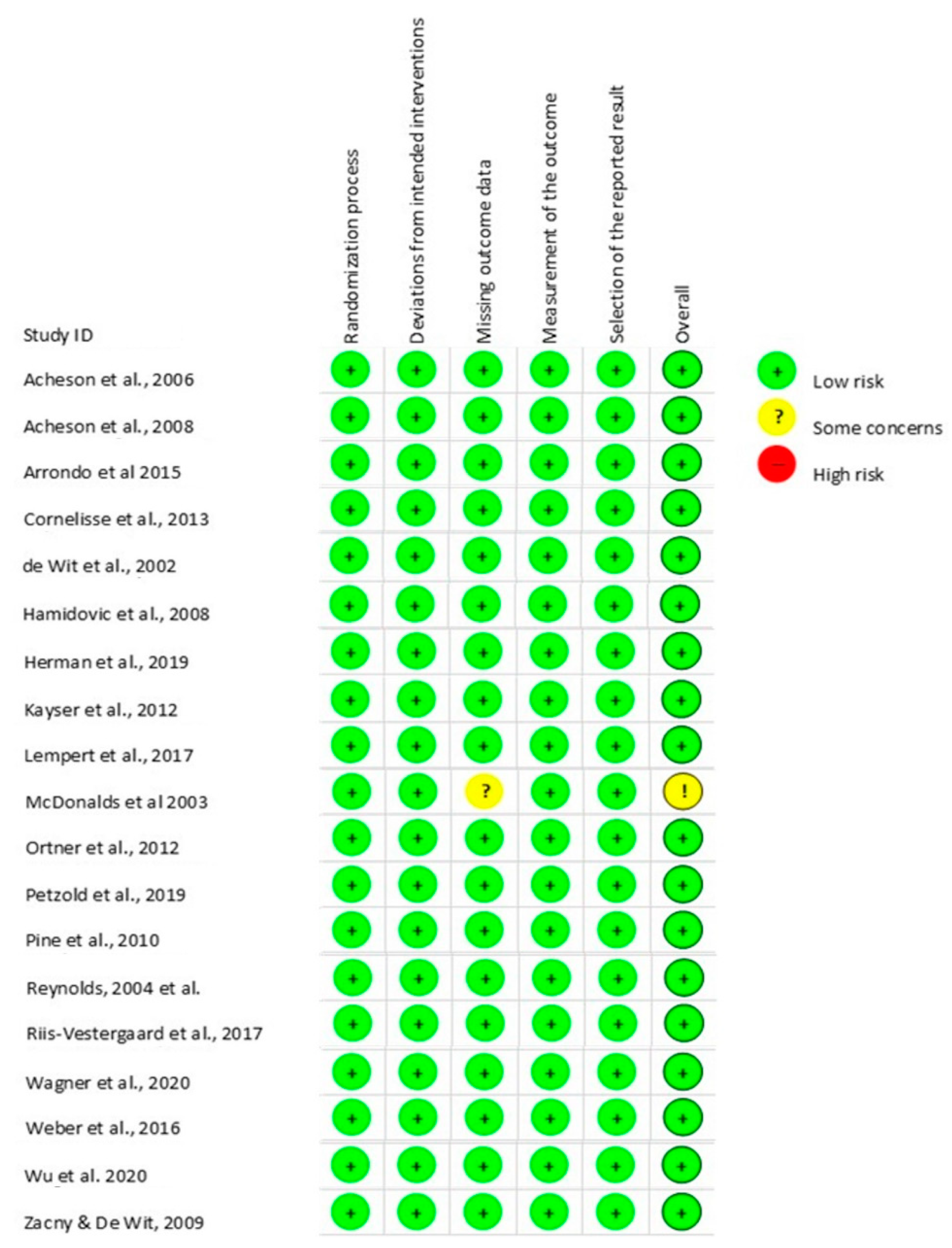
3.3. Risk of Bias
4. Discussion
4.1. Dopaminergic System
4.2. D-Amphetamine
4.3. Levodopa (L-Dopa)
4.4. Tolcapone
4.5. Hypothalamic-Pituitary-Adrenal Axis (HPA-Axis)
4.6. Sympathetic-Adreno-Medullar System (SAM-System)
4.7. Testosterone
4.8. Opioids and Endocannabinoids
4.9. Diazepam
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kalenscher, T.; Pennartz, C.M. Is a bird in the hand worth two in the future? The neuroeconomics of intertemporal decision-making. Prog. Neurobiol. 2008, 84, 284–315. [Google Scholar] [CrossRef]
- Riis-Vestergaard, M.I.; van Ast, V.; Cornelisse, S.; Joëls, M.; Haushofer, J. The effect of hydrocortisone administration on intertemporal choice. Psychoneuroendocrinology 2018, 88, 173–182. [Google Scholar] [CrossRef]
- Haushofer, J.; Jain, P.; Musau, A.; Ndetei, D. Stress May Increase Choice of Sooner Outcomes, But Not Temporal Discounting. J. Econ. Behav. Organ. 2021, 183, 377–396. [Google Scholar] [CrossRef]
- Madden, G.J.; Petry, N.M.; Johnson, P.S. Pathological gamblers discount probabilistic rewards less steeply than matched controls. Exp. Clin. Psychopharmacol. 2009, 17, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Scheres, A.; Tontsch, C.; Thoeny, A.L.; Kaczkurkin, A. Temporal reward discounting in attention-deficit/hyperactivity disorder: The contribution of symptom domains, reward magnitude, and session length. Biol. Psychiatry 2010, 67, 641–648. [Google Scholar] [CrossRef]
- Kalenscher, T.; Ohmann, T.; Güntürkün, O. The neuroscience of impulsive and self-controlled decisions. Int. J. Psychophysiol. 2006, 62, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Basile, A.G.; Toplak, M.E. Four converging measures of temporal discounting and their relationships with intelligence, executive functions, thinking dispositions, and behavioral outcomes. Front. Psychol. 2015, 6, 728. [Google Scholar] [CrossRef] [PubMed]
- Amlung, M.; Vedelago, L.; Acker, J.; Balodis, I.; MacKillop, J. Steep delay discounting and addictive behavior: A meta-analysis of continuous associations. Addiction 2017, 112, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Kirby, K.N.; Petry, N.M.; Bickel, W.K. Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. J. Exp. Psychol. Gen. 1999, 128, 78–87. [Google Scholar] [CrossRef]
- Coffey, S.F.; Gudleski, G.D.; Saladin, M.E.; Brady, K.T. Impulsivity and rapid discounting of delayed hypothetical rewards in cocaine-dependent individuals. Exp. Clin. Psychopharmacol. 2003, 11, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Petry, N.M. Delay discounting of money and alcohol in actively using alcoholics, currently abstinent alcoholics, and controls. Psychopharmacology 2001, 154, 243–250. [Google Scholar] [CrossRef]
- Karakula, S.L.; Weiss, R.D.; Griffin, M.L.; Borges, A.M.; Bailey, A.J.; McHugh, R.K. Delay discounting in opioid use disorder: Differences between heroin and prescription opioid users. Drug Alcohol Depend. 2016, 169, 68–72. [Google Scholar] [CrossRef]
- Reynolds, B.; Richards, J.B.; Horn, K.; Karraker, K. Delay discounting and probability discounting as related to cigarette smoking status in adults. Behav. Process. 2004, 65, 35–42. [Google Scholar] [CrossRef]
- Mason, L.; O’Sullivan, N.; Blackburn, M.; Bentall, R.; El-Deredy, W. I want it now! Neural correlates of hypersensitivity to immediate reward in hypomania. Biol. Psychiatry 2012, 71, 530–537. [Google Scholar] [CrossRef]
- Jackson, J.N.; MacKillop, J. Attention-deficit/hyperactivity disorder and monetary delay discounting: A meta-analysis of case-control studies. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2016, 1, 316–325. [Google Scholar] [CrossRef]
- Hurlemann, R.; Scheele, D.; Kinfe, T.M.; Berger, R.; Philipsen, A.; Voncken, M.J.; Kuypers, K.P.; Schruers, K. Increased temporal discounting in social anxiety disorder normalizes after oxytocin treatment. Psychother. Psychosom. 2019, 88, 55–57. [Google Scholar] [CrossRef]
- Berlin, H.A.; Rolls, E.T. Time perception, impulsivity, emotionality, and personality in self-harming borderline personality disorder patients. J. Pers. Disord. 2004, 18, 358–378. [Google Scholar] [CrossRef]
- Pulcu, E.; Trotter, P.D.; Thomas, E.J.; McFarquhar, M.; Juhász, G.; Sahakian, B.J.; Deakin, J.F.; Zahn, R.; Anderson, I.M.; Elliott, R. Temporal discounting in major depressive disorder. Psychol. Med. 2014, 44, 1825–1834. [Google Scholar] [CrossRef]
- Brown, H.E.; Hart, K.L.; Snapper, L.A.; Roffman, J.L.; Perlis, R.H. Impairment in delay discounting in schizophrenia and schizoaffective disorder but not primary mood disorders. NPJ Schizophr. 2018, 4, 9. [Google Scholar] [CrossRef]
- Sellitto, M.; Ciaramelli, E.; di Pellegrino, G. Myopic discounting of future rewards after medial orbitofrontal damage in humans. J. Neurosci. 2010, 30, 16429–16436. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Prisma Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Acheson, A.; Reynolds, B.; Richards, J.B.; de Wit, H. Diazepam impairs behavioral inhibition but not delay discounting or risk-taking in healthy adults. Exp. Clin. Psychopharmacol. 2006, 14, 190–198. [Google Scholar] [CrossRef]
- Richards, J.B.; Zhang, L.; Mitchell, S.H.; de Wit, H. Delay or probability discounting in a model of impulsive behavior: Effect of alcohol. J. Exp. Anal. Behav. 1999, 71, 121–143. [Google Scholar] [CrossRef]
- Reynolds, B.; Schiffbauer, R. Measuring state changes in human delay discounting: An experiential discounting task. Behav. Process. 2004, 67, 343–356. [Google Scholar] [CrossRef]
- Acheson, A.; de Wit, H. Bupropion improves attention but does not affect impulsive behavior in healthy young adults. Exp. Clin. Psychopharmacol. 2008, 16, 113–123. [Google Scholar] [CrossRef]
- Arrondo, G.; Aznárez-Sanado, M.; Fernández-Seara, M.A.; Goñi, J.; Loayza, F.R.; Salamon-Klobu, T.E.; Heukamp, F.H.; Pastor, M.A. Dopaminergic modulation of the trade-off between probability and time in economic decision-making. Eur. Neuropsychopharmacol. 2015, 25, 817–827. [Google Scholar] [CrossRef]
- Cornelisse, S.; van Ast, V.; Haushofer, J.; Seinstra, M.; Joels, M. Time-dependent effect of hydrocortisone administration on intertemporal choice. SSRN 2013, 1–22. [Google Scholar] [CrossRef]
- de Wit, H.; Enggasser, J.L.; Richards, J.B. Acute administration of d-amphetamine decreases impulsivity in healthy volunteers. Neuropsychopharmacology 2002, 27, 813–825. [Google Scholar] [CrossRef]
- Hamidovic, A.; Kang, U.J.; de Wit, H. Effects of low to moderate acute doses of pramipexole on impulsivity and cognition in healthy volunteers. J. Clin. Psychopharmacol. 2008, 28, 45–51. [Google Scholar] [CrossRef]
- Herman, A.M.; Critchley, H.D.; Duka, T. The impact of Yohimbine-induced arousal on facets of behavioral impulsivity. Psychopharmacology 2019, 236, 1783–1795. [Google Scholar] [CrossRef]
- Kayser, A.S.; Allen, D.C.; Navarro-Cebrian, A.; Mitchell, J.M.; Fields, H.L. Dopamine, corticostriatal connectivity, and intertemporal choice. J. Cogn. Neurosci. 2012, 32, 9402–9409. [Google Scholar] [CrossRef] [PubMed]
- Lempert, K.M.; Lackovic, S.F.; Tobe, R.H.; Glimcher, P.W.; Phelps, E.A. Propranolol reduces reference-dependence in intertemporal choice. Soc. Cogn. Affect. Neurosci. 2017, 12, 1394–1401. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.; Schleifer, L.; Richards, J.B.; de Wit, H. Effects of THC on behavioral measures of impulsivity in humans. Neuropsychopharmacology 2003, 28, 1356–1365. [Google Scholar] [CrossRef]
- Ortner, G.R.; Wibral, M.; Becker, A.; Dohmen, T.; Klingmüller, D.; Falk, A.; Weber, B. No evidence for an effect of testosterone administration on delay discounting in male university students. Psychoneuroendocrinology 2013, 38, 1814–1818. [Google Scholar] [CrossRef] [PubMed]
- Petzold, J.; Lee, Y.; Pooseh, S.; Oehme, L.; Beuthien-Baumann, B.; London, E.D.; Goschke, T.; Smolka, M.N. Presynaptic dopamine function measured with [18 F] fluorodopa and L-DOPA effects on impulsive choice. Sci. Rep. 2019, 9, 17927. [Google Scholar] [CrossRef]
- Pine, A.; Shiner, T.; Seymour, B.; Dolan, R.J. Dopamine, time, and impulsivity in humans. J. Neurosci. 2010, 30, 8888–8896. [Google Scholar] [CrossRef]
- Pine, A.; Seymour, B.; Roiser, J.P.; Bossaerts, P.; Friston, K.J.; Curran, H.V.; Dolan, R.J. Encoding of marginal utility across time in the human brain. J. Neurosci. 2009, 29, 9575–9581. [Google Scholar] [CrossRef]
- Wagner, B.; Clos, M.; Sommer, T.; Peters, J. Dopaminergic Modulation of Human Intertemporal Choice: A Diffusion Model Analysis Using the D2-Receptor Antagonist Haloperidol. J. Neurosci. 2020, 40, 7936–7948. [Google Scholar] [CrossRef]
- Weber, S.C.; Beck-Schimmer, B.; Kajdi, M.E.; Müller, D.; Tobler, P.N.; Quednow, B.B. Dopamine D2/3-and μ-opioid receptor antagonists reduce cue-induced responding and reward impulsivity in humans. Transl. Psychiatry 2016, 6, e850. [Google Scholar] [CrossRef]
- Gelkopf, M.; Bleich, A.; Hayward, R.; Bodner, G.; Adelson, M. Characteristics of benzodiazepine abuse in methadone maintenance treatment patients: A 1-year prospective study in an Israeli clinic. Drug Alcohol Depend. 1999, 55, 63–68. [Google Scholar] [CrossRef]
- Wu, Y.; Shen, B.; Liao, J.; Li, Y.; Zilioli, S.; Li, H. Single dose testosterone administration increases impulsivity in the intertemporal choice task among healthy males. Horm. Behav. 2020, 118, 104634. [Google Scholar] [CrossRef] [PubMed]
- Zacny, J.P.; de Wit, H. The prescription opioid, oxycodone, does not alter behavioral measures of impulsivity in healthy volunteers. Pharmacol. Biochem. Behav. 2009, 94, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Yin, Y.; Wang, J.; Zhou, X.; McClure, S.M.; Li, J. High-definition tDCS alters impulsivity in a baseline-dependent manner. Neuroimage 2016, 143, 343–352. [Google Scholar] [CrossRef]
- Cook, D.J.; Mulrow, C.D.; Haynes, R.B. Systematic reviews: Synthesis of best evidence for clinical decisions. Ann. Intern. Med. 1997, 126, 376–380. [Google Scholar] [CrossRef]
- Sanderson, S.; Tatt, I.D.; Higgins, J. Tools for assessing quality and susceptibility to bias in observational studies in epidemiology: A systematic review and annotated bibliography. Int. J. Epidemiol. 2007, 36, 666–676. [Google Scholar] [CrossRef]
- Milenkova, M.; Mohammadi, B.; Kollewe, K.; Schrader, C.; Fellbrich, A.; Wittfoth, M.; Dengler, R.; Münte, T.F. Intertemporal choice in Parkinson’s disease. Mov. Disord. 2011, 26, 2004–2010. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Yoon, S.; Nakajima, R.; Lee, H.J.; Lim, H.J.; Lee, Y.K.; Choi, J.S.; Yoon, B.J.; Augustine, G.J.; Baik, J.H. Dopamine D2 receptor-mediated circuit from the central amygdala to the bed nucleus of the stria terminalis regulates impulsive behavior. Proc. Natl. Acad. Sci. USA 2018, 115, E10730–E10739. [Google Scholar] [CrossRef]
- Bernosky-Smith, K.A.; Qiu, Y.Y.; Feja, M.; Lee, Y.B.; Loughlin, B.; Li, J.X.; Bass, C.E. Ventral tegmental area D2 receptor knockdown enhances choice impulsivity in a delay-discounting task in rats. Behav. Brain Res. 2018, 341, 129–134. [Google Scholar] [CrossRef]
- Dalley, J.W.; Fryer, T.D.; Brichard, L.; Robinson, E.S.; Theobald, D.E.; Lääne, K.; Peña, Y.; Murphy, E.R.; Shah, Y.; Probst, K.; et al. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science 2007, 315, 1267–1270. [Google Scholar] [CrossRef]
- Lee, B.; London, E.D.; Poldrack, R.A.; Farahi, J.; Nacca, A.; Monterosso, J.R.; Mumford, J.A.; Bokarius, A.V.; Dahlbom, M.; Mukherjee, J.; et al. Striatal dopamine D2/D3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. J. Neurosci. 2009, 29, 14734–14740. [Google Scholar] [CrossRef]
- Ballard, M.E.; Mandelkern, M.A.; Monterosso, J.R.; Hsu, E.; Robertson, C.L.; Ishibashi, K.; Dean, A.C.; London, E.D. Low dopamine D2/D3 receptor availability is associated with steep discounting of delayed rewards in methamphetamine dependence. Int. J. Neuropsychopharmacol. 2015, 18, pyu119. [Google Scholar] [CrossRef]
- Pennisi, P.; Salehinejad, M.A.; Corso, A.M.; Merlo, E.M.; Avenanti, A.; Vicario, C.M. Delay discounting in Parkinson’s disease: A systematic review and meta-analysis. Behav. Brain Res. 2022, 346, 114101. [Google Scholar] [CrossRef]
- Foerde, K.; Figner, B.; Doll, B.B.; Woyke, I.C.; Braun, E.K.; Weber, E.U.; Shohamy, D. Dopamine modulation of intertemporal decision-making: Evidence from Parkinson disease. J. Cogn. Neurosci. 2016, 28, 657–667. [Google Scholar] [CrossRef]
- Cools, R.; Barker, R.A.; Sahakian, B.J.; Robbins, T.W. L-Dopa medication remediates cognitive inflexibility but increases impulsivity in patients with Parkinson’s disease. Neuropsychology 2003, 41, 1431–1441. [Google Scholar] [CrossRef]
- Castrellon, J.J.; Meade, J.; Greenwald, L.; Hurst, K.; Samanez-Larkin, G.R. Dopaminergic modulation of reward discounting in healthy rats: A systematic review and meta-analysis. Psychopharmacology 2020, 238, 711–723. [Google Scholar] [CrossRef]
- Seeman, P. Parkinson’s disease treatment may cause impulse–control disorder via dopamine D3 receptors. Synapse 2015, 69, 183–189. [Google Scholar] [CrossRef]
- Onge, J.R.; Floresco, S.B. Dopaminergic modulation of risk-based decision making. Neuropsychopharmacology 2009, 34, 681–697. [Google Scholar] [CrossRef]
- Castells, X.; Blanco-Silvente, L.; Cunill, R. Amphetamines for attention deficit hyperactivity disorder (ADHD) in adults. Cochrane Database Syst. Rev. 2018, 8, CD007813. [Google Scholar] [CrossRef]
- Perry, J.L.; Stairs, D.J.; Bardo, M.T. Impulsive choice and environmental enrichment: Effects of d-amphetamine and methylphenidate. Behav. Brain Res. 2008, 193, 48–54. [Google Scholar] [CrossRef]
- Song, J.; Park, J.H.; Han, D.H.; Roh, S.; Son, J.H.; Choi, T.Y.; Lee, H.; Kim, T.H.; Lee, Y.S. Comparative study of the effects of bupropion and escitalopram on Internet gaming disorder. Psychiatry Clin. Neurosci. 2016, 70, 527–535. [Google Scholar] [CrossRef]
- Verbeeck, W.; Bekkering, G.E.; van den Noortgate, W.; Kramers, C. Bupropion for attention deficit hyperactivity disorder (ADHD) in adults. Cochrane Database Syst. Rev. 2017, 10, CD0095. [Google Scholar] [CrossRef] [PubMed]
- Cardinal, R.N.; Robbins, T.W.; Everitt, B.J. The effects of d-amphetamine, chlordiazepoxide, α-flupenthixol and behavioural manipulations on choice of signalled and unsignalled delayed reinforcement in rats. Psychopharmacology 2000, 152, 362–375. [Google Scholar] [CrossRef] [PubMed]
- Helms, C.M.; Reeves, J.M.; Mitchell, S.H. Impact of strain and D-amphetamine on impulsivity (delay discounting) in inbred mice. Psychopharmacology 2006, 188, 144–151. [Google Scholar] [CrossRef]
- Maguire, D.R.; Henson, C.; France, C.P. Effects of amphetamine on delay discounting in rats depend upon the manner in which delay is varied. Neuropharmacology 2014, 87, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.M.; Campos, F.L.; Marques, M.; Soares-Cunha, C.; Kokras, N.; Dalla, C.; Leite-Almeida, H.; Sousa, N.; Salgado, A.J. Effect of levodopa on reward and impulsivity in a rat model of Parkinson’s disease. Front. Behav. Neurosci. 2017, 11, 145. [Google Scholar] [CrossRef]
- Cools, R.; D’Esposito, M. Inverted-U–shaped dopamine actions on human working memory and cognitive control. Biol. Psychiatry 2011, 69, e113–e125. [Google Scholar] [CrossRef]
- Rivest, J.; Barclay, C.L.; Suchowersky, O. COMT Inhibitors in Parkinson’s Disease. Can. J. Neurol. Sci. 1999, 2, S34–S38. [Google Scholar] [CrossRef]
- Boettiger, C.A.; Mitchell, J.M.; Tavares, V.C.; Robertson, M.; Joslyn, G.; D’Esposito, M.; Fields, H.L. Immediate reward bias in humans: Front-parietal networks and a role for the catechol-O-methyltransferase 158Val/Val genotype. J. Neurosci. 2007, 27, 14383–14391. [Google Scholar] [CrossRef]
- Paloyelis, Y.; Asherson, P.; Mehta, M.A.; Faraone, S.V.; Kuntsi, J. DAT1 and COMT effects on delay discounting and trait impulsivity in male adolescents with attention deficit/hyperactivity disorder and healthy controls. Neuropsychopharmacology 2010, 35, 2414–2426. [Google Scholar] [CrossRef]
- Lempert, K.M.; Porcelli, A.J.; Delgado, M.R.; Tricomi, E. Individual differences in delay discounting under acute stress: The role of trait perceived stress. Front. Psychol. 2012, 3, 251. [Google Scholar] [CrossRef]
- Eisenegger, C.; Haushofer, J.; Fehr, E. The role of testosterone in social interaction. Trends Cogn. Sci. 2011, 15, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Kurath, J.; Mata, R. Individual differences in Risk-taking and endogenous levels of testosterone, estradiol, and cortisol: A systematic literature search and three independent meta-analyses. Neurosci. Biobehav. Rev. 2018, 90, 428–446. [Google Scholar] [CrossRef] [PubMed]
- Creutz, L.M.; Kritzer, M.F. Mesostriatal and mesolimbic projections of midbrain neurons immunoreactive for estrogen receptor beta or androgen receptors in rats. J. Comp. Neurol. 2004, 476, 348–362. [Google Scholar] [CrossRef]
- van Steenbergen, H.; Eikemo, M.; Leknes, S. The role of the opioid system in decision making and cognitive control: A review. Cogn. Affect. Behav. Neurosci. 2019, 19, 435–458. [Google Scholar] [CrossRef] [PubMed]
- Aizpurua-Olaizola, O.; Elezgarai, I.; Rico-Barrio, I.; Zarandona, I.; Etxebarria, N.; Usobiaga, A. Targeting the endocannabinoid system: Future therapeutic strategies. Drug Discov. Today 2017, 22, 105–110. [Google Scholar] [CrossRef]
- Kieres, A.K.; Hausknecht, K.A.; Farrar, A.M.; Acheson, A.; de Wit, H.; Richards, J.B. Effects of morphine and naltrexone on impulsive decision making in rats. Psychopharmacology 2004, 173, 167–174. [Google Scholar] [CrossRef]
- Pattij, T.; Schetters, D.; Janssen, M.C.; Wiskerke, J.; Schoffelmeer, A.N. Acute effects of morphine on distinct forms of impulsive behavior in rats. Psychopharmacology 2009, 205, 489–502. [Google Scholar] [CrossRef]
- de Wit, H.; Crean, J.; Richards, J.B. Effects of d-amphetamine and ethanol on a measure of behavioral inhibition in humans. Behav. Neurosci. 2000, 114, 830–837. [Google Scholar] [CrossRef]
- Ameri, A. The effects of cannabinoids on the brain. Prog. Neurobiol. 1999, 58, 315–348. [Google Scholar] [CrossRef]
- Chait, L.D.; Pierri, J. Effects of Smoked Marijuana on Human Performance: A Critical Review; Marijuana/Cannabinoids; CRC Press: Boca Raton, FL, USA, 1992. [Google Scholar]
- Evenden, J.; Ko, T. The psychopharmacology of impulsive behavior in rats VIII: Effects of amphetamine, methylphenidate, and other drugs on responding maintained by a fixed consecutive number avoidance schedule. Psychopharmacology 2005, 180, 294–305. [Google Scholar] [CrossRef]
- Deakin, J.B.; Aitken, M.R.; Dowson, J.H.; Robbins, T.W.; Sahakian, B.J. Diazepam produces disinhibitory cognitive effects in male volunteers. Psychopharmacology 2004, 173, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Fava, M. Psychopharmacologic treatment of pathologic aggression. Psychiatr. Clin. N. Am. 1997, 20, 427–451. [Google Scholar] [CrossRef] [PubMed]
- Dåderman, A.M.; Fredriksson, B.; Kristiansson, M.; Nilsson, L.H.; Lidberg, L. Violent behavior, impulsive decision-making, and anterograde amnesia while intoxicated with flunitrazepam and alcohol or other drugs: A case study in forensic psychiatric patients. J. Am. Acad. Psychiatry Law 2002, 30, 238–251. Available online: https://pubmed.ncbi.nlm.nih.gov/12108561/ (accessed on 13 December 2022). [PubMed]
| Author/Year/Study Design | Sample | Age (Mean ± SD), in Years | Intervention Type | Intertemporal Decision-Making Task | Results | |
|---|---|---|---|---|---|---|
| Source | Size | |||||
| Acheson et al. [22]. -United States -Randomized, within subject-design. | Males and females | 18 participants -9 Male -9 Female | Aged: 18–45 Mean: 20.9 ± 2.5 | Diazepam (Valium 20 mg). Placebo | Delay and probability discounting task [23]. Experiential discounting task [24]. | Diazepam did not affect Delay and probability discounting task neither Experiential discounting task, p <0.05. |
| Acheson & de Wit [25]. -United States -Randomized, within-subject design. | Male and female | 33 participants -Men:18 -Women:15 | Aged: 18–45 Mean: 23.45 ± 4.6 | Bupropion hydrochloride BH (75 mg) BH (100 mg) D-amphetamine sulfate (5 mg) Placebo | Delay and Probability Discounting Task [23]. | Neither bupropion nor amphetamine affected discounting of hypothetical delayed or probabilistic rewards on the DPD, p <0.05. |
| Arrondo et al. [26]. -Spain -Randomized, between-subject design. | Males and Females | 29 participants Intervention: 15 -Men:9 -Women:6 Placebo: 14 -Men:4 -Women:10 | Aged intervention group: 24.0 ± 3.4 years Age placebo group: 23 ±1.9 years | Metoclopramide (10 mg) (Primperan) Placebo | Decision-making task. | Intervention group was more willing to wait to increase the probability of the reward, p = 0.007. |
| Cornelisse et al. [27]. - Germany - Randomized, between subject design. | Males | 79 Participants | Aged 18 to 35 | Hydrocortisone (10 mg). Placebo (albochin) | Temporal discounting task [27]. | Hydrocortisone administration increased preference for immediate rewards after administration, but not three hours later. -p < 0.10 -p < 0.01 -p < 0.05 |
| de Wit et al. [28] -United States -Randomized, within subject-subject design. | Males and females | 36 participants -18 Male -18 Female | Aged 18–44 Mean: 24 ± 6.5 | D-amphetamine (10 & 20 mg). Placebo | Delay Discounting [23]. Delay of Gratification. Time perception. | Only high d-Amphetamine significantly decreased the discounting, p < 0.05. |
| Hamidovic et al. [29]. -United States -Randomized, within design. | Males and females | 10 Participants | Aged 18–28 | Pramipexole (0.25 & 0.50 mg Mirapex) Placebo. | Delay Discounting task. | Pramipexole did not significantly affect either delay or probability discounting, p <0.05. |
| Herman et al. [30]. -UK -Randomized, between subject design. | Male and female | 42 Participants Exposed: 21 -12 females -9 male Controls: 21 -11 females -10 Male | Aged 18–40 Exposed:21.29 Control: 23.19 | Yohimbine (20 mg). Placebo | Probability Discounting Task (PD [4]). Monetary Choice Questionnaire (MCQ [4]). | There were no group differences in performance on neither MCQ, PD. |
| Kayser et al. [31]. -United States -Randomized, within-subject design. | Males and females | 23 participants -13 females -10 males | Aged 19–41 | Tolcapone (200 mg). Placebo | Delay Discounting Task. | Tolcapone (200 mg) increased the choosing of the delayed reward ∆ICR = −0.04 p = 0.025. |
| Lempert et al. [32]. -United States -Randomized, within-subject design. | Male and female | 37 participants | Aged 27.8 ± 6.6 | Propranolol (80 mg) Placebo | Intertemporal choice task. | Propranolol did not reduce temporal discounting overall, p < 0.05. |
| McDonald et al. [33]. -United States -Randomized, within-subjects design. | Males and females | 37 participants -Men:18 -Women:19 | Aged 18–45 Mean 23 ± 4.48 | Marinols 7.5 mg Marinols 15 mg Placebo | The Delay discounting task [23]. | THC did not significantly affect either delay or probability discounting, p < 0.05. |
| Ortner et al. [34]. -Germany -Randomized, between subject design. | Males | 91 participants - Experimental group: 46 Placebo group: 45. | Aged 24.3 SD: 2.73 | Testogel ® 50 mg Placebo 50 mg | Monetary choice questionnaire [9]. | Testosterone does not have a significant effect on delay discounting in male university students, p = 0.538. |
| Petzold et al. [35]. -Germany -Randomized, within-subject design. | Males and females | 87 participants -65 males -22 females 44 Low-weight subjects -25 males -19 females | Aged: 20–40 Mean: 35.91 ± 3.8 | Madopar 187.5 mg L-dopa Booster 93.75 mg Placebo | Value-based decision-making test battery. | No significant differences between placebo and L-DOPA conditions for delay discounting, risk-seeking for gains and losses, p < 0.05. |
| Pine et al. [36]. -London -Randomized, within-subject design. | Males and females | 14 participants -Men: 6 -Women: 8 | Aged 18–30 Mean 21 | Haloperidol 1.5 mg Madopar (L-Dopa 150 mg) Placebo | Temporal discounting task [37]. | L-dopa 150 mg increased the number of sooner options chosen relative to the placebo condition. There was no significant difference between haloperidol and placebo conditions on this disposition, p < 0.05. |
| Reynolds et al. [13]. -United States -Randomized, within-subject design. | Male and female | 35 participants -Men 19 -Women 16 | Aged 18–45 | Diazepam (Valium, 5 mg) Diazepam 10 mg Placebo | Delay discounting task [23]. | Diazepam (5 mg & 10 mg) did not affect performance on any of the behavioral task measures of impulsivity, including k values on the discounting task. p < 0.05. |
| Riis-Vestergaard et al. [2]. -Netherlands -Randomized, between-subject design. | Males | 79 participants | Aged 18–35 | Hydrocortisone (10 mg; rapid and slow cort) Placebo | Intertemporal choice task. | Hydrocortisone administration increased preference for immediate rewards after administration, but not three hours later, p < 0.05. |
| Wagner et al. [38]. - Germany -Randomized, between subject design | Males | 54 participants: 27 for each group. | Placebo Group: 24.4. SD: 3.4 - Experimental group: 23.3 SD: 2.5 | Haloperidol 2 mg Placebo | Temporal discounting task. | The D2-receptor antagonist haloperidol attenuated temporal discounting and substantially shortened nondecision times. |
| Weber et al. [39]. - Switzerland - Randomized, between subject design | Male and Female | 121 participants - Experimental group: 81 Placebo group: 40 | Placebo group: 22.15 Amilsupride group: 21.46 Naltrexone group: 21.65 | Amisulpride 400 mg Naltrexone 50 mg Placebo | Monetary Choice Questionnaire [9]. | Amisulpride group chose the smaller immediate rewards significantly less often than the placebo group (t [40] = 2.58, p < 0.01). The difference between the naltrexone and the placebo groups did not reach significance (t [40] = 1.70. p = 0.09). |
| Wu et al. [41]. -China -Between subjects, placebo-controlled, double-blind intervention. | Males | 111 participants | Aged 18–27 Mean: 21.7 ± 1.9 | Androgel (150 mg) ®. Testosterone Placebo | Intertemporal choice (ITC) task. | Testosterone group (k value: M = 0.10, SD = 0.21) showed increased impulsivity than those in the placebo group (k value: M = 0.022, SD = 0.030), p = 0.001. |
| Zacny & de Wit [42]. -United States -Randomized, within-subject design. | Males and Females | 12 participants -6 Men -6 Women | Aged 21–39 Mean: 25.3 ± 3.6 | Oxycodone (5 mg, 10 mg & 20 mg). Placebo | Delay and probability discounting task (DPD [9]). | There were no discernible trends of an effect of oxycodone on the DPD. |
| Drug | Properties | Uses |
|---|---|---|
| Amisulpride | Amisulpride is a dopamine D2 receptor antagonist. At low doses Amisulpride blocks presynaptic dopamine D2 and D3 receptors, increasing dopaminergic levels in the synaptic cleft. | Used in schizophrenia, and to prevent and treat postoperative nausea and vomiting in adults |
| Pramipexole | Pramipexole is a non-ergot dopamine agonist. It shows specific and strong activity in D2 and D3 receptors. | Used to treat symptoms of Parkinson’s disease and Restless Legs Syndrome |
| Haloperidol | Haloperidol binds to dopamine D2 receptor. Blocking approximately 60–80% of D2 receptors in the brain. But it also has activity at a number of receptors in the brain. | Used to treat schizophrenia. Also, symptoms of agitation, irritability and delirium |
| Metoclopramide | Metoclopramide is a dopamine D2 antagonist. It inhibits dopamine D2 and serotonin 5-HT3 receptors in the chemoreceptor trigger zone located in the area postrema of the brain. | Used to treat gastroesophageal reflux disease, and prevention of nausea and vomiting |
| Levadopa | Levadopa is a dopamine precursor. While dopamine cannot cross the blood brain barrier, Levadopa is able to. After crossing the barrier Levadopa is converted to dopamine | Used in the treatment of Parkinson disease |
| Bupropion | Bupropion is a norepinephrine/dopamine reuptake inhibitor (NDRI). It inhibits the enzymes involved in reuptaking norepinephrine and dopamine prolonging their action. Specifically, bupropion binds to the norepinephrine transporter (NET) and the dopamine transporter (DAT) | Used in the treatment of major depressive disorder and seasonal affective disorder |
| Tolcapone | Tolcapone is a selective and reversible inhibitor of catechol-O-methyltransferase (COMT) -COMT is an enzyme responsible for the degradation of catecholamines-. | Used as adjunct therapy in the symptomatic management of idiopathic Parkinson’s disease. |
| D-amphetamine | D-amphetamine is a noncatecholamine, sympathomimetic amine that acts as Central Nervous System stimulant. | Used in the treatment of attention deficit hyperactivity disorder and narcolepsy. |
| Yohimbine | Yohimbine is a pre-synaptic alpha 2-adrenergic blocking agent. It increases norepinephrine release | Yohimbine is found in supplements. |
| Propranolol | Propranolol is a non-selective beta-adrenergic receptor antagonist. Means that it does not have preference for Beta receptors. It inhibits sympathetic stimulation of the heart. It reduces resting heart rate, cardiac output, blood pressure. | Used to treat hypertension, angina, atrial fibrillation, myocardial infarction, migraine, essential tremor, hypertrophic subaortic stenosis, and pheochromocytoma. |
| Hydrocortisone | Hydrocortisone, or cortisol, is a glucocorticoid secreted by the adrenal cortex. It is essential for life and supports many important cardiovascular, metabolic, immunologic, and homeostatic functions | Used to treat corticosteroid-responsive dermatoses, endocrine disorders, immune conditions, and allergic disorders. |
| Testosterone | Testosterone is a steroid sex hormone | Used to treat hypogonadism or breast carcinoma in women, |
| Marinols (Dronabinold) | Marinol (Dronabinol) is a cannabinoid. It’s a synthetic form of THC, which is an ingredient found in marijuana. | Treatment of anorexia and weight loss in people with acquired immunodeficiency syndrome. Also is used in treatment of nausea and vomiting from chemotherapy |
| Diazepam | Diazepam is a benzodiazepine that binds to receptors in the brain and spinal cord. This binding increases the inhibitory effects of GABA (gamma-aminobutyric acid). GABA is involved in sleep induction, memory, anxiety, epilepsy and neuronal excitability. | Used to treat panic disorders, severe anxiety, and seizures. |
| Oxycodone | Oxycodone is an opioid. It binds to the mu opioid receptor, and to the kappa and delta opioid receptor. | Used in the treatment of pain. |
| Naltrexone | Naltrexone is a opiate antagonist. It may block the effects of endogenous opioids. | Used in opioid overdose. |
| Drug | Mechanism | Study |
|---|---|---|
| Amisulpride | Dopamine D2 receptor antagonist. | Weber et al. [39] |
| Haloperidol | Dopamine antagonist. | Wagner et al. [38] |
| Pine et al. [36] | ||
| Metoclopramide | Dopamine D2 receptor antagonist. | Arrondo et al. [26] |
| Pramipexole | Dopamine agonist. | Hamidovic et al. [29] |
| Levodopa | Dopamine agonist. | Pine et al. [36] |
| Petzold et al. [35] | ||
| D-amphetamine | Indirect agonist of dopamine and norepinephrine. | Acheson et al. [25] |
| de Wit et al. [28] | ||
| de Wit et al. [28] | ||
| Bupropion | Norepinephrine/dopamine-reuptake inhibitor. | Acheson et al. [25] |
| Tolcapone | Inhibitor of catechol-O-methyltransferase (Enzyme responsible for the degradation of catecholamines). | Kayser et al. [31] |
| Hydrocortisone | Glucocorticoid (Cortisol). | Cornelisse et al. [27] |
| Riis-Verstergaard et al. [2] | ||
| Yohimbine | Alpha 2-adrenergic blocking agent. | Herman et al. [30] |
| Propranolol | Nonselective β-adrenergic receptor antagonist. | Lempert et al. [32] |
| Testosterone | Acts on Androgen receptor. | Wu et al. [41] |
| Ortner et al. [34] | ||
| Oxycodone | Opioid agonists. | Zacny et al. [42] |
| Naltrexone | Opioid antagonist. | Weber et al. [39] |
| Marinols | (THC). | McDonald et al. [33] |
| Diazepam | Benzodiazepine. Increases the inhibitory effects of gamma-aminobutyric acid (GABA). | Reynolds et al. [13] |
| Acheson et al. [22] | ||
| Acheson et al. [22] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarmiento, L.F.; Ríos-Flórez, J.A.; Paez-Ardila, H.A.; Lima de Sousa, P.S.; Olivera-La Rosa, A.; Oliveira da Silva, A.M.H.; Gouveia, A., Jr. Pharmacological Modulation of Temporal Discounting: A Systematic Review. Healthcare 2023, 11, 1046. https://doi.org/10.3390/healthcare11071046
Sarmiento LF, Ríos-Flórez JA, Paez-Ardila HA, Lima de Sousa PS, Olivera-La Rosa A, Oliveira da Silva AMH, Gouveia A Jr. Pharmacological Modulation of Temporal Discounting: A Systematic Review. Healthcare. 2023; 11(7):1046. https://doi.org/10.3390/healthcare11071046
Chicago/Turabian StyleSarmiento, Luis Felipe, Jorge Alexander Ríos-Flórez, Hector Andres Paez-Ardila, Pêssi Socorro Lima de Sousa, Antonio Olivera-La Rosa, Anderson Manoel Herculano Oliveira da Silva, and Amauri Gouveia, Jr. 2023. "Pharmacological Modulation of Temporal Discounting: A Systematic Review" Healthcare 11, no. 7: 1046. https://doi.org/10.3390/healthcare11071046
APA StyleSarmiento, L. F., Ríos-Flórez, J. A., Paez-Ardila, H. A., Lima de Sousa, P. S., Olivera-La Rosa, A., Oliveira da Silva, A. M. H., & Gouveia, A., Jr. (2023). Pharmacological Modulation of Temporal Discounting: A Systematic Review. Healthcare, 11(7), 1046. https://doi.org/10.3390/healthcare11071046






