A Case Series on Pain Accompanying Photoimmunotherapy for Head and Neck Cancer
Abstract
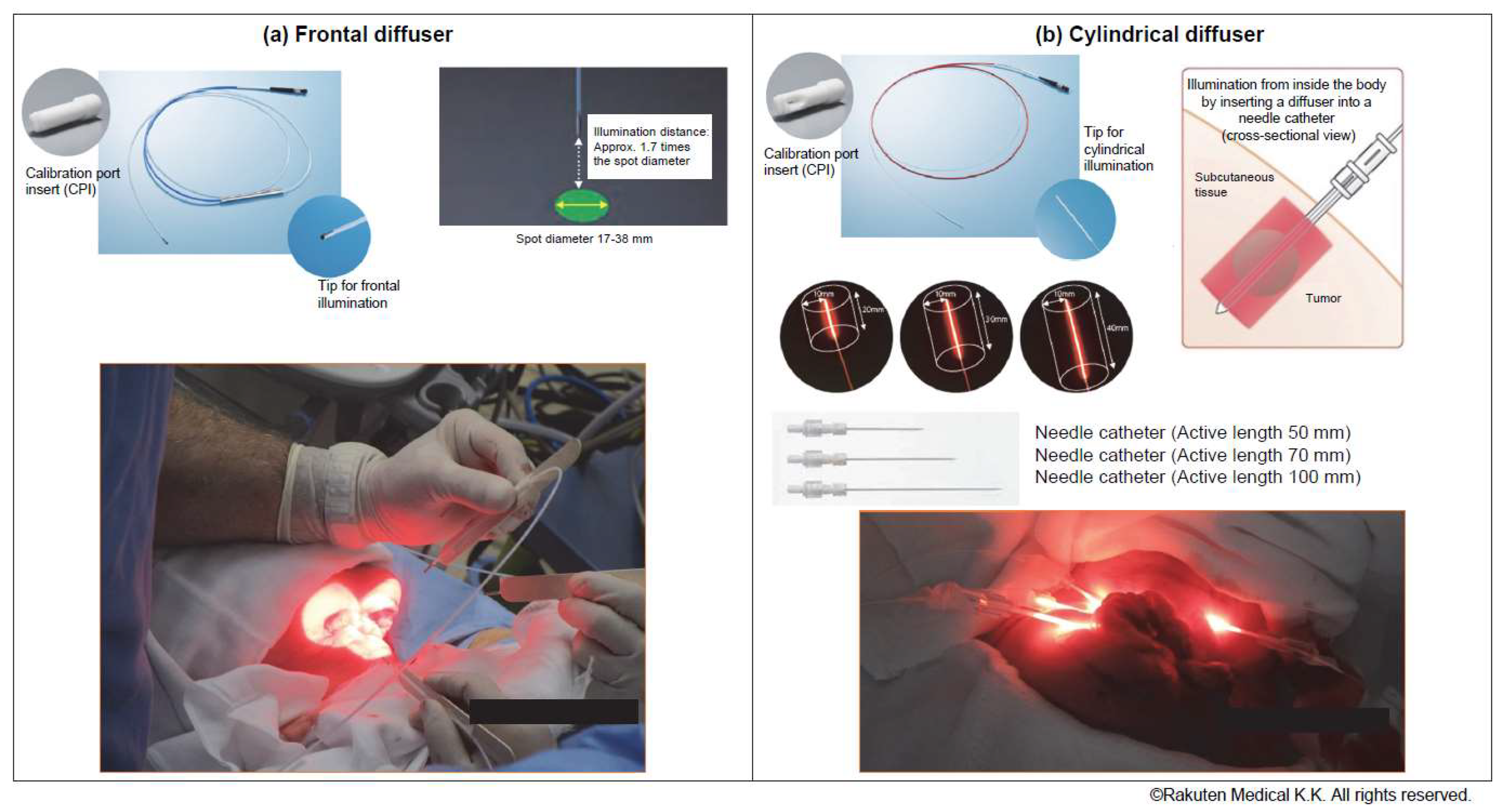
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Analgesic Management and Pain Assessment
2.3. Statistical Analysis
2.4. Ethical Considerations
3. Results
3.1. Pain after PIT
3.2. Opioid Therapy for Pain after PIT
3.3. Fluctuations in Laboratory Test Results after PIT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chow, L.Q.M. Head and neck cancer. N. Engl. J. Med. 2020, 382, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Mody, M.D.; Rocco, J.W.; Yom, S.S.; Haddad, R.I.; Saba, N.F. Head and neck cancer. Lancet 2021, 398, 2289–2299. [Google Scholar] [CrossRef] [PubMed]
- Mendenhall, W.M.; Mendenhall, C.M.; Malyapa, R.S.; Palta, J.R.; Mendenhall, N.P. Re-irradiation of head and neck carcinoma. Am. J. Clin. Oncol. 2008, 31, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Burtness, B.; Harrington, K.J.; Greil, R.; Soulières, D.; Tahara, M.; de Castro, G., Jr.; de Castro, G., Jr.; Psyrri, A.; Basté, N.; Neupane, P.; et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet 2019, 394, 1915–1928. [Google Scholar] [CrossRef] [PubMed]
- Vermorken, J.B.; Mesia, R.; Rivera, F.; Remenar, E.; Kawecki, A.; Rottey, S.; Erfan, J.; Zabolotnyy, D.; Kienzer, H.-R.; Cupissol, D.; et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N. Engl. J. Med. 2008, 359, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Park, J.C.; Krishnakumar, H.N.; Saladi, S.V. Current and future biomarkers for immune checkpoint inhibitors in head and neck squamous cell carcinoma. Curr. Oncol. 2022, 29, 4185–4198. [Google Scholar] [CrossRef] [PubMed]
- Tahara, M.; Okano, S.; Enokida, T.; Ueda, Y.; Fujisawa, T.; Shinozaki, T.; Tomioka, T.; Okano, W.; Biel, M.A.; Ishida, K.; et al. A phase I, single-center, open-label study of RM-1929 photoimmunotherapy in Japanese patients with recurrent head and neck squamous cell carcinoma. Int. J. Clin. Oncol. 2021, 26, 1812–1821. [Google Scholar] [CrossRef] [PubMed]
- Mitsunaga, M.; Ogawa, M.; Kosaka, N.; Rosenblum, L.T.; Choyke, P.L.; Kobayashi, H. Cancer cell–selective in vivo near infrared photoimmunotherapy targeting specific membrane molecules. Nat. Med. 2011, 17, 1685–1691. [Google Scholar] [CrossRef] [PubMed]
- Alsahafi, E.; Begg, K.; Amelio, I.; Raulf, N.; Lucarelli, P.; Sauter, T.; Tavassoli, M. Clinical update on head and neck cancer: Molecular biology and ongoing challenges. Cell Death Dis. 2019, 10, 540. [Google Scholar] [CrossRef] [PubMed]
- Grandis, J.R.; Tweardy, D.J. Elevated levels of transforming growth factor alpha and epidermal growth factor receptor messenger RNA are early markers of carcinogenesis in head and neck cancer. Cancer Res. 1993, 53, 3579–3584. [Google Scholar] [PubMed]
- Cognetti, D.M.; Johnson, J.M.; Curry, J.M.; Kochuparambil, S.T.; McDonald, D.; Mott, F.; Fidler, M.J.; Stenson, K.; Vasan, N.R.; Razaq, M.A.; et al. Phase 1/2a, open-label, multicenter study of RM-1929 photoimmunotherapy in patients with locoregional, recurrent head and neck squamous cell carcinoma. Head Neck 2021, 43, 3875–3887. [Google Scholar] [CrossRef] [PubMed]
- Aslakson, R.A.; Chandrashekaran, S.V.; Rickerson, E.; Fahy, B.N.; Johnston, F.M.; Miller, J.A.; Conca-Cheng, A.; Wang, S.; Morris, A.M.; Lorenz, K.; et al. A Multicenter, Randomized Controlled Trial of Perioperative Palliative Care Surrounding Cancer Surgery for Patients and Their Family Members (PERIOP-PC). J. Palliat. Med. 2019, 22, 44. [Google Scholar] [CrossRef] [PubMed]
- Gerbershagen, H.J.; Aduckathil, S.; van Wijck, A.J.; Peelen, L.M.; Kalkman, C.J.; Meissner, W. Pain intensity on the first day after surgery: A prospective cohort study comparing 179 surgical procedures. Anesthesiology 2013, 118, 934–944. [Google Scholar] [CrossRef] [PubMed]
- Hawker, G.A.; Mian, S.; Kendzerska, T.; French, M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res. 2011, 63 (Suppl. S11), S240–S252. [Google Scholar]
- Nakajima, K.; Takakura, H.; Shimizu, Y.; Ogawa, M. Changes in plasma membrane damage inducing cell death after treatment with near-infrared photoimmunotherapy. Cancer Sci. 2018, 109, 2889–2896. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Ando, K.; Okuyama, S.; Moriguchi, S.; Ogura, T.; Totoki, S.; Hanaoka, H.; Nagaya, T.; Kokawa, R.; Takakura, H.; et al. Photoinduced ligand release from a silicon phthalocyanine dye conjugated with monoclonal antibodies: A mechanism of cancer cell cytotoxicity after near-infrared photoimmunotherapy. ACS Cent. Sci. 2018, 4, 1559–1569. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, I.; Okada, T.; Tokashiki, K.; Tsukahara, K. Quality-of-Life Evaluation of Patients with Unresectable Locally Advanced or Locally Recurrent Head and Neck Carcinoma Treated with Head and Neck Photoimmunotherapy. Cancers 2022, 14, 4413. [Google Scholar] [CrossRef] [PubMed]


| N = 5 | % | ||
|---|---|---|---|
| Sex | Male | 2 | 40 |
| Female | 3 | 60 | |
| ECOG PS | 0 | 4 | 80 |
| 1 | 1 | 20 | |
| Age (years) | Median (range) | 60.5 (51–74) | |
| Tumor | Buccal mucosa cancer | 2 | 40 |
| Oropharyngeal cancer | 2 | 40 | |
| Nasopharyngeal cancer | 1 | 20 | |
| Number of PIT sessions | 1 | 2 | 40 |
| 2 | 2 | 40 | |
| 3 | 1 | 20 | |
| Treatment method | Frontal + cylindrical | 2 | 40 |
| Cylindrical | 1 | 20 | |
| Frontal | 2 | 40 |
| Case | Session | Sex | Tumor Site | Method | NRS | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Frontal | Cylindrical | Pre-Treatment | Immediately after Treatment | One Hour Post-Treatment | Three Hours Post-Treatment | Six Hours Post-Treatment | ||||
| 1 | 1 | Male | Left buccal mucosa | ◯ | ◯ | 3 | 10 | NE | 8 | 8 |
| 2 | NE | 10 | 9 | 8 | 9 | |||||
| 2 | Female | Right buccal mucosa | ◯ | ◯ | 1 | 0 | 0 | 0 | 1 | |
| 3 | 1 | Male | Oropharynx | ◯ | 4 | NE | NE | 8 | 6 | |
| 2 | 7 | NE | 10 | 10 | 6 | |||||
| 4 | 1 | Female | Oropharynx | ◯ | NE | 7 | 5 | NE | 3 | |
| 2 | 0 | NE | 10 | 4 | NE | |||||
| 3 | NE | 10 | 8 | 6 | 2 | |||||
| 5 | Female | Nasopharynx | ◯ | NE | 0 | NE | NE | NE | ||
| Case | Session | Sex | Tumor Site | Method | Maximum Opioid Dose (mg) * | Maximum NRS | Mean NRS ** | |
|---|---|---|---|---|---|---|---|---|
| Via Frontal Diffuser | Via Cylindrical Diffuser | |||||||
| 1 | 1 | Male | Buccal mucosa | ◯ | ◯ | 94 | 10 | 4.0 |
| 2 | 119 | 10 | 4.2 | |||||
| 2 | Female | Buccal mucosa | ◯ | ◯ | 60 | 3 | 2.1 | |
| 3 | 1 | Male | Oropharynx | ◯ | 330 | 8 | 6.0 | |
| 2 | 126 | 10 | 7.5 | |||||
| 4 | 1 | Female | Oropharynx | ◯ | 62.5 | 7 | 2.8 | |
| 2 | 65 | 10 | 3.4 | |||||
| 3 | 52.5 | 10 | 3.2 | |||||
| 5 | Female | Nasopharynx | ◯ | 0 | 3 | 1.0 | ||
| Method | Test Result Median | POD-2 | POD-1 | POD0 | POD1 | POD2 | POD3 | POD4 |
|---|---|---|---|---|---|---|---|---|
| Via frontal diffuser | Body temperature (°C) | 36.6 | 36.7 | 36.6 | 36.6 | 36.4 | 36.4 | 36.5 |
| WBC (102/μL) | 49 | NE | 110 | NE | ||||
| CRP (mg/dL) | 0.03 | 0.08 | ||||||
| Via cylindrical diffuser | Body temperature | 36.7 | 36.8 | 37.1 | 37.1 | 36.9 | 36.7 | 36.6 |
| WBC (102/μL) | 42 | 58 | ||||||
| CRP (mg/dL) | 0.59 | 4.42 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shibutani, Y.; Sato, H.; Suzuki, S.; Shinozaki, T.; Kamata, H.; Sugisaki, K.; Kawanobe, A.; Uozumi, S.; Kawasaki, T.; Hayashi, R. A Case Series on Pain Accompanying Photoimmunotherapy for Head and Neck Cancer. Healthcare 2023, 11, 924. https://doi.org/10.3390/healthcare11060924
Shibutani Y, Sato H, Suzuki S, Shinozaki T, Kamata H, Sugisaki K, Kawanobe A, Uozumi S, Kawasaki T, Hayashi R. A Case Series on Pain Accompanying Photoimmunotherapy for Head and Neck Cancer. Healthcare. 2023; 11(6):924. https://doi.org/10.3390/healthcare11060924
Chicago/Turabian StyleShibutani, Yuma, Haruna Sato, Shinya Suzuki, Takeshi Shinozaki, Hayato Kamata, Kazuki Sugisaki, Atushi Kawanobe, Shinya Uozumi, Toshikatsu Kawasaki, and Ryuichi Hayashi. 2023. "A Case Series on Pain Accompanying Photoimmunotherapy for Head and Neck Cancer" Healthcare 11, no. 6: 924. https://doi.org/10.3390/healthcare11060924
APA StyleShibutani, Y., Sato, H., Suzuki, S., Shinozaki, T., Kamata, H., Sugisaki, K., Kawanobe, A., Uozumi, S., Kawasaki, T., & Hayashi, R. (2023). A Case Series on Pain Accompanying Photoimmunotherapy for Head and Neck Cancer. Healthcare, 11(6), 924. https://doi.org/10.3390/healthcare11060924








