Factors Associated with Chronic Kidney Disease of Unknown Etiology (CKDu): A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion/ Exclusion Criteria
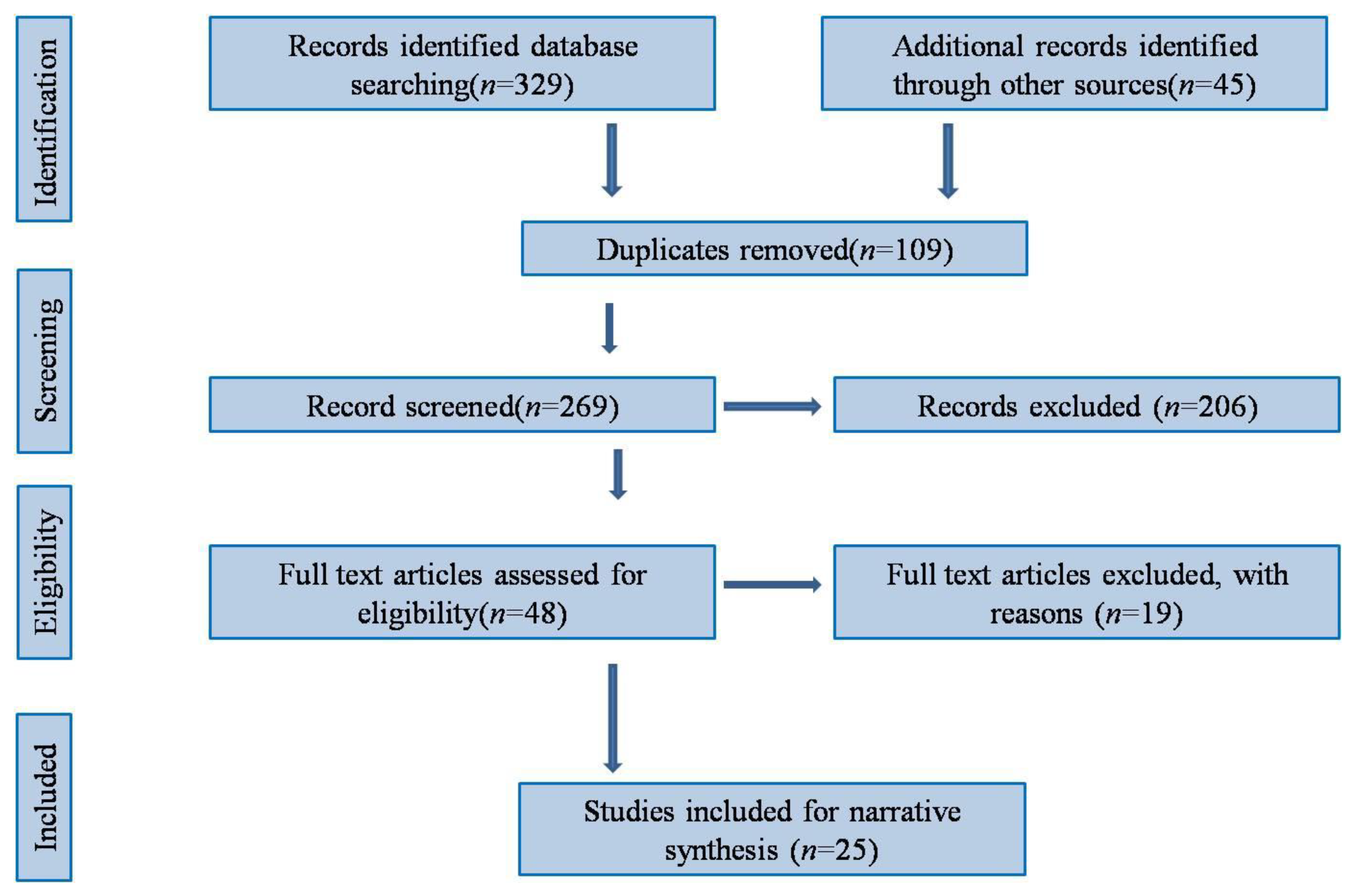
2.3. Study Selection
2.4. Quality Assessment
2.5. Data Extraction and Synthesis
3. Results
3.1. Characteristics of the Studies
3.2. Narrative Synthesis
4. Associated Factors
4.1. Farming
4.2. Water Sources
5. Miscellaneous
6. Discussion
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CKDu | Chronic kidney disease of unknown etiology |
| HTN | Hypertension |
| DM | Diabetes mellitus |
| B2M | Beta-2 microglobulin |
| A1M | Alpha1-microglobulin, and NAG:N-acetyl-B-D-glucosaminidase |
References
- Kassa, M.; Grace, J. The Global Burden and Perspectives on Noncommunicable Diseases (NCDs) and the Prevention, Data Availability and Systems Approach of NCDs in Low-resource Countries. In Public Health in Developing Countries—Challenges and Opportunities; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Coates, M.M.; Kintu, A.; Gupta, N.; Wroe, E.B.; Adler, A.J.; Kwan, G.F.; Park, P.H.; Rajbhandari, R.; Byrne, A.L.; Casey, D.C.; et al. Burden of non-communicable diseases from infectious causes in 2017: A modelling study. Lancet Glob. Health 2020, 8, e1489–e14982020. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, J. Modelling and prediction of global non-communicable diseases. BMC Public Health 2020, 20, 822. [Google Scholar] [CrossRef]
- Couser, W.G.; Remuzzi, G.; Mendis, S.; Tonelli, M. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int. 2011, 80, 1258–1270. [Google Scholar] [CrossRef]
- Carney, E.F. The impact of chronic kidney disease on global health. Nat. Rev. Nephrol. 2020, 16, 251. [Google Scholar] [CrossRef]
- Gunawardena, S.; Dayaratne, M.; Wijesinghe, H.; Wijewickrama, E. A Systematic Review of Renal Pathology in Chronic Kidney Disease of Uncertain Etiology. Kidney Int. Rep. 2021, 6, 1711–1728. [Google Scholar] [CrossRef] [PubMed]
- Torres, C.; Aragón, A.; González, M.; López, I.; Jakobsson, K.; Elinder, C.G.; Lundberg, I.; Wesseling, C. Decreased kidney function of unknown cause in Nicaragua: A community-based survey. Am. J. Kidney Dis. 2010, 55, 485–496. [Google Scholar] [CrossRef]
- McKinley, J.M.; Mueller, U.; Atkinson, P.M.; Ofterdinger, U.; Cox, S.F.; Doherty, R.; Fogarty, D.; Egozcue, J.J.; Pawlowsky-Glahn, V. Chronic kidney disease of unknown origin is associated with environmental urbanisation in Belfast, UK. Environ. Geochem. Health 2021, 43, 2597–2614. [Google Scholar] [CrossRef]
- Lunyera, J.; Mohottige, D.; Von Isenburg, M.; Jeuland, M.; Patel, U.D.; Stanifer, J.W. CKD of Uncertain Etiology: A Systematic Review. Clin. J. Am. Soc. Nephrol. 2016, 11, 379–385. [Google Scholar] [CrossRef]
- Gifford, F.J.; Gifford, R.M.; Eddleston, M.; Dhaun, N. Endemic Nephropathy Around the World. Kidney Int. Rep. 2017, 2, 282–292. [Google Scholar] [CrossRef]
- Du, H.; Le, G.; Hou, L.; Mao, X.; Liu, S.; Huang, K. Nontoxic Concentration of Ochratoxin A Aggravates Renal Fibrosis Induced by Adriamycin/Cyclosporine A Nephropathy via TGF-β1/SMAD2/3. J. Agric. Food Chem. 2022, 70, 14005–14014. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, D.; Liu, Z. Fine Particulate Matter (PM2.5) and Chronic Kidney Disease. Rev. Environ. Contam. Toxicol. 2021, 254, 183–215. [Google Scholar] [CrossRef]
- Li, G.; Huang, J.; Wang, J.; Zhao, M.; Liu, Y.; Guo, X.; Wu, S.; Zhang, L. Long-Term Exposure to Ambient PM2.5 and Increased Risk of CKD Prevalence in China. J. Am. Soc. Nephrol. 2021, 32, 448–458. [Google Scholar] [CrossRef]
- Weaver, V.M.; Fadrowski, J.J.; Jaar, B.G. Global dimensions of chronic kidney disease of unknown etiology (CKDu): A modern era environmental and/or occupational nephropathy? BMC Nephrol. 2015, 16, 145. [Google Scholar] [CrossRef] [PubMed]
- Lebov, J.F.; Valladares, E.; Peña, R.; Peña, E.M.; Sanoff, S.L.; Cisneros, E.C.; Colindres, R.E.; Morgan, D.R.; Hogan, S.L. A population-based study of prevalence and risk factors of chronic kidney disease in León, Nicaragua. Can. J. Kidney Health Dis. 2015, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Jayatilake, N.; Mendis, S.; Maheepala, P.; Mehta, F.R. CKDu National Research Project Team. Chronic kidney disease of uncertain aetiology: Prevalence and causative factors in a developing country. BMC Nephrol. 2013, 14, 180. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e10000972009. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Aromataris, E.; Munn, Z. (Eds.) JBI Manual for Evidence Synthesis; JBI: Adelaide, Australia, 2020; Available online: https://synthesismanual.jbi.global (accessed on 14 December 2022).
- Jayasumana, C.; Paranagama, P.; Agampodi, S.; Wijewardane, C.; Gunatilake, S.; Siribaddana, S. Drinking well water and occupational exposure to Herbicides is associated with chronic kidney disease, in Padavi-Sripura, Sri Lanka. Environ. Health 2015, 14, 6. [Google Scholar] [CrossRef]
- VanDervort, D.R.; López, D.L.; Orantes, C.M.; Rodríguez, D.S. Spatial distribution of unspecified chronic kidney disease in El Salvador by crop area cultivated and ambient temperature. MEDICC Rev. 2014, 16, 31–38. [Google Scholar] [CrossRef]
- Hamilton, S.A.; Nakanga, W.P.; Prynn, J.E.; Crampin, A.C.; Fecht, D.; Vineis, P.; Caplin, B.; Pearce, N.; Nyirenda, M.J. Prevalence and risk factors for chronic kidney disease of unknown cause in Malawi: A cross-sectional analysis in a rural and urban population. BMC Nephrol. 2020, 21, 387. [Google Scholar] [CrossRef]
- Gonzalez-Quiroz, M.; Smpokou, E.T.; Silverwood, R.J.; Camacho, A.; Faber, D.; Garcia, B.R.; Oomatia, A.; Hill, M.; Glaser, J.; Le Blond, J.; et al. Decline in Kidney Function among Apparently Healthy Young Adults at Risk of Mesoamerican Nephropathy. J. Am. Soc. Nephrol. 2018, 29, 2200–2212. [Google Scholar] [CrossRef]
- Siriwardhana, E.A.; Perera, P.A.; Sivakanesan, R.; Abeysekara, T.; Nugegoda, D.B.; Jayaweera, J.A. Dehydration and malaria augment the risk of developing chronic kidney disease in Sri Lanka. Indian J. Nephrol. 2015, 25, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Nanayakkara, S.; Senevirathna, S.T.; Abeysekera, T.; Chandrajith, R.; Ratnatunga, N.; Gunarathne, E.D.; Yan, J.; Hitomi, T.; Muso, E.; Komiya, T.; et al. An integrative study of the genetic, social and environmental determinants of chronic kidney disease characterized by tubulointerstitial damages in the North Central Region of Sri Lanka. J. Occup. Health 2014, 56, 28–38. [Google Scholar] [CrossRef]
- López-Marín, L.; Chávez, Y.; García, X.A.; Flores, W.M.; García, Y.M.; Herrera, R.; Almaguer, M.; Orantes, C.M.; Calero, D.; Bayarre, H.D.; et al. Histopathology of chronic kidney disease of unknown etiology in Salvadoran agricultural communities. MEDICC Rev. 2014, 16, 49–54. [Google Scholar] [CrossRef]
- Rango, T.; Jeuland, M.; Manthrithilake, H.; McCornick, P. Nephrotoxic contaminants in drinking water and urine, and chronic kidney disease in rural Sri Lanka. Sci. Total Environ. 2015, 518–519, 574–585. [Google Scholar] [CrossRef]
- Gobalarajah, K.; Subramaniam, P.; Jayawardena, U.A.; Rasiah, G.; Rajendra, S.; Prabagar, J. Impact of water quality on Chronic Kidney Disease of unknown etiology (CKDu) in Thunukkai Division in Mullaitivu District, Sri Lanka. BMC Nephrol. 2020, 21, 507. [Google Scholar] [CrossRef]
- Mascarenhas, S.; Mutnuri, S.; Ganguly, A. Deleterious role of trace elements—Silica and lead in the development of chronic kidney disease. Chemosphere 2017, 177, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Jayasekara, J.M.; Dissanayake, D.M.; Adhikari, S.B.; Bandara, P. Geographical distribution of chronic kidney disease of unknown origin in North Central Region of Sri Lanka. Ceylon Med. J. 2013, 58, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Siriwardhana, E.A.; Perera, P.A.; Sivakanesan, R.; Abeysekara, T.; Nugegoda, D.B.; Weerakoon, K.G. Is the staple diet eaten in Medawachchiya, Sri Lanka, a predisposing factor in the development of chronic kidney disease of unknown etiology?—A comparison based on urinary β2-microglobulin measurements. BMC Nephrol. 2014, 15, 103. [Google Scholar] [CrossRef]
- Xing, X.; Lu, J.; Wang, Z. Associated risk factors for chronic kidney disease of unknown etiologies in 241 patients. Int. J. Artif. Organs. 2015, 38, 184–191. [Google Scholar] [CrossRef]
- Robles-Osorio, M.L.; Pérez-Maldonado, I.N.; Martín del Campo, D.; Montero-Perea, D.; Avilés-Romo, I.; Sabath-Silva, E.; Sabath, E. Urinary arsenic levels and risk of renal injury in a cross-sectional study in open population. Rev. Invest. Clin. 2012, 64, 609–614. [Google Scholar] [PubMed]
- Athuraliya, N.T.; Abeysekera, T.D.; Amerasinghe, P.H.; Kumarasiri, R.; Bandara, P.; Karunaratne, U.; Milton, A.H.; Jones, A.L. Uncertain etiologies of proteinuric-chronic kidney disease in rural Sri Lanka. Kidney Int. 2011, 80, 1212–1221. [Google Scholar] [CrossRef] [PubMed]
- Jayasumana, C.; Paranagama, P.A.; Amarasinghe, M.D.; Wijewardane, K.M.; Dahanayake, K.S.; Fonseka, S.I. Possible link of chronic arsenic toxicity with chronic kidney disease of unknown etiology in Sri Lanka. J. Nat. Sci. Res. 2013, 3, 64–73. [Google Scholar]
- Chandrajith, R.; Nanayakkara, S.; Itai, K.; Aturaliya, T.N.; Dissanayake, C.B.; Abeysekera, T.; Harada, K.; Watanabe, T.; Koizumi, A. Chronic kidney diseases of uncertain etiology (CKDue) in Sri Lanka: Geographic distribution and environmental implications. Environ. Geochem. Health 2011, 33, 267–278. [Google Scholar] [CrossRef]
- Selvarajah, M.; Weeratunga, P.; Sivayoganthan, S.; Rathnatunga, N.; Rajapakse, S. Clinicopathological correlates of chronic kidney disease of unknown etiology in Sri Lanka. Indian J. Nephrol. 2016, 26, 357–363. [Google Scholar] [CrossRef]
- de Silva, M.W.; Albert, S.M.; Jayasekara, J.M. Structural violence and chronic kidney disease of unknown etiology in Sri Lanka. Soc. Sci. Med. 2017, 178, 184–195. [Google Scholar] [CrossRef]
- Wijetunge, S.; Ratnatunga, N.V.; Abeysekera, D.T.; Wazil, A.W.; Selvarajah, M.; Ratnatunga, C.N. Retrospective analysis of renal histology in asymptomatic patients with probable chronic kidney disease of unknown aetiology in Sri Lanka. Ceylon Med. J. 2013, 58, 142–147. [Google Scholar] [CrossRef]
- Siddarth, M.; Datta, S.K.; Ahmed, R.S.; Banerjee, B.D.; Kalra, O.P.; Tripathi, A.K. Association of CYP1A1 gene polymorphism with chronic kidney disease: A case control study. Environ. Toxicol. Pharmacol. 2013, 36, 164–170. [Google Scholar] [CrossRef]
- Gutiérrez-Amavizca, B.E.; Orozco-Castellanos, R.; Ortíz-Orozco, R.; Padilla-Gutiérrez, J.; Valle, Y.; Gutiérrez-Gutiérrez, N.; García-García, G.; Gallegos-Arreola, M.; Figuera, L.E. Contribution of GSTM1, GSTT1, and MTHFR polymorphisms to end-stage renal disease of unknown etiology in Mexicans. Indian J. Nephrol. 2013, 23, 438–443. [Google Scholar] [CrossRef]
- Sayanthooran, S.; Magana-Arachchi, D.N.; Gunerathne, L.; Abeysekera, T. Potential diagnostic biomarkers for chronic kidney disease of unknown etiology (CKDu) in Sri Lanka: A pilot study. BMC Nephrol. 2017, 18, 31. [Google Scholar] [CrossRef] [PubMed]
- Nanayakkara, S.; Senevirathna, S.T.; Karunaratne, U.; Chandrajith, R.; Harada, K.H.; Hitomi, T.; Watanabe, T.; Abeysekera, T.; Aturaliya, T.N.; Koizumi, A. Evidence of tubular damage in the very early stage of chronic kidney disease of uncertain etiology in the North Central Province of Sri Lanka: A cross-sectional study. Environ. Health Prev. Med. 2012, 17, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.L. Indian Agriculture and Agricultural Bill. J. Interdiscipl. Cycle Res. 2020, XII. [Google Scholar]
- Sarkar, S.; Sinha, R.; Chaudhury, A.R.; Maduwage, K.; Abeyagunawardena, A.; Bose, N.; Pradhan, S.; Bresolin, N.L.; Garcia, B.A.; McCulloch, M. Snake bite associated with acute kidney injury. Pediatr. Nephrol. 2021, 36, 3829–3840. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, M.; Ghoochani, M.; Hossein Mahvi, A. Health risk assessment to fluoride in drinking water of rural residents living in the Poldasht city, Northwest of Iran. Ecotoxicol. Environ. Saf. 2018, 148, 426–430. [Google Scholar] [CrossRef]
- Kaur, G.; Kumar, R.; Mittal, S.; Sahoo, P.K.; Vaid, U. Ground/drinking water contaminants and cancer incidence: A case study of rural areas of South West Punjab, India. Hum Ecol. Risk Assess. 2021, 27, 205–226. [Google Scholar] [CrossRef]
- Roncal-Jimenez, C.; García-Trabanino, R.; Barregard, L.; Lanaspa, M.A.; Wesseling, C.; Harra, T.; Aragón, A.; Grases, F.; Jarquin, E.R.; González, M.A.; et al. Heat stress nephropathy from exercise-induced uric acid crystalluria: A perspective on Mesoamerican nephropathy. Am. J. Kidney Dis. 2016, 67, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Correa-Rotter, R.; García-Trabanino, R. Mesoamerican Nephropathy. Semin. Nephrol. 2019, 39, 263–271. [Google Scholar] [CrossRef]
- Polhuis, K.C.M.M.; Wijnen, A.H.C.; Sierksma, A.; Calame, W.; Tieland, M. The Diuretic Action of Weak and Strong Alcoholic Beverages in Elderly Men: A Randomized Diet-Controlled Crossover Trial. Nutrients 2017, 9, 660. [Google Scholar] [CrossRef]
- Piasentin, A.; Claps, F.; Silvestri, T.; Rebez, G.; Traunero, F.; Mir, M.C.; Rizzo, M.; Celia, A.; Cicero, C.; Urbani, M.; et al. Assessing Trifecta Achievement after Percutaneous Cryoablation of Small Renal Masses: Results from a Multi-Institutional Collaboration. Medicina 2022, 3, 1041. [Google Scholar] [CrossRef]
- Lucignani, G.; Rizzo, M.; Ierardi, A.M.; Piasentin, A.; De Lorenzis, E.; Trombetta, C.; Liguori, G.; Bertolotto, M.; Carrafiello, G.; Montanari, E.; et al. Percutaneous Microwave Ablation is Comparable to Cryoablation for the Treatment of T1a Renal Masses: Results From a Cross-Sectional Study. Clin. Genitourin. Cancer. 2022, 20, e506–e511. [Google Scholar] [CrossRef]
- Ljungberg, B.; Bensalah, K.; Canfield, S.; Dabestani, S.; Hofmann, F.; Hora, M.; Kuczyk, M.A.; Lam, T.; Marconi, L.; Merseburger, A.S.; et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur. Urol. 2015, 67, 913–924. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.; Uzzo, R.G.; Allaf, M.E.; Bass, E.B.; Cadeddu, J.A.; Chang, A.; Clark, P.E.; Davis, B.J.; Derweesh, I.H.; Giambarresi, L.; et al. Renal Mass and Localized Renal Cancer: AUA Guideline. J. Urol. 2017, 198, 520–529. [Google Scholar] [CrossRef] [PubMed]

| Authors | Country | Year | Study setting | LMICs/HMICs | Design | Sample Size | Male | Female | Age |
|---|---|---|---|---|---|---|---|---|---|
| ChannaJayasumana et al. [20] | Sri Lanka | 2015 | Hospital | LMICs | Case-control | 305 | 98 | 82 | Mean (SD) = 45.51 (19.78) years |
| VanDervort DR. [21] | El-Salvador | 2014 | Community | LMICs | Cross-sectional | 24,726 | NA | NA | NA |
| SA. Hamilton et al. [22] | Malawi | 2020 | Community | LMICs | Cross-sectional | 821 | 317 | 504 | Mean (SD) = 33.5 (12.7) years |
| M Gonzalez et al. [23] | Nicaragua | 2018 | Community | LMICs | Cohort | 350 | 263 | 87 | Age range = 18–30 years, Mean (SD) = 23.9 (3.7) years |
| E Siriwardhana et al. [24] | Sri Lanka | 2015 | Community | LMICs | Case-control | 200 | 59 | 41 | Mean (SD) = 47.8 (9.6) years |
| N Jayatilake et al. [16] | Sri Lanka | 2013 | Community | LMICs | cross-sectional | 627 | Not mentioned | Not mentioned | Mean (SD) = 39.1 (14.2) years |
| S Nanayakkara [25] | Sri Lanka | 2014 | Community | LMICs | Case-control | 311 | 311 (total male) | 0 | Mean (SD) =46.6 (9.0) years |
| L Lopez et al. [26] | El-Salvador | 2014 | Hospital | LMICs | Cross-sectional | 46 | 36 | 10 | Mean =45 years |
| T Rango et al. [27] | Sri Lanka | 2015 | Community | LMICs | Case-control | 134 | 82 | 52 | Mean (SD)= 37.5 (16.6) years |
| K Gobalarajah et al. [28] | Sri Lanka | 2020 | Community | LMICs | Cross-sectional | 35 | 28 | 7 | Age range = 30–80 years |
| S Mascarenhas et al. [29] | India | 2017 | Community | LMICs | Cohort | 266 | 58 | 56 | Not mentioned |
| JM jayaseka ra et al. [30] | Sri Lanka | 2013 | Community | LMICs | Cross-sectional | 863 | 609 | 254 | Mean (SD)= 54.7 (8) years |
| E Siriwardhana et al. [31] | Sri Lanka | 2014 | Hospital | LMICs | case-control | 200 | 59 | 41 | Mean (SD)= 46.3 ( 5.9) years |
| MA Jayasumana [32] | Sri Lanka | 2013 | Community | LMICs | Case-control | 305 | Not mentioned | Not mentioned | Not mentioned |
| R Osorio et al. [33] | Mexico | 2012 | Community-based | LMICs | cross-sectional | 90 | 20 | 70 | Mean (SD) = 40.9 (12.9) years |
| X Xing et al. [34] | China | 2015 | Hospital | LMICs | Cross-sectional | 700 | 150 | 91 | Mean (SD) = 45.02 (15.97) years |
| R Chandrajith et al. [35] | Sri Lanka | 2010 | Community | LMICs | case-control | 135 | NA | NA | NA |
| M Selvarajah et al. [36] | Sri Lanka | 2016 | Hospital | LMICs | Cross-sectional | 125 | 92 | 33 | Mean (SD) = 46.2 (11.64)years |
| Nanayakkara et al. [37] | Sri Lanka | 2012 | Community | LMICs | Case control | 237 | 73 | 33 | Mean (SD) = [stage1 = 29 (12), stage2 = 45 (7), stage3 = 44 (13), stage4 = 50 (7), stage5 =4 9 (14)] |
| N Athuraliya et al [38] | Sri Lanka | 2011 | Community | LMICs | Cross-sectional | 6153 | 66 | 43 | Mean (SD) = 45.05 (14.79) years |
| de Silva MW et al. [39] | Sri Lanka | 2017 | Community | LMICs | Case-control | 548 | 184 | 90 | Mean (SD) = 56.1 (10.9) years |
| S Wijetunge et al. [40] | Sri Lanka | 2013 | Community | LMICs | Retrospective cohort | 211 | 153 | 58 | Mean (SD) = 36.8 (14) years |
| Siddarth et al. [41] | India | 2013 | Hospital | LMICs | case-control | 668 | 170 | 164 | Mean (SD) = 46.0 (7.3) years |
| B Guttierrez et al. [42] | Mexico | 2013 | Hospital | LMICs | Case-control | 235 | 178 | 57 | Mean (SD) = 29.24 (15.48) years |
| S Sayanthooran et al. [43] | Sri Lanka | 2017 | Community | LMICs | Case-control | 60 | 51 | 9 | Mean age = 51 (12) years |
| Authors | Excluded Comorbidity to Define CKDu | Comorbidity Status |
|---|---|---|
| ChannaJayasumana et al. [20] | DM or chronic and/or severe HTN, history of snakebite, urological disease of known etiology, glomerulonephritis | Not mentioned |
| VanDervort DR. [21] | Not mentioned | Not mentioned |
| SA. Hamilton et al. [22] | DM, HTN, and Heavy Proteinuria | Obese = 54, HIV positive = 3, Overweight = 177 |
| M Gonzalez et al. [23] | Self-reported CKD, DM, or HTN | Not mentioned |
| E Siriwardhana et al. [24] | DM, long-standing HTN, glomerular nephritis, urolithiasis, congenital kidney diseases, history of snake bite and leptospirosis | Not mentioned |
| N Jayatilake et al. [16] | Glomerulonephritis, pyelonephritis, renal calculi or snake bite, hypertension | Not mentioned |
| S Nanayakkara [25] | History of DM and HbA1c >6.5% or HTNor other known renal diseases such as autoimmune diseases, glomerular nephritis, Fanconi syndrome or IgA nephropathy | NCDs = 43% |
| L Lopez et al. [26] | HTN, DM, glomerulopathies, polycystic kidney disease and obstructive kidney disease, HIV positivity | Glomerulomegally(73.3%), Tubular atrophy(89.1%), Mono nuclear inflammatory infiltration, Intimal proliferation, thickening of the tunica media in blood vessels |
| T Rango et al. [27] | Not mentioned | Normal weight =72 , Underweight = 34). Overweight = 27, and Obese= 1. |
| K Gobalarajah et al. [28] | The patients’ disease history revealed that they were secondarily diagnosed with DM & HTN only after they developed CKDu. | DM & HTN, but these are secondarily developed. |
| S Mascarenhas et al. [29] | DM & HTN | |
| JM jayaseka ra et al. [30] | DM, HTN, UTI or other renal diseases in the history | Not mentioned |
| E Siriwardhana et al. [31] | DM, long-standing HTN, glomerular nephritis, urolithiasis, and congenital kidney diseases and having a history of snake bite and leptospirosis | Not mentioned |
| MA Jayasumana [32] | Not mentioned | Not mentioned |
| R Osorio et al. [33] | Antecedents of renal disease, DM, HTN, UTI | Not mentioned |
| X Xing et al. [34] | Secondary renal damage, chronic glomerulonephritis, nephritic syndrome, polycystic kidney disease | Hepatitis, tuberculosis, acute and chronic glomerulonephritis, respiratory infections, Urinary calculus and urinary tract infection (UTI), hydronephrosis(50 individuals) |
| R Chandrajith et al. [35] | Not mentioned | Anaemia is mild in the early stage of CKD, HTN in the late stage, edema is a late feature.Tubular atrophy and glomerular sclerosis |
| M Selvarajah et al. [36] | DM, chronic or severe HTN, snake bite, glomerulonephritis or urological diseases, active renal or peri-renal infection, structural and anatomical renal abnormalities of the kidney, cysts and renal masses and those with a solitary kidney, coagulopathy, and uncontrolled HTN. | Not mentioned |
| Nanayakkara et al. [37] | Not clearly stated | Not mentioned |
| N Athuraliya et al. [38] | HTN, DM | Co-morbidity status among CKDu: Not Mentioned |
| de Silva MW et al. [39] | DM, HTN, UTI or other diseases likely toaffect renal function | Interstitial nephritis in all CKDu cases |
| S Wijetunge et al. [40] | DM and long-standingessential HTN | Glomerular sclerosis (GS), Interstitial fibrosis (IF), Interstitial inflammation (II), Tubular atrophy (TA) and Hypertension associated changes in blood vessels |
| Siddarth et al. [41] | DM and any other known causes of CKD such as chronic glomerulonephritis, hypertensive nephrosclerosis, autosomal dominant polycystic kidney disease, chronic tubulointerstitial nephropathy or evidence of systemic/local (UTI) infection | Not mentioned |
| B Guttierrez et al. [42] | T2DM, essential HTN, glomerulonephritis, infections, drugs | Not mentioned |
| S Sayanthooran et al. [43] | DM, chronic or severe HTN snake bite, glomerulonephritis or urological diseases | Asthma = 3 |
| Authors Name | Diagnosis | Cutoff eGFR during the Inclusion of the Study Participants | Dialysis Status | How Is the Diagnosis Confirmed |
|---|---|---|---|---|
| ChannaJayasumana et al. [20] | CKDu | Less than 90 | Not mentioned | Prediagnosed CKD |
| VanDervort DR. [21] | Not mentioned | Not mentioned | ICD-10 definitions were used to classify CKD and ESRD | |
| SA.Hamilton et al. [22] | CKDu | <90 mL/min/1.73 m2 | Not mentioned | Heavily proteinuric if the albumin: creatinine ratio (ACR) was ≥30 mg/mmol |
| M Gonzalez et al. [23] | MeN | >90 mL/min/1.73 m2 | No | Serum creatinine, cysteine C |
| E.Siriwardhana et al. [24] | CKDu | <60 mL/min | Not mentioned | Proteinuria, elevated levels of serum creatinine and abdominal ultrasound scan reports |
| N Jayatilake et al. [16] | CKDu | ≤90 mL/min/1.73 m2 | Not mentioned | Renal biopsy. |
| S.Nanayakkara [25] | CKDu | Not mentioned | Not mentioned | Not mentioned |
| L Lopez et al. [26] | CKDu | 89 mL/min/ 1.73 m2 to 30 mL/min/ 1.73 m2 | Not mentioned | eGFR calculation. |
| T Rango et al. [27] | CKDu | Not mentioned | Not mentioned | Data brought from health centers |
| K Gobalarajah et al. [28] | CKDu | Not mentioned | Not mentioned | Not mentioned |
| S Mascarenhas et al. [29] | CKDu | Not mentioned | Not mentioned | Serum creatinine, eGFR calculation, Sr urea, Plasma albumin, Sr uric acid, FBS |
| JM jayaseka ra et al. [30] | CKDu | Not mentioned | Not mentioned | Presence of urinary protein one plus or more in sulphosalicylic acid test on two occasions, and the presence of radiological/pathological evidence of chronic kidney disease. |
| E Siriwardhana et al. [31] | CKDu | Not mentioned | Not mentioned | Presence of proteinuria, elevated levels of serum Creatinine (>1.2 mg/dL) and confirmed abdominal ultrasound scan /renal biopsy reports |
| MA Jayasumana [32] | CKDu | Not mentioned | Not mentioned | Not mentioned |
| R Osorio et al. [33] | CKDu | <60 mL/min | Not mentioned | Not mentioned |
| X Xing et al. [34] | CKDu | s ≥ 90 mL/min/1.73 m2 | 73% | Kidney damage is more than or equal to 3 years. |
| R Chandrajith et al. [35] | CKDu | Not mentioned | Not mentioned | Not mentioned |
| M Selvarajah et al. [36] | CKDu | <90 mL/min/1.73 m2 | Not mentioned | Renal biopsy and pre-diagnosed, and the presence of proteinuria |
| Nanayakkara et al. [37] | CKDu | ≤90 mL/min/1.73 m2 | Not mentioned | House-to-house screening using dipstick proteinuria on threeoccasions |
| N Athuraliya et al. [38] | CKDu | Not mentioned | Not mentioned | Proteinurea |
| de Silva MW et al [39] | CKDu with interstitial nephritis | Not mentioned | Not mentioned | Who showed proteinuria on two occasions along with other features of kidney disease (decreased eGFR, radiological changes in kidney size, increased cortical echogenicity, and loss of corticomedullary demarcation) |
| S Wijetunge et al. [40] | CKDu | Not mentioned | Not included | Dipstick albuminuria positive(compulsory), renal biopsy(in patients of whom positive albuminuria +1 or above on at least two consecutive dipstick tests and proteinuria more than 500 mg/24 h, or proteinuria less than 500 mg/24 h with haematuria, or proteinuria less than 500 mg/24 h with renal insufficiency) |
| Siddarth et al. [41] | CKDu | <90 mL/min/1.73 m2 | Not mentioned | Not mentioned |
| B Guttierrez et al. [42] | ESRD of unknown causes | Less than 15 mL/min | Peritoneal dialysis, automated peritoneal dialysis, hemodialysis = 32% | eGFR calculation |
| S Sayanthooran et al. [43] | CKDu | ≤90 mL/min/1.73 m2 | Not mentioned | EGFR calculation |
| Authors | Analysis for Association | Associated Factors |
|---|---|---|
| ChannaJayasumana et al. [20] | Unadjusted odds ratio, Mann–Whitney test | Farming, use of herbicide during farming, Well |
| VanDervort DR. [21] | Geographically weighted regression | People residing near sugarcane agriculture field areas based on the global information system (GIS) technique |
| SA. Hamilton et al. [22] | Linear regression, logistic regression | Regular meat eater, Age per 10-year increase, Gender |
| M Gonzalez et al. [23] | Compared with rapid decline, probability-weighted logistic regression | Current or former employees of banana plantations, sugarcane farming, cane cutters, seeders, duration of farming, Use of Herbicide during farming, substance abuse, availability of shade during working hours, people who frequently work in a hot environment, Consuming pipe water supply |
| E Siriwardhana et al. [24] | Linear logistic model analysis | Farming, paddy farming, Herbicide, substance abuse, Working under the sun for more than 6 h per day and consuming less than 3 litres of water per day, consumption of NSAID drugs, history of malaria |
| N Jayatilake et al. [16] | Logistic regression | Farming, Substance abuse, Smoking, Age more than 39 years, Gender |
| S Nanayakkara [25] | Univariate and multiple logistic analyses, Student-Newman-Keuls (SNK) multiple range test, student t-test | History of Malaria |
| L Lopez et al. [26] | F test | Sugarcane workers, tobacco use |
| T Rango et al. [27] | Logistic regression | Presence of trace elements such as cadmium (Cd), arsenic (As), lead (Pb), and uranium (U) in the available water sources |
| K Gobalarajah et al. [28] | Regression analysis between creatinine of CKDu and explanatory variables | Dissolved solids and Arsenic, Phosphate content |
| S Mascarenhas et al. [29] | Descriptive statistic was performed (Differences at p < 0.05 were considered to be significant.) | Blood lead level in affected individuals, pH of groundwater of endemic areas and its seasonal variation |
| JM jayaseka ra et al. [30] | GIS distribution mapping | Gender, age group over 40 years, farmers. The source of drinking water (shallow wells, tube wells and water reservoirs), patients who consumed boiled water, Clustering of the disease |
| E Siriwardhana et al. [31] | Fisher’s exact test, chi-square test v | Urine B2M level, Food habits, Consumption of foods which are locally produced, Surface water used for consumption by the local community |
| MA Jayasumana [32] | Logistic regression and proportion | Arsenic level, Chronic Arsenic Toxicity |
| R Osorio et al. [33] | Linear correlation analysis with Pearson and coefficient of determination | Age more than 50 years. |
| X Xing et al. [34] | Multiple logistic regression. | Age more than 60 years, Nephrotoxic drugs, Alcohol consumption |
| R Chandrajith et al. [35] | T-test | Heavy metals in water bodies (Cadmium, fluoride level) |
| M Selvarajah et al. [36] | Chi-square test and descriptive statistics | Age, Gender, Family history of CKD |
| Nanayakkara et al. [37] | Spearman’s rank correlation, Welch’s t-test | N-acetyl-B-D-glucosaminidase (NAG) and alpha1-microglobulin (A1M) excretion |
| N Athuraliya et al. [38] | Logistic regression | a1-microglobulin (A1M) excretion |
| de Silva MW et al. [39] | Correlation | Gender (wage labourers) |
| S Wijetunge et al. [40] | Corellation test | Consuming water from abandoned water sources and well water, the presence of heavy metals in abandoned wells [such as Calcium (Ca), Magnesium (Mg), Barium (Ba), Strontium (Sr), Iron (Fe), Titanium (Ti), and Vanadium (V)], family history of CKD |
| Siddarth et al. [41] | Chi square test, multinomial logistic regression | Genetic factor |
| B Guttierrez et al. [42] | χ2 test or Fisher’s exact test, binary logistic regression | Genetic factors |
| S Sayanthooran et al. [43] | Logistic regression | Genetic factors |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nayak, S.; Rehman, T.; Patel, K.; Dash, P.; Alice, A.; Kanungo, S.; Palo, S.K.; Pati, S. Factors Associated with Chronic Kidney Disease of Unknown Etiology (CKDu): A Systematic Review. Healthcare 2023, 11, 551. https://doi.org/10.3390/healthcare11040551
Nayak S, Rehman T, Patel K, Dash P, Alice A, Kanungo S, Palo SK, Pati S. Factors Associated with Chronic Kidney Disease of Unknown Etiology (CKDu): A Systematic Review. Healthcare. 2023; 11(4):551. https://doi.org/10.3390/healthcare11040551
Chicago/Turabian StyleNayak, Swetalina, Tanveer Rehman, Kripalini Patel, Pujarini Dash, Alice Alice, Srikanta Kanungo, Subrata Kumar Palo, and Sanghamitra Pati. 2023. "Factors Associated with Chronic Kidney Disease of Unknown Etiology (CKDu): A Systematic Review" Healthcare 11, no. 4: 551. https://doi.org/10.3390/healthcare11040551
APA StyleNayak, S., Rehman, T., Patel, K., Dash, P., Alice, A., Kanungo, S., Palo, S. K., & Pati, S. (2023). Factors Associated with Chronic Kidney Disease of Unknown Etiology (CKDu): A Systematic Review. Healthcare, 11(4), 551. https://doi.org/10.3390/healthcare11040551




