Relationship between Locomotive Syndrome and Musculoskeletal Pain and Generalized Joint Laxity in Young Chinese Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measurement and Measuring Equipment
Physical Measurement
2.3. Locomotive Syndrome (LS) Risk Tests
2.4. Musculoskeletal Pain
2.5. General Joint Laxity Test
2.6. Statistical Analysis
3. Results
4. Discussion
4.1. Prevalence of LS in Young Adults
4.2. Musculoskeletal Pain and LS
4.3. GJL and LS
4.4. Strength and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bao, J.; Zhou, L.; Liu, G.; Tang, J.; Lu, X.; Cheng, C.; Jin, Y.; Bai, J. Current state of care for the elderly in China in the context of an aging population. Biosci. Trends 2022, 16, 107–118. [Google Scholar] [CrossRef]
- Ramos, G.F.; Ribeiro, V.P.; Mercadante, M.P.; Ribeiro, M.P.; Delgado, A.F.; Farhat, S.C.L.; Leal, M.M.; Marques, H.H.; Odone-Filho, V.; Tannuri, U.; et al. Mortality in adolescents and young adults with chronic diseases during 16 years: A study in a Latin American tertiary hospital. J. Pediatr. 2019, 95, 667–673. [Google Scholar] [CrossRef]
- Nakamura, K. A “super-aged” society and the “locomotive syndrome”. J. Orthop. Sci. 2008, 13, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Ogata, T. Locomotive Syndrome: Definition and Management. Clin. Rev. Bone Miner Metab. 2016, 14, 56–67. [Google Scholar] [CrossRef]
- Yoshimura, N.M.S.; Oka, H.; Tanaka, S.; Ogata, T.; Kawaguchi, H.; Akune, T.; Nakamura, K. Association between new indices in the locomotive syndrome risk test and decline in mobility: Third survey of the ROAD study. J. Orthop. Sci. 2015, 20, 896–905. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Ishijima, M.; Ishibashi, M.; Liu, L.; Arikawa-Hirasawa, E.; Machida, S.; Naito, H.; Hamada, C.; Kominami, E. A nationwide observational study of locomotive syndrome in Japan using the ResearchKit: The Locomonitor study. J. Orthop. Sci. 2019, 24, 1094–1104. [Google Scholar] [CrossRef]
- Kato, T.; Nishimura, A.; Ohtsuki, M.; Wakasugi, Y.; Nagao-Nishiwaki, R.; Fukuda, A.; Kato, K.; Sudo, A. Is musculoskeletal pain related to locomotive syndrome even in young and middle-aged adults? Mod. Rheumatol. 2022, 32, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Ito, Y.M.; Akagi, M.; Chosa, E.; Fuji, T.; Hirano, K.; Ikeda, S.; Ishibashi, H.; Ishibashi, Y.; Ishijima, M.; et al. Reference values for the locomotive syndrome risk test quantifying mobility of 8681 adults aged 20-89 years: A cross-sectional nationwide study in Japan. J. Orthop. Sci. 2020, 25, 1084–1092. [Google Scholar] [CrossRef]
- Yasuda, T. Identifying preventative measures against frailty, locomotive syndrome, and sarcopenia in young adults: A pilot study. J. Phys. Ther. Sci. 2021, 33, 823–827. [Google Scholar] [CrossRef] [PubMed]
- Suetta, C.; Haddock, B.; Alcazar, J.; Noerst, T.; Hansen, O.M.; Ludvig, H.; Kamper, R.S.; Schnohr, P.; Prescott, E.; Andersen, L.L.; et al. The Copenhagen Sarcopenia Study: Lean mass, strength, power, and physical function in a Danish cohort aged 20–93 years. J. Cachexia Sarcopenia Muscle 2019, 10, 1316–1329. [Google Scholar] [CrossRef]
- Nakamura, K. Locomotive syndrome: Disability-free life expectancy and locomotive organ health in a “super-aged” society. J. Orthop. Sci. 2009, 14, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Uesugi, Y.; Kanaya, S.; Nakanishi, H.; Naito, Y. The Relationship between Locomotive Syndrome Risk, Gait Pattern, and Standing Posture in Young Japanese Women: A Cross-Sectional Study. Healthcare 2020, 8, 565. [Google Scholar] [CrossRef]
- Nishimura, A.; Ohtsuki, M.; Kato, T.; Nagao, R.; Ito, N.; Kato, K.; Ogura, T.; Sudo, A. Locomotive syndrome testing in young and middle adulthood. Mod. Rheumatol. 2020, 30, 178–183. [Google Scholar] [CrossRef]
- Shen, S.; Suzuki, K.; Kohmura, Y.; Fuku, N.; Someya, Y.; Naito, H. Association of physical fitness and motor ability at young age with locomotive syndrome risk in middle-aged and older men: J-Fit(+) Study. BMC Geriatr. 2021, 21, 89. [Google Scholar] [CrossRef]
- Arbex, M.; Okazaki, J.E.F.; Tavares, D.R.B.; Figueiredo Bersani, A.L.; Santos, F.C. Locomotive Syndrome is associated with chronic pain and poor quality of life in Brazilian oldest old: LOCOMOV Project. J. Orthop. Sci. 2021, 26, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Chiba, D.; Tsuda, E.; Wada, K.; Kumagai, G.; Sasaki, E.; Nawata, A.; Nakagomi, S.; Takahashi, I.; Nakaji, S.; Ishibashi, Y. Lumbar spondylosis, lumbar spinal stenosis, knee pain, back muscle strength are associated with the locomotive syndrome: Rural population study in Japan. J. Orthop. Sci. 2016, 21, 366–372. [Google Scholar] [CrossRef]
- Tsuji, H.; Tetsunaga, T.; Tetsunaga, T.; Misawa, H.; Nishida, K.; Ozaki, T. Cognitive factors associated with locomotive syndrome in chronic pain patients: A retrospective study. J. Orthop. Sci. 2021, 26, 896–901. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, T.; Ikari, K.; Yano, K.; Okazaki, K. Evaluation of factors associated with locomotive syndrome in Japanese elderly and younger patients with rheumatoid arthritis. Mod. Rheumatol. 2019, 29, 733–736. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yao, C.; Wu, R.; Yan, W.; Yao, Y.; Song, K.; Jiang, Q.; Shi, D. Prevalence of patellofemoral pain and knee pain in the general population of Chinese young adults: A community-based questionnaire survey. BMC Musculoskelet Disord. 2018, 19, 165. [Google Scholar] [CrossRef]
- Aydin, E.; Metin Tellioglu, A.; Kurt Omurlu, I.; Turan, Y. Impact of Generalized Joint Laxity on Plantar Loading Patterns in Young Females. Foot Ankle Int. 2017, 38, 909–915. [Google Scholar] [CrossRef]
- Wolf, J.M.; Cameron, K.L.; Owens, B.D. Impact of joint laxity and hypermobility on the musculoskeletal system. J. Am. Acad. Orthop. Surg. 2011, 19, 463–471. [Google Scholar] [CrossRef]
- Sahin, N.; Baskent, A.; Ugurlu, H.; Berker, E. Isokinetic evaluation of knee extensor/flexor muscle strength in patients with hypermobility syndrome. Rheumatol. Int. 2008, 28, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Seichi, A.; Hoshino, Y.; Doi, T.; Akai, M.; Tobimatsu, Y.; Iwaya, T. Development of a screening tool for risk of locomotive syndrome in the elderly: The 25-question Geriatric Locomotive Function Scale. J. Orthop. Sci. 2012, 17, 163–172. [Google Scholar] [CrossRef]
- Muraki, S.; Akune, T.; Oka, H.; En-yo, Y.; Yoshida, M.; Saika, A.; Suzuki, T.; Yoshida, H.; Ishibashi, H.; Tokimura, F.; et al. Association of radiographic and symptomatic knee osteoarthritis with health-related quality of life in a population-based cohort study in Japan: The ROAD study. Osteoarthr. Cartil. 2010, 18, 1227–1234. [Google Scholar] [CrossRef]
- Yamazaki, T.; Maruyama, S.; Sato, Y.; Suzuki, Y.; Shimizu, S.; Kaneko, F.; Ikezu, M.; Matsuzawa, K.; Edama, M. A preliminary study exploring the change in ankle joint laxity and general joint laxity during the menstrual cycle in cis women. J. Foot Ankle Res. 2021, 14, 21. [Google Scholar] [CrossRef]
- Motohashi, M. Profile of bilateral anterior cruciate ligament injuries: A retrospective follow-up study. J. Orthop. Surg. 2004, 12, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Zhai, X.; Li, S.; Shi, Y.; Fan, X. The Relationship between Physical Activity, Mobile Phone Addiction, and Irrational Procrastination in Chinese College Students. Int. J. Environ. Res. Public Health 2021, 18, 5325. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Ma, X.; Di, Q. Mental Health Problems during the COVID-19 Pandemics and the Mitigation Effects of Exercise: A Longitudinal Study of College Students in China. Int. J. Environ. Res. Public Health 2020, 17, 3722. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, A.; Ito, N.; Asanuma, K.; Akeda, K.; Ogura, T.; Sudo, A. Do exercise habits during middle age affect locomotive syndrome in old age? Mod. Rheumatol. 2018, 28, 334–338. [Google Scholar] [CrossRef]
- Akahane, M.; Yoshihara, S.; Maeyashiki, A.; Tanaka, Y.; Imamura, T. Lifestyle factors are significantly associated with the locomotive syndrome: A cross-sectional study. BMC Geriatr. 2017, 17, 241. [Google Scholar] [CrossRef]
- Nakatoh, S. Relationships between chronic pain with locomotive syndrome and somatic symptom disorder in general community-dwelling population: A cross-sectional evaluation of individuals aged 50 years or older undergoing primary specific health screening. Mod. Rheumatol. 2020, 30, 1067–1073. [Google Scholar] [CrossRef]
- Iizuka, Y.; Iizuka, H.; Mieda, T.; Tajika, T.; Yamamoto, A.; Takagishi, K. Population-based study of the association of osteoporosis and chronic musculoskeletal pain and locomotive syndrome: The Katashina study. J. Orthop. Sci. 2015, 20, 1085–1089. [Google Scholar] [CrossRef]
- Akahane, M.; Maeyashiki, A.; Tanaka, Y.; Imamura, T. The impact of musculoskeletal diseases on the presence of locomotive syndrome. Mod. Rheumatol. 2019, 29, 151–156. [Google Scholar] [CrossRef]
- de Schepper, E.I.; Damen, J.; van Meurs, J.B.; Ginai, A.Z.; Popham, M.; Hofman, A.; Koes, B.W.; Bierma-Zeinstra, S.M. The association between lumbar disc degeneration and low back pain: The influence of age, gender, and individual radiographic features. Spine 2010, 35, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Muraki, S.; Akune, T.; Oka, H.; En-Yo, Y.; Yoshida, M.; Saika, A.; Suzuki, T.; Yoshida, H.; Ishibashi, H.; Tokimura, F.; et al. Health-related quality of life in subjects with low back pain and knee pain in a population-based cohort study of Japanese men: The Research on Osteoarthritis Against Disability study. Spine 2011, 36, 1312–1319. [Google Scholar] [CrossRef] [PubMed]
- Decoster, L.C.; Bernier, J.N.; Lindsay, R.H.; Vailas, J.C. Generalized Joint Hypermobility and Its Relationship to Injury Patterns Among NCAA Lacrosse Players. J. Athl. Train. 1999, 34, 99–105. [Google Scholar]
- Grahame, R.; Hakim, A.J. Hypermobility. Curr. Opin. Rheumatol. 2008, 20, 106–110. [Google Scholar] [CrossRef]
- Roberts, D.; Ageberg, E.; Andersson, G.; Friden, T. Clinical measurements of proprioception, muscle strength and laxity in relation to function in the ACL-injured knee. Knee Surg. Sport. Traumatol. Arthrosc. 2007, 15, 9–16. [Google Scholar] [CrossRef]
- Griffin, L.Y.; Agel, J.; Albohm, M.J.; Arendt, E.A.; Dick, R.W.; Garrett, W.E.; Garrick, J.G.; Hewett, T.E.; Huston, L.; Ireland, M.L.; et al. Noncontact anterior cruciate ligament injuries: Risk factors and prevention strategies. J. Am. Acad. Orthop. Surg. 2000, 8, 141–150. [Google Scholar] [CrossRef] [PubMed]

| Total (n = 157) | No-LS (n = 123) | LS (n = 34) | p-Value | ||
|---|---|---|---|---|---|
| Age (years) | 19.8 ± 1.2 | 19.8 ± 1.0 | 20.0 ± 1.6 | 0.325 | |
| Sex (n, %) | Male | 100 (63.7) | 84 (68.3) | 16 (47.1) | * 0.027 |
| Female | 57 (36.3) | 39 (31.7) | 18 (52.9) | ||
| Height (cm) | 171.4 ± 8.1 | 171.7 ± 7.8 | 170.5 ± 9.3 | 0.449 | |
| Weight (kg) | 63.6 ± 12.0 | 63.7 ± 11.8 | 63.5 ± 12.8 | 0.955 | |
| BMI (kg/m2) | 21.6 ± 3.0 | 21.6 ± 3.0 | 21.7 ± 3.0 | 0.795 | |
| Total number of pain sites (n) | 1.8 ± 2.3 | 1.4 ± 2.1 | 3.1 ± 2.6 | *** <0.001 | |
| Pain VAS | 3.92 ± 6.01 | 2.81 ± 4.96 | 7.94 ± 7.64 | *** <0.001 | |
| GJL score | 2.63 ± 1.49 | 2.46 ± 1.44 | 3.27 ± 1.50 | ** 0.005 | |
| Grip strength (kg) | 30.6 ± 8.4 | 31.5 ± 8.1 | 27.7 ± 9.1 | * 0.022 | |
| Grip strength/weight | 0.48 ± 0.1 | 0.50 ± 0.1 | 0.43 ± 0.1 | ** 0.003 | |
| SMI (kg/m2) | 7.90 ± 1.51 | 7.93 ± 1.62 | 7.79 ± 1.04 | 0.624 | |
| ASMI (kg/BMI × 100) | 37.5 ± 4.8 | 38.0 ± 5.1 | 35.9 ± 3.1 | * 0.030 | |
| PBF (%) | 21.0 ± 6.6 | 20.2 ± 6.4 | 23.4 ± 6.8 | * 0.012 | |
| TBW (%) | 37.4 ± 7.6 | 37.9 ± 7.4 | 35.7 ± 8.0 | 0.129 | |
| Stand-up test (score) | 7.4 ± 1.1 | 7.5 ± 0.9 | 7.0 ± 1.5 | * 0.028 | |
| Two-step test (m/m) | 1.54 ± 0.2 | 1.55 ± 0.2 | 1.53 ± 0.1 | 0.569 | |
| GLFS-25 (unit) | 4.0 ± 4.9 | 2.2 ± 1.9 | 10.0 ± 7.0 | *** <0.001 | |
| Usual gait speed (s/6 m) | 0.78 ± 0.1 | 0.76 ± 0.1 | 0.81 ± 0.2 | 0.181 | |
| 5xSST (s) | 5.4 ± 1.0 | 5.4 ± 1.0 | 5.6 ± 0.9 | 0.261 | |
| IPAQ (Mets × min/wk) | 1996.2 ± 1620.1 | 2009.2 ± 1513.8 | 1948.9 ± 1985.6 | 0.850 | |
| Sedentary time (min × weeks) | 3662.4 ± 1104.6 | 3603.6 ± 1096.2 | 3867.2 ± 1129.8 | 0.223 | |
| Drinking (%) | 48.0 | 46.7 | 52.9 | 0.734 | |
| Smoking (%) | 1.3 | 1.6 | 0 | 1.000 | |
| Pain Sites | No-LS Group n (%) | LS Group n (%) | Unadjusted | Adjusted Model | ||||
|---|---|---|---|---|---|---|---|---|
| (Crude) | (Model 1) | (Model 2) | ||||||
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | |||
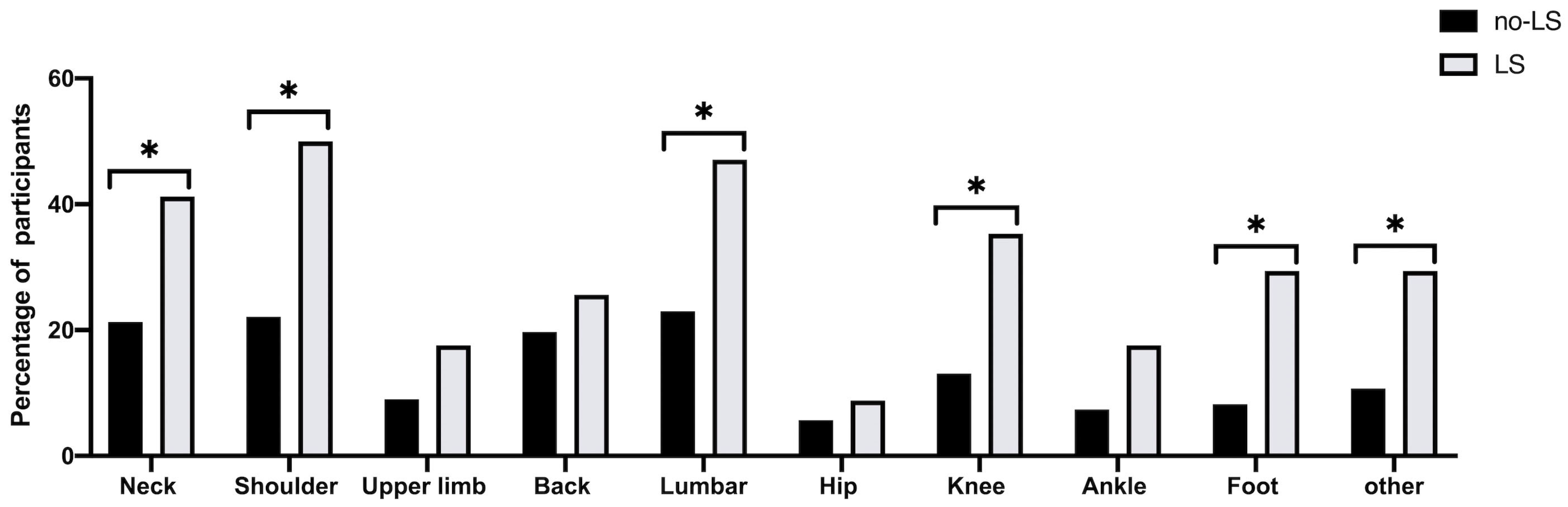
| Neck | 26 (21.3%) | 14 (41.2%) | 2.59 (1.52–5.80) | * 0.021 | 2.13 (0.92–4.93) | 0.076 | 2.53 (0.10–6.41) | 0.051 |
| Shoulder | 27 (22.1%) | 17 (50.0%) | 3.52 (1.59–7.80) | ** 0.002 | 3.11 (1.38–7.04) | ** 0.006 | 2.99 (1.18–7.59) | * 0.021 |
| Upper limb | 11 (9.0%) | 6 (17.6%) | 2.16 (0.74–6.35) | 0.161 | 2.00 (0.66–6.11) | 0.223 | 2.53 (0.73–8.77) | 0.144 |
| Back | 24 (19.7%) | 9 (26.5%) | 1.47 (0.61–3.56) | 0.392 | 1.26 (0.51–3.13) | 0.619 | 1.49 (0.54–4.10) | 0.444 |
| Lumbar | 28 (23.0%) | 16 (47.1%) | 2.99 (1.35–6.61) | ** 0.007 | 2.57 (1.14–5.82) | * 0.023 | 2.93 (1.19–7.21) | * 0.019 |
| Hip | 7 (5.7%) | 3 (8.8%) | 1.59 (0.39–6.51) | 0.519 | 1.31 (0.30–5.62) | 0.718 | 1.92 (0.37–9.98) | 0.440 |
| Knee | 16 (13.1%) | 12 (35.3%) | 3.61 (1.50–8.70) | ** 0.004 | 2.97 (1.20–7.38) | * 0.019 | 3.19 (1.16–8.73) | * 0.024 |
| Ankle | 9 (7.4%) | 6 (17.6%) | 2.69 (0.88–8.19) | 0.081 | 2.60 (0.83–8.16) | 0.102 | 4.14 (0.10–17.3) | 0.051 |
| Foot | 10 (8.2%) | 10 (29.4%) | 4.67 (1.75–12.4) | ** 0.002 | 4.41 (1.61–12.1) | ** 0.004 | 6.18 (1.95–19.6) | ** 0.002 |
| Other | 13 (10.7%) | 10 (29.4%) | 3.49 (1.37–8.90) | ** 0.009 | 3.34 (1.23–8.67) | * 0.013 | 3.64 (1.27–10.6) | * 0.017 |
| Pain Sites | No-LS Group | LS Group | Unadjusted | Adjusted Model | ||||
|---|---|---|---|---|---|---|---|---|
| (Crude) | (Model 1) | (Model 2) | ||||||
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | |||
| Total score | 2.81 ± 4.96 | 7.94 ± 7.64 | 1.14(1.06–1.21) | *** 0.001 | 1.13(1.05–1.21) | ** 0.001 | 1.13 (1.05–1.22) | ** 0.002 |
| Neck | 0.40 ± 0.94 | 1.41 ± 2.23 | 1.56 (1.19–2.03) | ** 0.001 | 1.48 (1.12–1.94) | ** 0.005 | 1.57 (1.13–2.19) | ** 0.008 |
| Shoulder | 0.42 ± 0.99 | 1.32 ± 1.63 | 1.69 (1.25–2.29) | *** 0.001 | 1.62 (1.19–2.20) | ** 0.002 | 1.67 (1.15–2.42) | ** 0.008 |
| Upper limb | 0.17 ± 0.66 | 0.53 ± 1.54 | 1.39 (0.96–2.03) | 0.084 | 1.43 (0.99–2.07) | 0.056 | 1.46 (0.97–2.18) | 0.070 |
| Back | 0.37 ± 0.92 | 0.53 ± 1.08 | 1.17 (0.82–1.68) | 0.390 | 1.08 (0.74–1.58) | 0.687 | 1.09 (0.74–1.61) | 0.671 |
| Lumbar | 0.57 ± 1.47 | 1.32 ± 1.77 | 1.30 (1.04–1.62) | * 0.022 | 1.24 (0.99–1.55) | 0.060 | 1.24 (0.98–1.57) | 0.073 |
| Hip | 0.12 ± 0.62 | 0.50 ± 0.09 | 1.07 (0.59–1.95) | 0.835 | 0.94 (0.51–1.74) | 0.838 | 1.00 (0.54–1.88) | 0.990 |
| Knee | 0.25 ± 0.87 | 0.62 ± 1.13 | 1.41 (0.98–2.01) | 0.062 | 1.32 (0.90–1.91) | 0.153 | 1.62 (0.99–2.67) | 0.054 |
| Ankle | 0.14 ± 0.55 | 0.56 ± 1.64 | 1.51 (1.00–2.28) | 0.048 | 1.55 (1.00–2.41) | 0.050 | 1.80 (1.04–3.11) | * 0.034 |
| Foot | 0.09 ± 0.32 | 0.62 ± 1.23 | 3.16 (1.60–6.68) | ** 0.002 | 3.07 (1.43–6.59) | ** 0.004 | 3.55 (1.47–8.56) | ** 0.005 |
| Other | 0.31 ± 1.37 | 0.88 ± 2.19 | 1.19 (0.98–1.46) | 0.091 | 1.17 (0.95–1.45) | 0.149 | 1.17 (0.93–1.47) | 0.187 |
| No-LS Group n (%) | LS Group n (%) | Unadjusted | Adjusted Model | |||||
|---|---|---|---|---|---|---|---|---|
| (Crude) | (Model 1) | (Model 2) | ||||||
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | |||
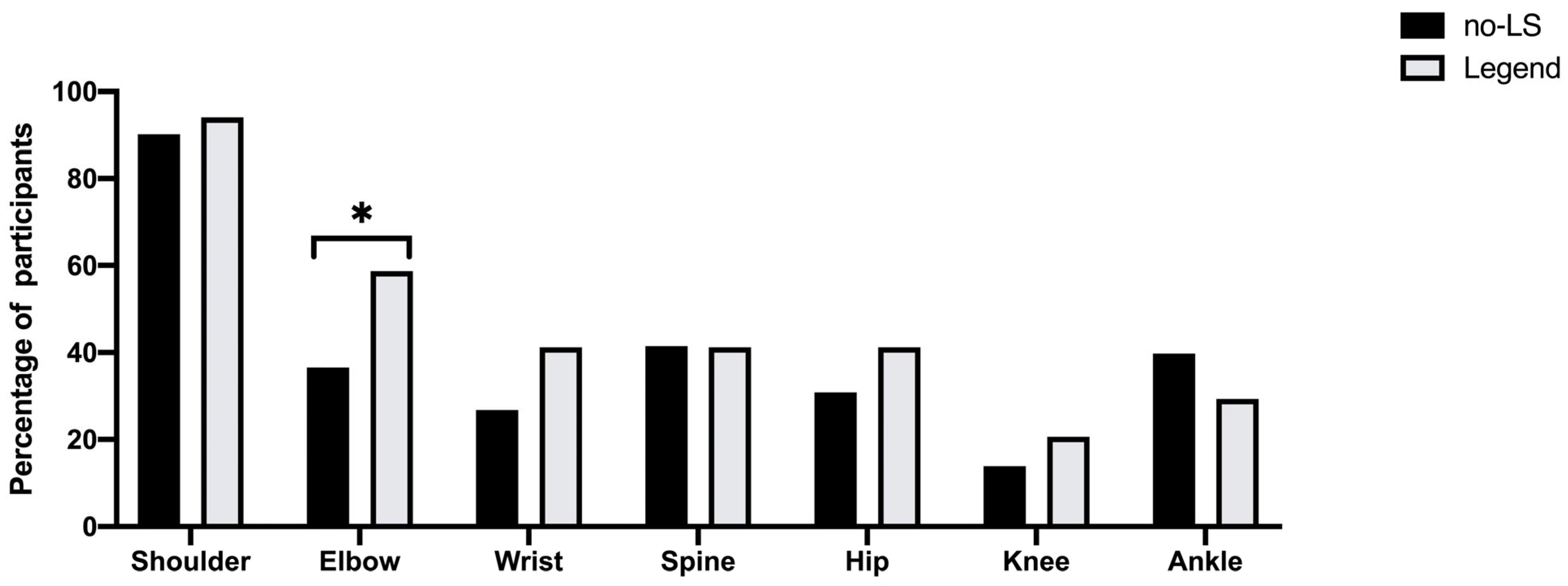
| Wrist | 33 (26.8%) | 14 (41.2%) | 1.91 (0.87–4.21) | 0.109 | 1.70 (0.75–3.84) | 0.204 | 1.79 (0.67–4.78) | 0.245 |
| Elbow | 45 (36.6%) | 20 (58.5%) | 2.48 (1.14–5.38) | * 0.022 | 2.06 (0.91–4.60) | 0.083 | 1.93 (0.74–5.01) | 0.179 |
| Shoulder | 111 (90.2%) | 32 (94.1%) | 1.73 (0.37–8.13) | 0.448 | 1.45 (0.30–7.02) | 0.641 | 2.24 (0.20–25.1) | 0.513 |
| Knee | 17 (13.9%) | 7 (20.6%) | 1.60 (0.60–4.25) | 0.345 | 1.84 (0.67–5.06) | 0.235 | 1.33 (0.39–4.55) | 0.652 |
| Ankle | 49 (39.8%) | 10 (29.4%) | 0.63 (0.28–1.43) | 0.269 | 0.63 (0.27–1.46) | 0.280 | 0.74 (0.29–1.88) | 0.525 |
| Spine | 51 (41.5%) | 14 (41.2%) | 0.99 (0.46–2.14) | 0.976 | 0.85 (0.38–1.89) | 0.683 | 0.88 (0.34–2.27) | 0.789 |
| Hip | 38 (30.9%) | 14 (41.2%) | 1.57 (0.72–3.43) | 0.262 | 1.51 (0.68–3.36) | 0.314 | 1.59 (0.62–4.09) | 0.339 |
| No-LS Group | LS Group n (%) | Unadjusted | Adjusted Model | |||||
|---|---|---|---|---|---|---|---|---|
| (Crude) | (Model 1) | (Model 2) | ||||||
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | |||
| General joint laxity score | 2.46 ± 1.44 | 3.27 ± 1.50 | 1.44 (1.11–1.86) | ** 0.006 | 1.38 (1.05–1.80) | * 0.019 | 1.42 (1.06–1.91) | * 0.019 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Wu, X.; Shen, S.; Hong, W.; Qin, Y.; Sun, M.; Luan, Y.; Zhou, X.; Zhang, B. Relationship between Locomotive Syndrome and Musculoskeletal Pain and Generalized Joint Laxity in Young Chinese Adults. Healthcare 2023, 11, 532. https://doi.org/10.3390/healthcare11040532
Ma Y, Wu X, Shen S, Hong W, Qin Y, Sun M, Luan Y, Zhou X, Zhang B. Relationship between Locomotive Syndrome and Musculoskeletal Pain and Generalized Joint Laxity in Young Chinese Adults. Healthcare. 2023; 11(4):532. https://doi.org/10.3390/healthcare11040532
Chicago/Turabian StyleMa, Yixuan, Xinze Wu, Shaoshuai Shen, Weihao Hong, Ying Qin, Mingyue Sun, Yisheng Luan, Xiao Zhou, and Bing Zhang. 2023. "Relationship between Locomotive Syndrome and Musculoskeletal Pain and Generalized Joint Laxity in Young Chinese Adults" Healthcare 11, no. 4: 532. https://doi.org/10.3390/healthcare11040532
APA StyleMa, Y., Wu, X., Shen, S., Hong, W., Qin, Y., Sun, M., Luan, Y., Zhou, X., & Zhang, B. (2023). Relationship between Locomotive Syndrome and Musculoskeletal Pain and Generalized Joint Laxity in Young Chinese Adults. Healthcare, 11(4), 532. https://doi.org/10.3390/healthcare11040532





