Manual Lymph Drainage for Post-COVID-19 Related Cough, Breathlessness, and Fatigue; Two Case Reports
Abstract
:1. Introduction
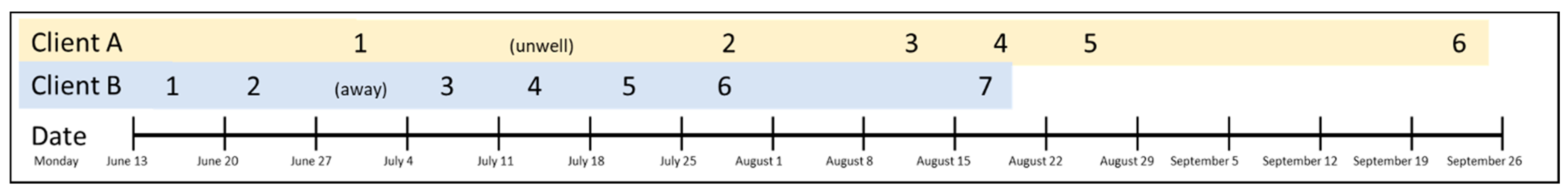
2. Case Presentation, Treatment, and Outcome
3. Clinical Assessments
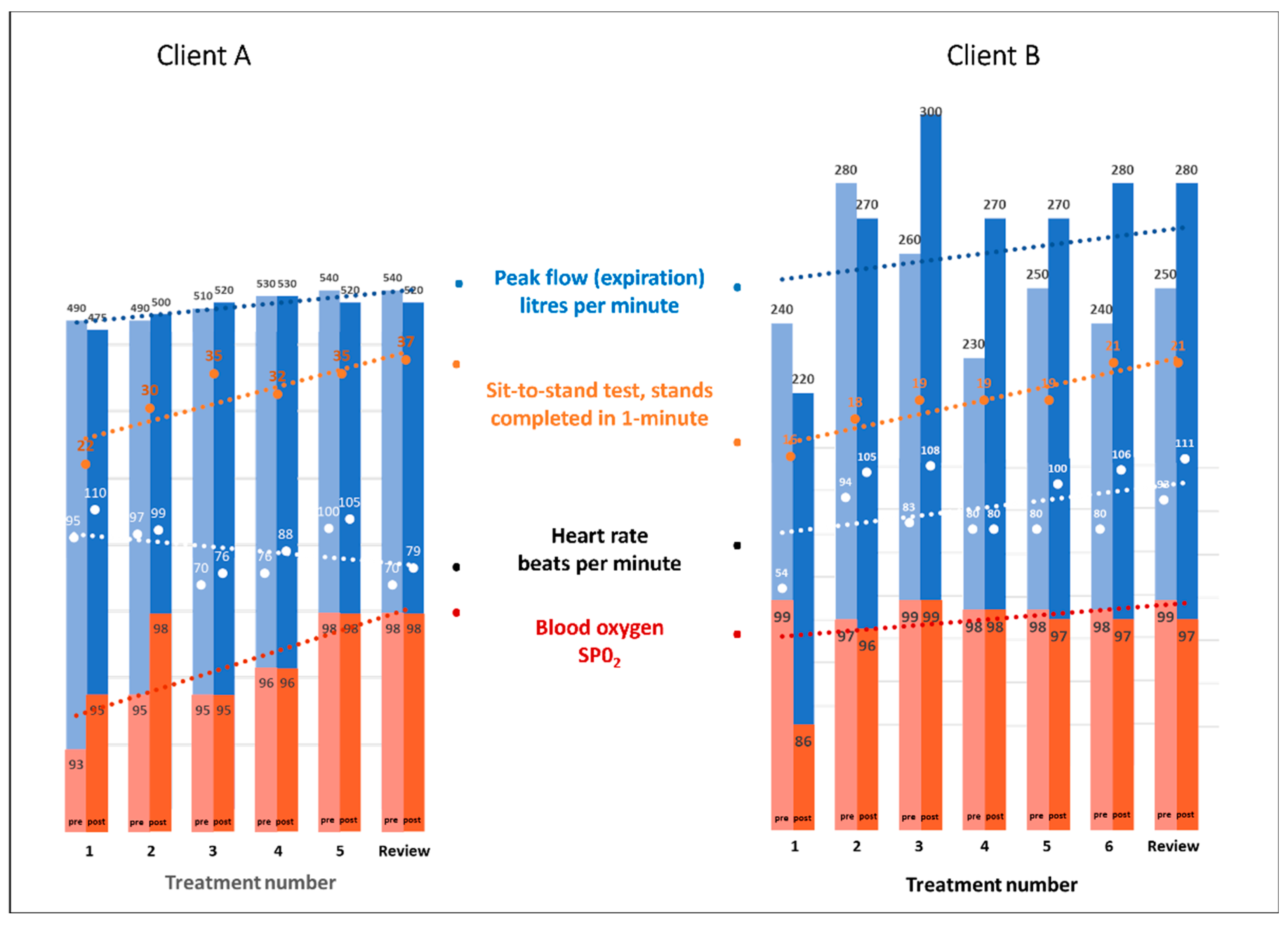
- The Peak Flow Meter (Phillips Respironics, Farnborough, UK) measures peak expiratory flow from the lungs. The device is Therapeutic Goods Administration (TGA) approved and endorsed by Asthma Australia [18]. The best of three attempts at the hardest fastest possible breath into the measuring tube was recorded;
- The Heart Sure Pulse Oximeter (Aeon Technology, Victoria, Australia) is a digital device with a small cuff that slides onto a finger and detects heart rate and blood oxygen levels [19];
- For the sit-to-stand test, the subject repeatedly sits down and stands up from a straight-backed chair without using their arms (arms folded across the torso). The number of ‘stands’ completed in one minute was recorded.
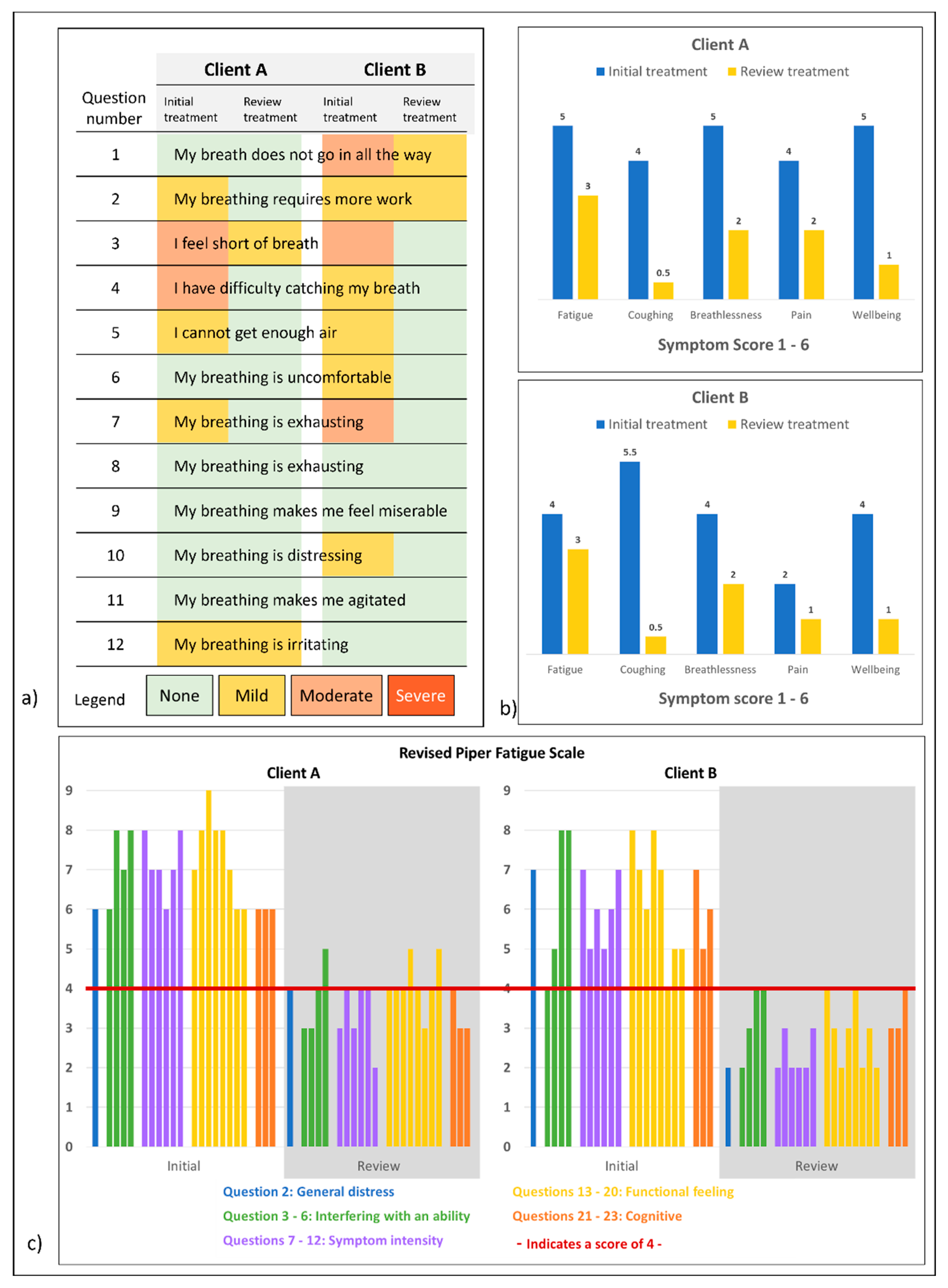
- The Dyspnea-12 Questionnaire [20]: the patient rated twelve statements on physical and affective problems related to breathing as none, mild, moderate, or severe;
- The Revised Piper Fatigue Scale [21], the patient scored 22 questions on unusual symptoms of tiredness and fatigue on a numeric scale between zero and ten, where ten was the most severe;
- Other self-identified symptoms of tiredness, fatigue, coughing, breathlessness, pain, wellbeing, asthma symptoms, and inactivity were collected on Likert scales from one to six where six was the most severe.
4. Therapeutic Intervention
5. Outcomes
5.1. Therapist-Assessed Outcomes
5.2. Patient-Assessed Outcomes
5.3. Intervention Adherence and Adverse Events
6. Discussion
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Worldometer. Available online: https://www.worldometers.info/ (accessed on 27 December 2022).
- Post COVID-19 Condition. Available online: https://www.who.int/teams/health-care-readiness/post-covid-19-condition (accessed on 27 December 2022).
- Hentsch, L.; Cocetta, S.; Allali, G.; Santana, I.; Eason, R.; Adam, E.; Janssens, J.P. Breathlessness and COVID-19: A Call for Research. Respiration 2021, 100, 1016–1026. [Google Scholar] [CrossRef]
- Visco, V.; Vitale, C.; Rispoli, A.; Izzo, C.; Virtuoso, N.; Ferruzzi, G.J.; Santopietro, M.; Melfi, A.; Rusciano, M.R.; Maglio, A.; et al. Post-COVID-19 Syndrome: Involvement and Interactions between Respiratory, Cardiovascular and Nervous Systems. J. Clin. Med. 2022, 11, 524. [Google Scholar] [CrossRef] [PubMed]
- Barbagelata, L.; Masson, W.; Iglesias, D.; Lillo, E.; Migone, J.F.; Orazi, M.L.; Furcada, J.M. Cardiopulmonary exercise testing in patients with post-COVID-19 syndrome. Med. Clín. (Engl. Ed.) 2022, 159, 6–11. [Google Scholar] [PubMed]
- Holdsworth, D.A.; Chamley, R.; Barker-Davies, R.; O’Sullivan, O.; Ladlow, P.; Mitchell, J.L.; Dewson, D.; Mills, D.; May, S.L.; Cranley, M. Comprehensive clinical assessment identifies specific neurocognitive deficits in working-age patients with long-COVID. PLoS ONE 2022, 17, e0267392. [Google Scholar] [CrossRef] [PubMed]
- Battistella, L.R.; Imamura, M.; De Pretto, L.R.; Van Cauwenbergh, S.K.; Ramos, V.D.; Uchiyama, S.S.T.; Matheus, D.; Kuhn, F.; de Oliveira, A.A.A.; Naves, G.S. Long-term functioning status of COVID-19 survivors: A prospective observational evaluation of a cohort of patients surviving hospitalisation. BMJ Open 2022, 12, e057246. [Google Scholar] [CrossRef]
- Kołodziej, M.; Wyszyńska, J.; Bal-Bocheńska, M. COVID-19: A New Challenge for Pulmonary Rehabilitation? J. Clin. Med. 2021, 10, 3361. [Google Scholar] [CrossRef]
- Maes, M.; Al-Rubaye, H.T.; Almulla, A.F.; Al-Hadrawi, D.S.; Stoyanova, K.; Kubera, M.; Al-Hakeim, H.K. Lowered Quality of Life in Long COVID Is Predicted by Affective Symptoms, Chronic Fatigue Syndrome, Inflammation and Neuroimmunotoxic Pathways. Int. J. Environ. Res. Public Health 2022, 19, 10362. [Google Scholar] [CrossRef]
- International Society of Lymphology (ISL). The Diagnosis and Treatment of Peripheral Lymphedema: 2020 Consensus Document of the International Society of Lymphology. Lymphology 2020, 53, 3–19. [Google Scholar]
- Schingale, F.J.; Esmer, M.; Küpeli, B.; Ünal, D. Investigation of the Less Known Effects of Manual Lymphatic Drainage: A Narrative Review. Lymphat. Res. Biol. 2022, 20, 7–10. [Google Scholar] [CrossRef]
- Kim, S.-J.; Kwon, O.-Y.; Yi, C.-H. Effects of Manual Lymph Drainage on Cardiac Autonomic Tone in Healthy Subjects. Int. J. Neurosci. 2009, 119, 1105–1117. [Google Scholar] [CrossRef]
- Foldi, E.; Sauerwald, A.; Hennig, B. Effect of complex decongestive physiotherapy on gene expression for the inflammatory response in peripheral lymphedema. Lymphology 2000, 33, 19–23. [Google Scholar] [PubMed]
- Lopera, C.; Worsley, P.R.; Bader, D.L.; Fenlon, D. Investigating the Short-Term Effects of Manual Lymphatic Drainage and Compression Garment Therapies on Lymphatic Function Using Near-Infrared Imaging. Lymphat. Res. Biol. 2017, 15, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Wittlinger, H.; Wittlinger, D.; Wittlinger, A. Textbook of Dr. Vodder’s Manual Lymph Drainage: A Practical Guide, 2nd ed.; Theime: New York, NY, USA, 2018. [Google Scholar]
- Sun, B.L.; Wang, L.H.; Yang, T.; Sun, J.Y.; Mao, L.L.; Yang, M.F.; Yuan, H.; Colvin, R.A.; Yang, X.Y. Lymphatic drainage system of the brain: A novel target for intervention of neurological diseases. Prog. Neurobiol. 2018, 163–164, 118–143. [Google Scholar] [CrossRef]
- Heald, A.; Perrin, R.; Walther, A.; Stedman, M.; Hann, M.; Mukherjee, A.; Riste, L. Reducing fatigue-related symptoms in Long COVID-19: A preliminary report of a lymphatic drainage intervention. Cardiovasc. Endocrinol. Metab. 2022, 11, e0261. [Google Scholar] [CrossRef] [PubMed]
- What You Need to Know about Peak Flow and Spirometry Testing during COVID-19. Available online: https://asthma.org.au/blog/peak-flow-and-spirometry/ (accessed on 27 December 2022).
- Limitations of Pulse Oximeters and the Effect of Skin Pigmentation. Available online: https://www.tga.gov.au/news/safety-updates/limitations-pulse-oximeters-and-effect-skin-pigmentation (accessed on 27 December 2022).
- Olsson, M.; Ekström, M. Validation of the Dyspnoea-12 and Multidimensional Dyspnea profile among older Swedish men in the population. BMC Geriatrics 2022, 22, 477. [Google Scholar] [CrossRef]
- Piper, B.F.; Dibble, S.L.; Dodd, M.J.; Weiss, M.C.; Slaughter, R.E.; Paul, S.M. The revised Piper Fatigue Scale: Psychometric evaluation in women with breast cancer. Oncol. Nurs. Forum 1998, 25, 677–684. [Google Scholar]
- Gagnier, J.J.; Riley, D.; Altman, D.G.; Moher, D.; Sox, H.; Kienle, G. The CARE Guidelines. Dtsch. Ärzteblatt Int. 2013, 110, 603–608. [Google Scholar] [CrossRef]
- Bretas, D.C.; Leite, A.S.; Mancuzo, E.V.; Prata, T.A.; Andrade, B.H.; Oliveira, J.d.G.F.; Batista, A.P.; Machado-Coelho, G.L.L.; Augusto, V.M.; Marinho, C.C. Lung function six months after severe COVID-19: Does time, in fact, heal all wounds? Braz. J. Infect. Dis. 2022, 26, 102352. [Google Scholar] [CrossRef]
- Williams, A.F.; Vadgama, A.; Franks, P.J.; Mortimer, P.S. A randomized controlled crossover study of manual lymphatic drainage therapy in women with breast cancer-related lymphoedema. Eur. J. Cancer Care 2002, 11, 254–261. [Google Scholar] [CrossRef]
- dos Santos Crisostomo, R.S.; Costa, D.S.; de Luz Belo Martins, C.; Fernandes, T.I.; Armada-da-Silva, P.A. Influence of manual lymphatic drainage on health-related quality of life and symptoms of chronic venous insufficiency: A randomized controlled trial. Arch. Phys. Med. Rehabil. 2015, 96, 283–291. [Google Scholar] [CrossRef]
- Molski, P.; Kruczyński, J.; Molski, A.; Molski, S. Manual lymphatic drainage improves the quality of life in patients with chronic venous disease: A randomized controlled trial. Arch. Med. Sci. 2013, 9, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Asplund, R. Manual lymph drainage therapy using light massage for fibromyalgia sufferers: A pilot study. J. Orthop. Nurs. 2003, 7, 192–196. [Google Scholar] [CrossRef]
- Stefanou, M.-I.; Palaiodimou, L.; Bakola, E.; Smyrnis, N.; Papadopoulou, M.; Paraskevas, G.P.; Rizos, E.; Boutati, E.; Grigoriadis, N.; Krogias, C. Neurological manifestations of long-COVID syndrome: A narrative review. Ther. Adv. Chronic Dis. 2022, 13, 20406223221076890. [Google Scholar] [CrossRef] [PubMed]
| Participant | Case A | Case B |
|---|---|---|
| Patient | ||
| Age in years | 50 | 50 |
| Sex | Female | Female |
| Occupation | Registered Nurse and Practice Manager (full-time) | Primary School Administration Officer (part-time) |
| General health | Good | Good |
| Usual activity level | Farmer/active | Fit/active/hiker |
| Co-morbidities/medication | None/none | None/none |
| Time since +ve COVID-19 test | 11 weeks | 15 weeks |
| Medication required since infection | BP Telmisartan 40 mg Mobic 15 mg | Symbicort inhaler and Ventolin for daily use |
| Presenting concerns | Tiredness Joint and back pain Breathlessness | Reduce asthma symptoms/medication |
| Presenting symptoms | Sinus congestion Headaches Fatigue Brain fog Joint pain (knees, ankles, wrists) Breathlessness Lack of social drive | Fatigue Asthma symptoms Weight gain (due to inactivity and comfort eating) |
| Therapist | ||
| Age | 56 | 50 |
| Sex | Female | Female |
| Substantive qualification | Dip Remedial Massage | Dip Remedial Massage |
| Years using MLD | 5 years since 2017 | 5 years since 2017 |
| Before the First Treatment | Before the Review Treatment | ||
|---|---|---|---|
| Overall, what do you believe is MOST directly contributing to or causing your fatigue? | Case A | Thought it was normal due to my workload, but it has not improved. | Work demands |
| Case B | I don’t think my body is working properly. | Increase in energy from getting chest/breathing under control, so doing more activities which is making me tired. | |
| Overall, the BEST thing you have found to relieve your fatigue is: | Case A | Typically, going to bed early, not socialising or doing anything too active over the weekend. | Good night sleep. Trying to let go of work. Gentle exercise. Relaxation. Deep breathing. |
| Case B | Not get stressed about it, be mindful to do restful things to get through the day. | Not doing so much and taking a bit of time out. | |
| Is there anything else you would like to add that would describe your fatigue better to us? | Case A | Usually really tired around 3–4 pm. | Slept exceptionally well after treatment and when using shakti mat. |
| Case B | When I do a job or even driving in a car, I have to be very careful I don’t fall asleep. | It’s a feeling of being tired rather than being fatigued because I was unwell and struggle with breathing. | |
| Are you experiencing any other symptoms right now? | Case A | Blurry vision when tired and headaches. | Not really. |
| Case B | None given. | No—so very much improved. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Overall, B.; Langley, K.; Douglass, J. Manual Lymph Drainage for Post-COVID-19 Related Cough, Breathlessness, and Fatigue; Two Case Reports. Healthcare 2023, 11, 3085. https://doi.org/10.3390/healthcare11233085
Overall B, Langley K, Douglass J. Manual Lymph Drainage for Post-COVID-19 Related Cough, Breathlessness, and Fatigue; Two Case Reports. Healthcare. 2023; 11(23):3085. https://doi.org/10.3390/healthcare11233085
Chicago/Turabian StyleOverall, Bronwyn, Kaori Langley, and Janet Douglass. 2023. "Manual Lymph Drainage for Post-COVID-19 Related Cough, Breathlessness, and Fatigue; Two Case Reports" Healthcare 11, no. 23: 3085. https://doi.org/10.3390/healthcare11233085
APA StyleOverall, B., Langley, K., & Douglass, J. (2023). Manual Lymph Drainage for Post-COVID-19 Related Cough, Breathlessness, and Fatigue; Two Case Reports. Healthcare, 11(23), 3085. https://doi.org/10.3390/healthcare11233085






