A Network of Sites and Upskilled Therapists to Deliver Best-Practice Stroke Rehabilitation of the Arm: Protocol for a Knowledge Translation Study
Abstract
:1. Introduction
- Increase access to evidence-based SENSe therapy delivered via a network of specialist SENSe therapy centers and skilled therapists.
- Improve outcomes for survivors of stroke with somatosensory impairment of the arm/hand (primary outcome—somatosensory function; secondary outcomes—performance of self-selected valued activities, arm use, and quality of life).
- Achieve high treatment fidelity for therapists in the delivery of upper-limb sensory rehabilitation following a tailored, evidence-informed knowledge-transfer intervention.
- Explore the association of the amount of therapist experience in SENSe delivery with outcomes for stroke survivors.
- Evaluate the cost-effectiveness of the knowledge-translation intervention in terms of the amount of improvement in SENSe therapy outcomes, i.e., somatosensory function, performance in valued activities, arm use, and quality of life.
2. Materials and Methods
2.1. Study Design
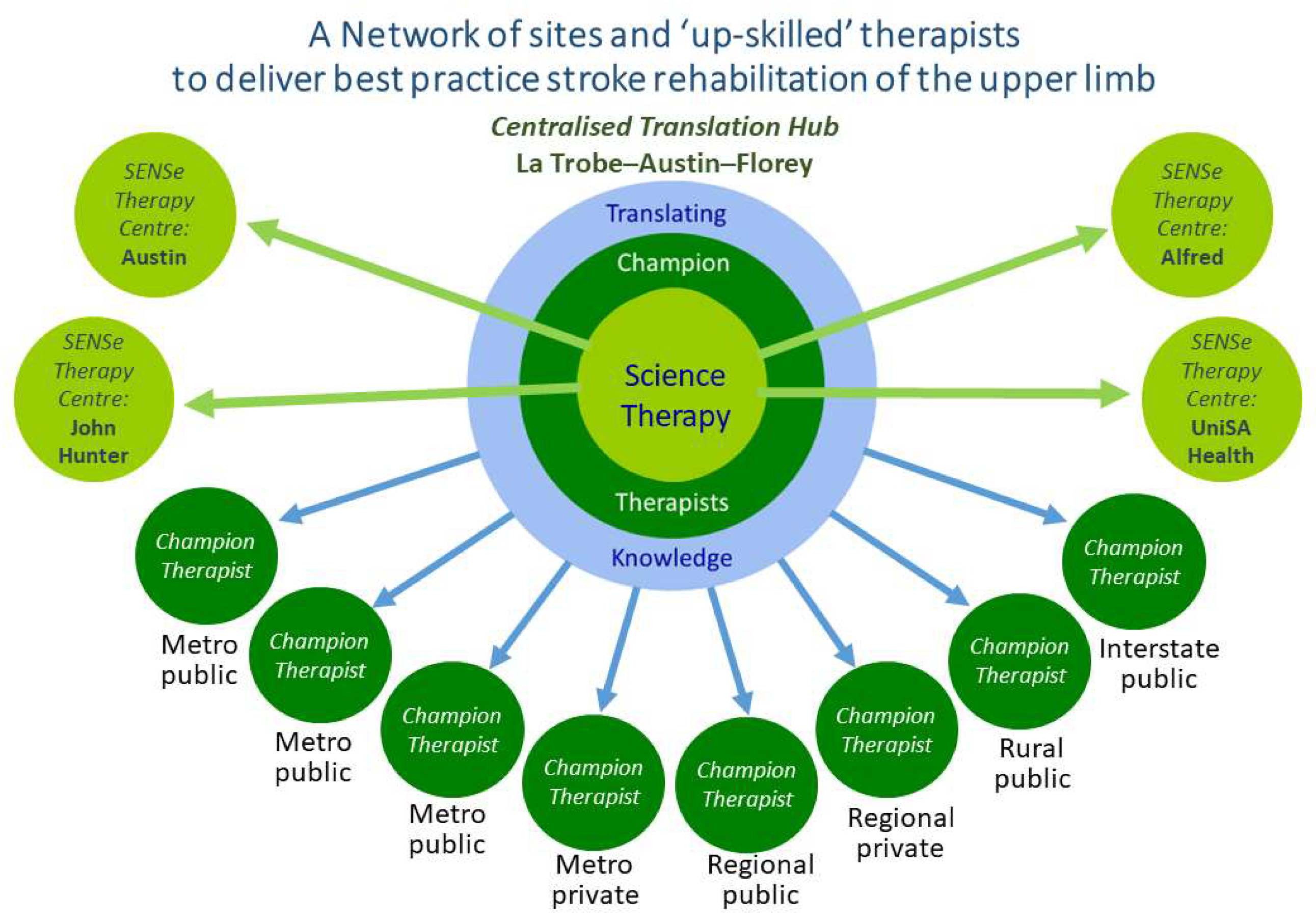
2.1.1. Centralized Translation Hub
2.1.2. Specialist SENSe Therapy Centers and SENSe CONNECT Protocol
2.2. Participants
2.3. Setting
2.4. Outcomes
2.5. Implementation Intervention
2.6. SENSe Therapy Intervention
2.7. Methods to Facilitate Sustainability
2.8. Data Analysis
2.9. Research Impact Evaluation
2.10. Patient and Public Involvement
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duncan, P.W.; Horner, R.D.; Reker, D.M.; Samsa, G.P.; Hoenig, H.; Hamilton, B.; LaClair, B.J.; Dudley, T.K. Adherence to postacute rehabilitation guidelines is associated with functional recovery in stroke. Stroke 2002, 33, 167–177. [Google Scholar] [CrossRef]
- Donnellan, C.; Sweetman, S.; Shelley, E. Health professionals’ adherence to stroke clinical guidelines: A review of the literature. Health Policy 2013, 111, 245–263. [Google Scholar] [CrossRef]
- Stroke Foundation. Clinical Guidelines for Stroke Management 2022 (Living Guidelines); Stroke Foundation: Melbourne, Australia, 2022; Available online: https://informme.org.au/en/Guidelines/Clinical-Guidelines-for-Stroke-Management (accessed on 21 October 2023).
- Intercollegiate Stroke Working Party. National Clinical Guideline for Stroke for the United Kingdom and Ireland; Royal College of Physicians: London, UK, 2023. [Google Scholar]
- Wolf, S.L.; Kwakkel, G.; Bayley, M.; McDonnell, M.N.; For the Upper Extremity Stroke Algorithm Working Group. Best practice for arm recovery post stroke: An international application. Physiotherapy 2016, 102, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Mead, G.E.; Sposato, L.A.; Sampaio Silva, G.; Yperzeele, L.; Wu, S.; Kutlubaev, M.; Cheyne, J.; Wahab, K.; Urrutia, V.C.; Sharma, V.K.; et al. A systematic review and synthesis of global stroke guidelines on behalf of the World Stroke Organization. Int. J. Stroke 2023, 18, 499–531. [Google Scholar] [CrossRef] [PubMed]
- Sentinel Stroke National Audit Programme (SSNAP). National Results Clinical Audit, UK; Kings College: London, UK, 2023; Available online: https://www.strokeaudit.org/ (accessed on 18 August 2023).
- World Health Organization. Rehabilitation 2030 Initiative. 2023. Available online: https://www.who.int/initiatives/rehabilitation-2030 (accessed on 18 August 2023).
- Gagliardi, A.R.; Alhabib, S. Trends in guideline implementation: A scoping systematic review. Implement. Sci. 2015, 10, 54. [Google Scholar] [CrossRef] [PubMed]
- Platz, T. Evidence-based guidelines and clinical pathways in stroke rehabilitation-an international perspective. Front. Neurol. 2019, 10, 200. [Google Scholar] [CrossRef] [PubMed]
- Cahill, L.S.; Carey, L.M.; Lannin, N.A.; Turville, M.; Neilson, C.L.; Lynch, E.A.; McKinstry, C.E.; Han, J.X.; O’Connor, D. Implementation interventions to promote the uptake of evidence-based practices in stroke rehabilitation. Cochrane Database Syst. Rev. 2020, 10, 1–154. [Google Scholar] [CrossRef]
- Cahill, L.S.; Lannin, N.A.; Mak-Yuen, Y.Y.; Turville, M.L.; Carey, L.M. Changing practice in the assessment and treatment of somatosensory loss in stroke survivors: Protocol for a knowledge translation study. BMC Health Serv. Res. 2018, 18, 34. [Google Scholar] [CrossRef]
- Michie, S.; Johnston, M.; Abraham, C.; Lawton, R.; Parker, D.; Walker, A.; on behalf of the “Psychological Theory” group. Making psychological theory useful for implementing evidence based practice: A consensus approach. Qual. Saf. Health Care 2005, 14, 26–33. [Google Scholar] [CrossRef]
- Cane, J.; O’Connor, D.; Michie, S. Validation of the theoretical domains framework for use in behaviour change and implementation research. Implement. Sci. 2012, 7, 37. [Google Scholar] [CrossRef]
- Atkins, L.; Francis, J.; Islam, R.; O’Connor, D.; Patey, A.; Ivers, N.; Foy, R.; Duncan, E.M.; Colquhoun, H.; Grimshaw, J.M.; et al. A guide to using the theoretical domains framework of behaviour change to investigate implementation problems. Implement. Sci. 2017, 12, 77. [Google Scholar] [CrossRef] [PubMed]
- Michie, S.; van Stralen, M.M.; West, R. The behavior change wheel: A new method for characterising and designing behavior change interventions. Implement. Sci. 2011, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Pumpa, L.; Cahill, L.S.; Carey, L.M. Somatosensory assessment and treatment after stroke: An evidence-practice gap. Aust. Occup. Ther. J. 2015, 62, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Doyle, S.; Bennett, S.; Gustafsson, L. Occupational therapy for upper limb post-stroke sensory impairments: A survey. Br. J. Occup. Ther. 2013, 76, 434–442. [Google Scholar] [CrossRef]
- Meyer, S.; Karttunen, A.H.; Thijs, V.; Feys, H.; Verheyden, G. How do somatosensory deficits in the arm and hand relate to upper limb impairment, activity and participation problems after stroke? A systematic review. Phys. Ther. 2014, 94, 14. [Google Scholar] [CrossRef]
- Carey, L.M.; Lamp, G.; Turville, M. The state of the science of somatosensory function and its impact on daily life in adults and older adults, and following stroke: A scoping review. OTJR Occup. Particip. Health 2016, 36 (Suppl. S2), 27S–41S. [Google Scholar] [CrossRef]
- Carlsson, H.; Gard, G.; Brogårdh, C. Upper-limb sensory impairments after stroke: Self-reported experiences of daily life and rehabilitation. J. Rehabil. Med. 2018, 50, 45–51. [Google Scholar] [CrossRef]
- Carey, L.M.; Matyas, T.A. Frequency of discriminative sensory loss in the hand after stroke. J. Rehabil. Med. 2011, 43, 257–263. [Google Scholar] [CrossRef]
- Kim, J.S.; Choi-Kwon, S. Discriminative sensory dysfunction after unilateral stroke. Stroke 1996, 27, 677–682. [Google Scholar] [CrossRef]
- Tyson, S.F.; Hanley, M.; Chillala, J.; Selley, A.B.; Tallis, R.C. Sensory loss in hospital-admitted people with stroke: Characteristics, associated factors, and relationship with function. Neurorehabil. Neural Repair 2008, 22, 166–172. [Google Scholar] [CrossRef]
- Kessner, S.S.; Schlemm, E.; Cheng, B.; Bingel, U.; Fiehler, J.; Gerloff, C.; Thomalla, G. Somatosensory deficits after ischemic stroke. Stroke 2019, 50, 1116–1123. [Google Scholar] [CrossRef] [PubMed]
- Turville, M.L.; Walker, J.; Blennerhassett, J.M.; Carey, L.M. Experiences of upper limb somatosensory retraining in persons with stroke: An interpretative phenomenological analysis. Front. Neurosci. 2019, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Carey, L.M.; Matyas, T.; Baum, C. Effects of somatosensory impairment on participation after stroke. Am. J. Occup. Ther. 2018, 72, 7203205100p1–7203205100p10. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.T.; Duncan, P.W.; Lai, S.-M.; Studenski, S. The relation between impairments and functional outcomes poststroke. Arch. Phys. Med. Rehabil. 2000, 81, 1357–1363. [Google Scholar] [CrossRef] [PubMed]
- Reding, M.J.; Potes, E. Rehabilitation outcome following initial unilateral hemispheric stroke: Life table analysis approach. Stroke 1988, 19, 1354–1358. [Google Scholar] [CrossRef] [PubMed]
- Borstad, A.L.; Nichols-Larsen, D.S. Assessing and treating higher level somatosensory impairments post stroke. Top. Stroke Rehabil. 2014, 21, 290–295. [Google Scholar] [CrossRef]
- Zandvliet, S.B.; Kwakkel, G.; Nijland, R.H.M.; van Wegen, E.E.H.; Meskers, C.G.M. Is recovery of somatosensory impairment conditional for upper-limb motor recovery early after stroke? Neurorehabil. Neural Repair 2020, 34, 403–416. [Google Scholar] [CrossRef]
- Kalra, L. Stroke rehabilitation 2009: Old chestnuts and new insights. Stroke 2010, 41, e88–e90. [Google Scholar] [CrossRef]
- Moncion, K.; Biasin, L.; Jagroop, D.; Bayley, M.; Danells, C.; Mansfield, A.; Tang, A. Barriers and facilitators to aerobic exercise implementation in stroke rehabilitation: A scoping review. J. Neurol Phys. Ther. 2020, 44, 179–187. [Google Scholar] [CrossRef]
- Carey, L.; Macdonell, R.; Matyas, T.A. SENSe: Study of the Effectiveness of Neurorehabilitation on Sensation: A randomized controlled trial. Neurorehabil. Neural Repair 2011, 25, 304–313. [Google Scholar] [CrossRef]
- Medical Research Council. A Framework for Development and Evaluation of RCTs for Complex Interventions to Improve Health; Medical Research Council: London, UK, 2000. [Google Scholar]
- Carey, L.M.; Matyas, T.A.; Oke, L.E. Sensory loss in stroke patients: Effective training of tactile and proprioceptive discrimination. Arch. Phys. Med. Rehabil. 1993, 74, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Carey, L.M.; Matyas, T.A. Training of somatosensory discrimination after stroke: Facilitation of stimulus generalization. Am. J. Phys. Med. Rehabil. 2005, 84, 428–442. [Google Scholar] [CrossRef] [PubMed]
- Juckett, L.A.; Wengerd, L.R.; Faieta, J.; Griffin, C.E. Evidence-based practice implementation in stroke rehabilitation: A scoping review of barriers and facilitators. Am. J. Occup. Ther. 2019, 74, 7401205050p1–7401205050p14. [Google Scholar] [CrossRef] [PubMed]
- Squires, J.E.; Sullivan, K.J.; Eccles, M.P.; Worswick, J.; Grimshaw, J.M. Are multifaceted interventions more effective than single-component interventions in changing health-care professionals’ behaviours? An overview of systematic reviews. Implement. Sci. 2014, 9, 152. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-A.; Jeffs, L.; Barwick, M.; Stevens, B. Organizational contextual features that influence the implementation of evidence-based practices across healthcare settings: A systematic integrative review. Syst. Rev. 2018, 7, 72. [Google Scholar] [CrossRef]
- Nilsen, P.; Bernhardsson, S. Context matters in implementation science: A scoping review of determinant frameworks that describe contextual determinants for implementation outcomes. BMC Health Serv. Res. 2019, 19, 189. [Google Scholar] [CrossRef]
- Hart, T.; Bagiella, E. Design and implementation of clinical trials in rehabilitation research. Arch. Phys. Med. Rehabil. 2012, 93, S117–S126. [Google Scholar] [CrossRef]
- Nielsen, B.; Tse, T.; Haslam, B.; Carey, L.M. Development of an audit checklist to evaluate treatment fidelity of a complex rehabilitation intervention. Disabil. Rehabil. 2023, 45, 1131–1138. [Google Scholar] [CrossRef]
- Cahill, L.S.; Lannin, N.A.; Purvis, T.; Cadilhac, D.A.; Mak-Yuen, Y.; O’Connor, D.A.; Carey, L.M. What is “usual care” in the rehabilitation of upper limb sensory loss after stroke? Results from a national audit and knowledge translation study. Disabil. Rehabil. 2022, 44, 6462–6470. [Google Scholar] [CrossRef]
- Matyas, T.A.; Mak-Yuen, Y.Y.K.; Boelsen-Robinson, T.P.; Carey, L.M. Calibration of impairment severity to enable comparison across somatosensory domains. Brain Sci. 2023, 13, 654. [Google Scholar] [CrossRef]
- Mak-Yuen, Y.Y.K.; Matyas, T.A.; Carey, L.M. Characterizing touch discrimination impairment from pooled stroke samples using the Tactile Discrimination Test: Updated criteria for interpretation and brief test version for use in clinical practice settings. Brain Sci. 2023, 13, 533. [Google Scholar] [CrossRef]
- Carey, L.M.; Oke, L.E.; Matyas, T.A. Impaired limb position sense after stroke: A quantitative test for clinical use. Arch. Phys. Med. Rehabil. 1996, 77, 1271–1278. [Google Scholar] [CrossRef] [PubMed]
- Carey, L.M.; Mak-Yuen, Y.Y.K.; Matyas, T.A. The functional Tactile Object Recognition Test: A unidimensional measure with excellent internal consistency for haptic sensing of real objects after stroke. Front. Neurosci. 2020, 14, 542590. [Google Scholar] [CrossRef] [PubMed]
- Law, M.; Baptise, S.; Carswell, A.; McColl, M.A.; Polatajko, H.J.; Pollock, N. Canadian Occupational Performance Measure, 4th ed.; CAOT Publications ACE: Ottawa, ON, Cananda, 2005. [Google Scholar]
- Wanklyn, T.; Webster, K.E.; Nielsen, B.; Mak-Yuen, Y.; Haslam, B.S.; Carey, L.M. The performance quality rating scale for somatosensation after stroke: A pilot study. OTJR Occup. Particip. Health 2023, 43, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Uswatte, G.; Taub, E.; Morris, D.; Vignolo, M.; McCulloch, K. Reliability and validity of the upper-extremity motor activity log-14 for measuring real-world arm use. Stroke 2005, 36, 2493–2496. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.R.J.; Peacock, S.J.; Hawthorne, G.; Iezzi, A.A.; Elsworth, G.; Day, N. Construction of the descriptive system for the assessment of quality of life AQoL-6D utility instrument. Health Qual. Life Outcomes 2012, 10, 38. [Google Scholar] [CrossRef] [PubMed]
- Australian Government, Australian Institute of Health and Wellbeing. Health Expenditure Australia 2019–2020. 2021. Available online: https://www.aihw.gov.au/reports/health-welfare-expenditure/health-expenditure (accessed on 2 July 2023).
- May, C.; Finch, T. Implementing, embedding, and integrating practices: An outline of normalization process theory. Sociology 2009, 43, 535–554. [Google Scholar] [CrossRef]
- Carey, L. Sense: Helping Stroke Survivors Regain a Sense of Touch: A Manual and DVD for Therapists; Florey Institute of Neuroscience and Mental Health: Melbourne, Australia, 2012. [Google Scholar]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (redcap)--A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inf. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Turville, M.L.; Matyas, T.A.; Blennerhassett, J.M.; Carey, L.M. Initial severity of somatosensory impairment influences response to upper limb sensory retraining post-stroke. NeuroRehabilitation 2018, 43, 413–423. [Google Scholar] [CrossRef]
- Szewczyk, Z.; Reeves, P.; Kingsland, M.; Doherty, E.; Elliott, E.; Wolfenden, L.; Tsang, T.W.; Dunlop, A.; Searles, A.; Wiggers, J. Cost, cost-consequence and cost-effectiveness evaluation of a practice change intervention to increase routine provision of antenatal care addressing maternal alcohol consumption. Implement. Sci. 2022, 17, 14. [Google Scholar] [CrossRef]
- Husereau, D.; Drummond, M.; Petrou, S.; Carswell, C.; Moher, D.; Greenberg, D.; Augustovski, F.; Briggs, A.H.; Mauskopf, J.; Loder, E. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. BMJ 2013, 346, f1049. [Google Scholar] [CrossRef] [PubMed]
- Husereau, D.; Drummond, M.; Augustovski, F.; de Bekker-Grob, E.; Briggs, A.H.; Carswell, C.; Caulley, L.; Chaiyakunapruk, N.; Greenberg, D.; Loder, E.; et al. Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) statement: Updated reporting guidance for health economic evaluations. BMC Med. 2022, 20, 23. [Google Scholar] [CrossRef] [PubMed]
- Searles, A.M.; Doran, C.; Attia, J.; Knight, D.; Wiggers, J.; Deeming, S.; Mattes, J.; Webb, B.; Hannan, S.; Ling, R.; et al. An approach to measuring and encouraging research translation and research impact. Health Res. Policy Syst. 2016, 14, 60. [Google Scholar] [CrossRef]
- Teasell, R.; Meyer, M.J.; McClure, A.; Pan, C.; Murie-Fernandez, M.; Foley, N.; Salter, K. Stroke rehabilitation: An international perspective. Top. Stroke Rehabil. 2009, 16, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Cramer, S.C.; Wolf, S.L.; Adams, H.P.; Chen, D.; Dromerick, A.W.; Dunning, K.; Ellerbe, C.; Grande, A.; Janis, S.; Lansberg, M.G.; et al. Stroke recovery and rehabilitation research. Stroke 2017, 48, 813–819. [Google Scholar] [CrossRef]
- World Health Organization. The Need to Scale Up Rehabilitation; World Health Organization: Geneva, Switzerland, 2017; Available online: https://apps.who.int/iris/handle/10665/331210 (accessed on 2 July 2023).
- Walker, M.F.; Hoffmann, T.C.; Brady, M.C.; Dean, C.M.; Eng, J.J.; Farrin, A.J.; Felix, C.; Forster, A.; Langhorne, P.; Lynch, E.A.; et al. Improving the development, monitoring and reporting of stroke rehabilitation research: Consensus-based core recommendations from the stroke recovery and rehabilitation roundtable. Int. J. Stroke 2017, 12, 472–479. [Google Scholar] [CrossRef]
- Ford, I.; Norrie, J. Pragmatic trials. NEJM 2016, 375, 454–463. [Google Scholar] [CrossRef]

| Outcome | Measure | A1 (Baseline) | A2 (6 Weeks Post A1) | A3 (6 Weeks Post A2) | A4 (12 Weeks Post A3) |
|---|---|---|---|---|---|
| Survivor of Stroke | |||||
| Arm somatosensory function | Tactile Discrimination Test (TDT) | X | X | X | X |
| Wrist Position Sense Test (WPST) | X | X | X | X | |
| Functional Tactile Object Recognition Test (fTORT) | X | X | X | X | |
| Performance of valued activities | Canadian Occupational Performance Measure (COPM) Performance Quality Rating Scale for Somatosensation after Stroke | X X | X X | X X | X X |
| Arm use | Motor Activity Log (MAL)-14 item version | X | X | X | X |
| Health-related quality of life | Australian Quality of Life (AQoL-6D) | X | X | X | X |
| Resource utilization | Resource Use and Productivity Questionnaire | X | X | X | X |
| Other | National Institute of Health Stroke Scale (NIHSS) | X | |||
| Modified Rankin Scale (mRS) | X | ||||
| Montreal Cognitive Assessment (MoCA) | X | ||||
| Jebsen Taylor Hand Function Test (JTHFT) | X | ||||
| SENSe Therapist | |||||
| Treatment fidelity | Customized Documentation Audit Checklist | Post delivery of SENSe therapy to each survivor of stroke. | |||
| Practice behavior change | Pre–Post Implementation Questionnaires | Prior to first delivery of SENSe therapy and after delivery of therapy to 12th survivor of stroke, or last scheduled delivery for that therapist. | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carey, L.M.; Cahill, L.S.; Blennerhassett, J.M.; Nilsson, M.; Lannin, N.A.; Thijs, V.; Hillier, S.; Cadilhac, D.A.; Donnan, G.A.; Morris, M.E.; et al. A Network of Sites and Upskilled Therapists to Deliver Best-Practice Stroke Rehabilitation of the Arm: Protocol for a Knowledge Translation Study. Healthcare 2023, 11, 3080. https://doi.org/10.3390/healthcare11233080
Carey LM, Cahill LS, Blennerhassett JM, Nilsson M, Lannin NA, Thijs V, Hillier S, Cadilhac DA, Donnan GA, Morris ME, et al. A Network of Sites and Upskilled Therapists to Deliver Best-Practice Stroke Rehabilitation of the Arm: Protocol for a Knowledge Translation Study. Healthcare. 2023; 11(23):3080. https://doi.org/10.3390/healthcare11233080
Chicago/Turabian StyleCarey, Leeanne M., Liana S. Cahill, Jannette M. Blennerhassett, Michael Nilsson, Natasha A. Lannin, Vincent Thijs, Susan Hillier, Dominique A. Cadilhac, Geoffrey A. Donnan, Meg E. Morris, and et al. 2023. "A Network of Sites and Upskilled Therapists to Deliver Best-Practice Stroke Rehabilitation of the Arm: Protocol for a Knowledge Translation Study" Healthcare 11, no. 23: 3080. https://doi.org/10.3390/healthcare11233080
APA StyleCarey, L. M., Cahill, L. S., Blennerhassett, J. M., Nilsson, M., Lannin, N. A., Thijs, V., Hillier, S., Cadilhac, D. A., Donnan, G. A., Morris, M. E., Churilov, L., Walker, M., Ramanathan, S., Pollack, M., May, E., Cloud, G. C., McGowan, S., Wijeratne, T., Budge, M., ... Matyas, T. A. (2023). A Network of Sites and Upskilled Therapists to Deliver Best-Practice Stroke Rehabilitation of the Arm: Protocol for a Knowledge Translation Study. Healthcare, 11(23), 3080. https://doi.org/10.3390/healthcare11233080









