Outcomes and Challenges in Noncommunicable Disease Care Provision in Health Facilities Supported by Primary Health Care System Strengthening Project in Sri Lanka: A Mixed-Methods Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Setting
2.2.1. General Setting
2.2.2. Specific Setting
- Empanelment of the population: Through this process, the population of a given GN division is assigned to a PMCI. Each individual in the identified catchment population is assigned a unique personal health number (PHN), and a personal health record (PHR) is created. The PHR includes information on the clinical profile at the time of registration and needs to be updated at every encounter with a healthcare provider. Currently, the PHR is a paper record with the unique identifier (PHN) made available to patients at the time of registration. The PHR is available with the patient and brought for each facility visit.
- Screening: During empanelment, the PMCIs actively screen individuals aged ≥35 years for intermediate risk factors for NCDs such as raised blood pressure (BP), raised blood glucose levels, abnormal blood lipids, overweight, and obesity, through either outreach activities or opportunistic screening at facilities. Laboratory investigations as part of the screening are expected to be available at the PMCI or through an external laboratory (sample collection and transfer linkage with PMCI). The demographic details of all the individuals screened, the results of investigations conducted, and the unit referred for further follow-up are documented in the “participants register” maintained at the PMCI. The 1 year and 2 year targets were to screen at least 25% and 50% of individuals aged 35 years and more, respectively [23].
- Follow-up and referral: Those with a low level of cardiovascular disease (CVD) risk and no intermediate risk factors are followed up at HLCs for risk reduction through lifestyle modification. Individuals followed up in the HLC are provided with a card similar to the PHR, which should note progress on the detected risk factors. The details of follow-up are recorded in the paper-based “HLC follow-up register”, in addition to being updated in the HLC module of electronic PHR. At least 25% of those enrolled at HLC are expected to have made a minimum of one follow-up visit within 1 year of the initial visit. The HLCs receive services from health promotion officers, counsellors, dieticians, instructors in physical education, and nutritionists.
2.3. Study Population
2.3.1. Quantitative Component
Assessment of Care Cascade
Assessment of Quality of Follow-Up Care
2.3.2. Qualitative Component
2.4. Data Collection, Study Variables, Data Source, and Study Tools
2.4.1. Quantitative Component
Assessment of Care Cascade
Cutoffs and definitions for parameters used during screening for NCD risk factors
- Body mass index (BMI):
- New version: <18.5 kg/m2, underweight; 18.5 to 24.9 kg/m2, normal; 25 to 29.9 kg/m2, overweight; ≥30 kg/m2, obese
- Old version: The categories as assigned in the register were retained, i.e., entries recorded as “underweight” were considered underweight and so on.
- High blood pressure (BP):
- New version: systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg
- Old version: entries recorded as “above or equal to 140/90 mmHg” or “above or equal to 160/100 mmHg”
- High blood glucose:
- New version: fasting blood glucose ≥ 126 mg/dL or random blood glucose ≥ 200 mg/dL
- Old version: entries recorded as “fasting blood glucose ≥ 126 mg/dL”
- High total cholesterol: ≥200 mg/dL
- High serum creatinine: >1.2 mg/dL
Indicators assessing process of care in monitoring of complications in individuals with diabetes and/or hypertension
- BP measurement taken in the last visit to PMCI
- Blood glucose test conducted in last 3 months
- Lipid profile taken (either total cholesterol alone or complete lipid profile) in the last year
- Renal function test conducted (either serum creatinine or serum urea) in the last year
- Electrocardiography (ECG) performed in the last year
- Fundus examination conducted in the last year
- Foot examination conducted in the last year
Indicators assessing the status of disease control
- BP control: individuals with systolic blood pressure < 140 mmHg and diastolic blood pressure < 90 mmHg on BP measurement during the last visit to PMCI
- Blood glucose control: individuals with fasting blood glucose < 126 mg/dL or random blood glucose < 200 mg/dL in the last 3 months
- Lipid profile within normal limits: individuals with total cholesterol < 200 mg/dL, LDL < 100 mg/dL, HDL ≥ 40 mg/dL, and triglycerides < 150 in the last year
- Renal function within normal limits: individuals with serum creatinine < 1.3 mg/dL in the last year
Assessment of Quality of Follow-Up Care
2.4.2. Qualitative Component
2.5. Data analysis
2.5.1. Quantitative Component
Assessment of Care Cascade
- Percentage eligible for follow-up at the medical clinic among those screened,
- Percentage indicated as referred to the medical clinic in the participant register,
- Percentage registered in the medical clinic calculated with the aggregate number registered in the medical clinic as the numerator and the total eligible for referral as the denominator,
- Percentage with at least one follow-up visit at HLC calculated with the aggregate number of individuals making at least one visit as the numerator and the individuals referred to HLC as the denominator.
Assessment of Quality of Follow-Up Care
2.5.2. Qualitative Component
3. Results
3.1. Screening for NCD Risk Factors and Outcomes
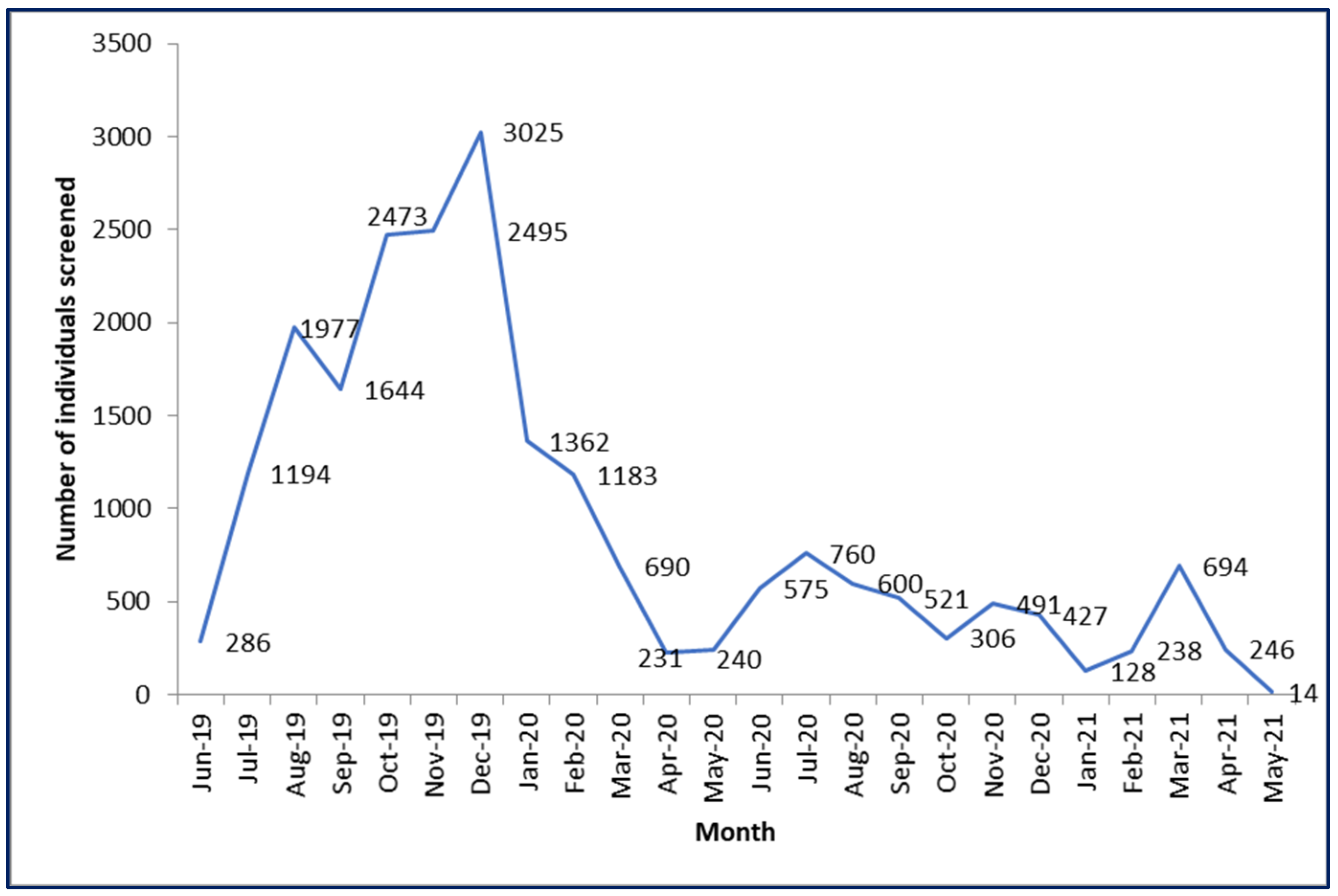
3.1.1. Coverage of Screening (Quantitative Component)
3.1.2. Completeness of Screening (Quantitative Component)
3.1.3. Prevalence of Risk Factors (Quantitative Component)
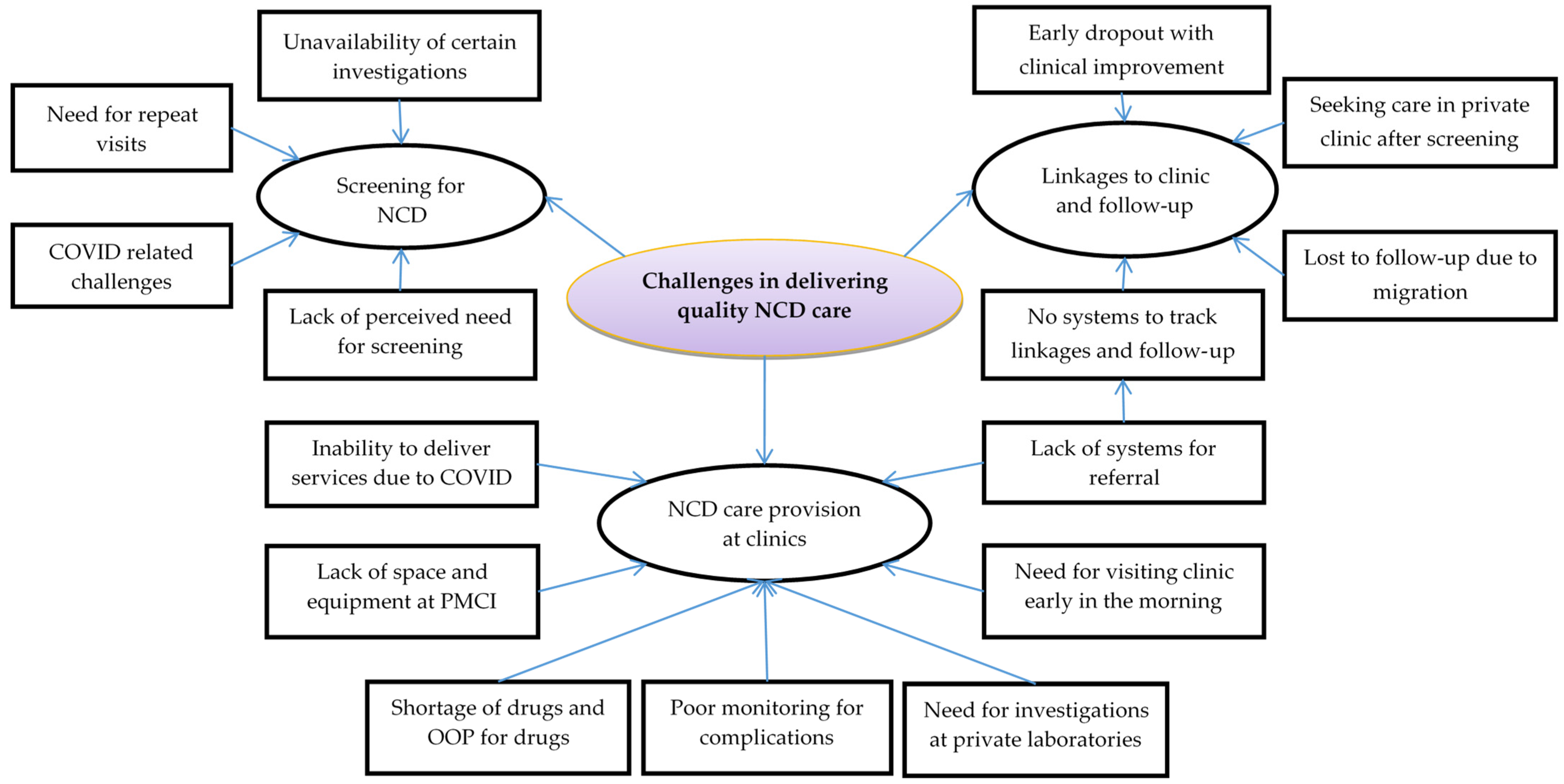
3.1.4. Challenges in Screening for NCDs (Qualitative Component)
Lack of Perceived Need for Screening (Qualitative Component)
“I feel that they may not have perceived the true value of the screening process. And I’m not quite sure when they’re invited through the phone, whether the importance of the screening was clearly conveyed to them… And then for people who are not with any illnesses. I mean they don’t get something big in return.”—Medical officer
Requirement of Repeat Visits (Qualitative Component)
“When checking glucose, we take ‘blood’, we check and then we inform the patients immediately about the results. But we cannot do this for cholesterol (as it needs to be performed in an apex laboratory through sample collection and transportation). Then, there is the probability of missing a few patients. That means, even though the patient participated in the screening activity, they might not like to spend another day to come and collect results due to personal reasons.”—Medical officer
COVID-19-Related Challenges (Qualitative Component)
“But I think due to this COVID-19 situation, services have gone down. Last year, in the fourth quarter, I went for supervision, and only nine patients were screened. And they said due to the COVID-19 situation, we also can’t do much. But this year I told them to screen, and, in the first quarter, they screened more than 50 patients”—Program manager
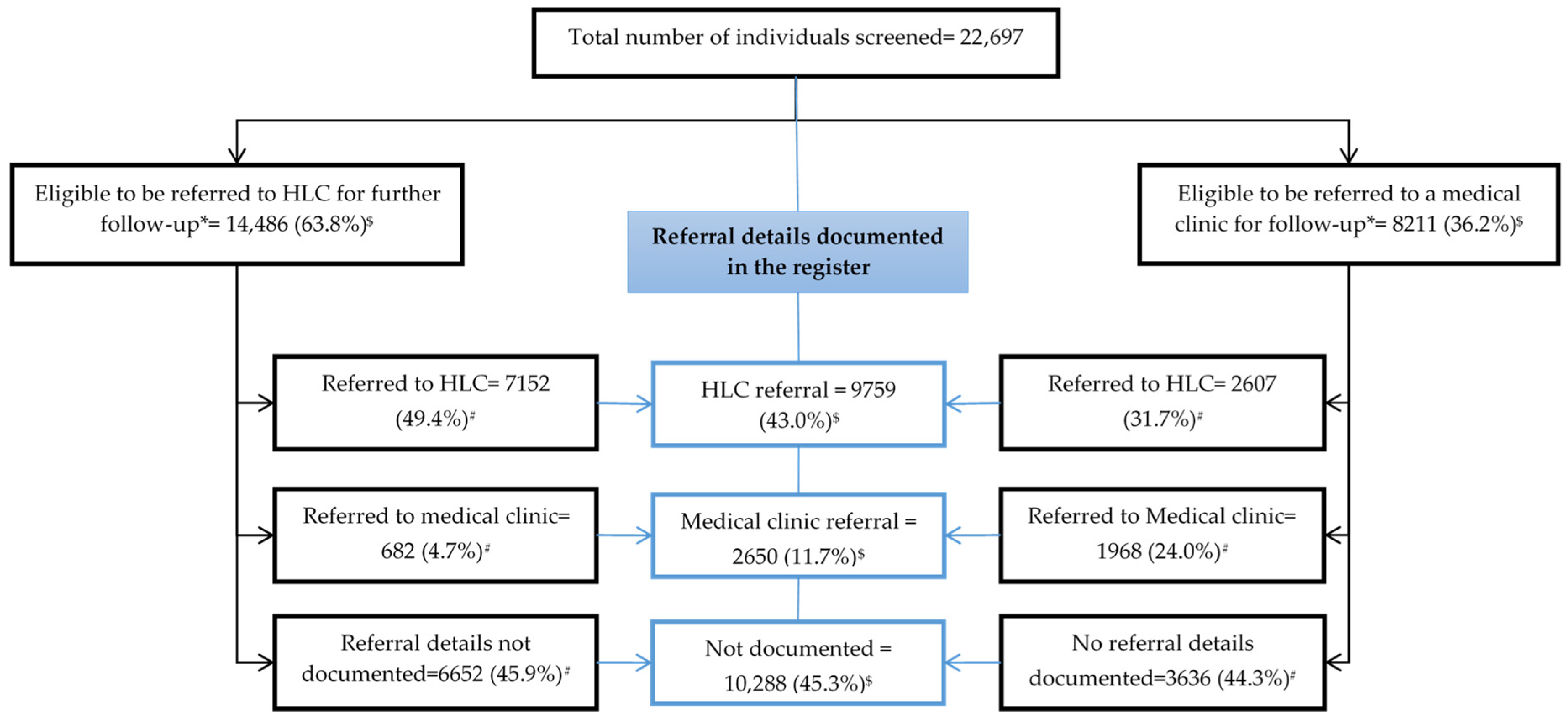
3.2. Linkage to Care
3.2.1. Documentation of Referral, Eligibility, and Registration at Referred Clinics (Quantitative Component)
3.2.2. Challenges around Linkages to Clinic and Follow-Up (Qualitative Component)
No Systems to Track Linkages and Follow-Up (Qualitative Component)
“Actually, we are not maintaining registers for documenting follow-up details of patients followed up in the medical clinic. We will do it in future. Once medical clinic details are entered in the HMIS, we will be able to track the patients. We have the register for the second HLC follow-up visit; we need to enter it into HMIS. We will do it. All these are because of COVID-19”—Medical officer
“They said that there would be a review examination after 1 year, when I was screened in 2019. But they didn’t call me thereafter.”—Patient
Seeking Care in Private Clinics after Screening (Qualitative Component)
“Yes, then, they go to the private clinic. These are practical issues; they (patients) get frightened about their blood glucose and go for private treatment. Then, they go XXX (bigger private hospital) or somewhere continuously. That is their choice.”—Medical officer
“I feel that most of the people who go to the private sector are people who are working and educated, so they look for the quality of the drugs that are issued. Now, when it comes to the drugs that we issue, we know we give them loose pills. We don’t even give them in a blister pack. And some people always go for the brand names or the quality of the drug, and they’re not happy with the type of the drugs that we give at our hospitals.”—Medical officer
“People are not very much welcomed in the government system, I mean, when a patient arrives, in the first few minutes. We are not like the private sector, only in a few hospitals (government hospitals) does the attendant (HCWs) at least look at the patient and pleasantly smiles at the patient, saying ‘Good Morning!’ There is no such greeting system… Most of our people (staff members) are behaving like they have a lot of work and a burden on their minds.”—Medical officer
“Some people go to the private sector just because they have money, and, because they are high and mighty, they go to private places without appreciating the dispensary.”—Patient
Early Dropouts with Clinical Improvement (Qualitative Component)
“You know there are dropouts. For example, some people start with a BMI of 30 or 40. Blood pressure too needs to be corrected. Then, sometimes blood glucose needs correction. So, they come to clinics for 2 or 3 months and then when things become a bit smoother, they forget about the pills and don’t come to the clinic”—Medical officer
Lost to Follow-Up due to Migration (Qualitative Component)
“No. I couldn’t. I was away from here (staying in city) for several months because of my mother’s illness. They had given me a scheduled date (for follow-up visit) during that time, and I couldn’t go on that date.”—Patient
3.3. Assessment of Quality of Follow-Up Care at Medical Clinic
3.3.1. Regularity of Follow-Up Visits and Monitoring for Complications (Quantitative Component)
3.3.2. Challenges around NCD Care Provision at Clinics (Qualitative Component)
Inability to Deliver Service due to COVID-19 (Qualitative Component)
“I can’t ask my staff to draw blood or do investigations due to COVID-19. Even in those who are having serious illnesses in hospitals, even the blood pressure is not measured because of the fear of COVID-19. In that context, how can I make my staff go very close to people and do it? I mean, that is a risk.”—Medical officer
“It was during the curfew (COVID-19 related lockdown), and three-wheelers were charging double the fare. So, I just visited a private medical center near my house.”—Patient
Lack of Space and Equipment at PMCI (Qualitative Component)
“There are people who are willing to do exercise while they are here. There should be a place for that and there should be a ‘washroom’. Further, if any small lecture is given, there should be a small space for that. Actually, the place should be built by keeping all these issues in mind. In the currently available clinic space, we cannot do those things. At a time, we have to arrange chairs to do that and then we need to rearrange the chairs. That is a waste of time. The place should be well designed.”—Medical officer
Shortage of Drugs and OOP Payment for Drugs (Qualitative Component)
“Sometimes in the past few months some medicines were not available. It was for around 4–5 months. I bought medicine for diabetes and high blood pressure from the pharmacy. So, there were issues like this.”—Patient
Poor Monitoring for Complications (Qualitative Component)
“No, they did not do any test after detecting high blood pressure, they did not tell me anything (to get the follow-up investigations). Dr XXXX (family doctor) asked me to do them (blood investigations—blood sugar, serum creatinine, and cholesterol) 4 months back.”—Patient
“No, I did not check them here (registered PMCI). I went to XXX hospital (secondary hospital). I went myself there around 2 years ago and got it (fundus examination) checked.”—Patient with diabetes and on treatment for the last 5 years
“We are asking patients to come for investigations, but if they don’t come, we can’t do anything.”—Medical officer
“We missed only nephropathy (serum creatinine), and we checked diabetes retinopathy through the eye clinic.”—Medical officer
Dependence on Private Laboratories for Investigations (Qualitative Component)
“We are also doing clinics but once in every 6 months they need to go to private laboratories for renal function investigations”—Medical officer
“People do not know even if we are doing those tests here (PMCI). We need to inform them that we have enough facilities, and we are doing tests. Now this is changing because people are aware that the laboratory is available in our hospitals.”—Medical officer
Need for Visiting Clinic Early in the Morning (Qualitative Component)
“There are patients who arrive by 5–5:30 a.m. So, I get 20–25th place in the queue. The doctor checks my blood pressure and writes medicines quickly. Time is spent waiting to see the doctor and then to obtain medicines. After reaching the pharmacy, they give us medicines quickly”—Patient
Lack of Systems for Referral (Qualitative Component)
“A major thing is the waste of time for the patient when they are referred. There is no value for referral there (apex hospital). Here, as only the medical officer and the consultant check them, we send them there (apex hospital). But, the patient has to go through the OPD again there (apex hospital). There is no ‘quota’ given to us to enable a direct referral to a consultant. If a ‘quota’ is given to us, that would be helpful”—Medical officer
3.4. Positive Aspects of NCD Care Provision through PMCI
“All (villagers) were happy about this (screening outreach at village) because they did it in the village. We can’t go to the hospital due to our jobs. But as they came to us, we could get everything done.”—Patient
“Now it is really good compared to how it was before. The medicine is good, the doctor is good, and the staff are also good.”—Patient
“Now the people who were with NCD clinics of XXX hospital (secondary hospital) and private clinics, when they got to know that the dispensary is working well, they all got their clinic books transferred to our clinic… They get all their drugs here. And even the investigations are conducted here… I think the PSSP project has improved the laboratory a lot. They provided the laboratories (apex laboratories to which samples are sent from PMCI for investigations) with machinery and human resources, so now the laboratory has the potential to perform the investigations for all the hospitals allocated to it.”—Medical officer
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization Third United Nations High-Level Meeting on NCDs. Available online: http://www3.paho.org/hq/index.php?option=com_content&view=article&id=14416:un-generalassembly-third-high-level-meeting-ncds-2018&Itemid=0&lang=en#gsc.tab=0 (accessed on 6 January 2021).
- United Nations Sustainable Development Goals. Goal 3: Good Health and Well-Being. Available online: https://www.undp.org/content/undp/en/home/sustainable-development-goals/goal-3-good-health-and-well-being.html (accessed on 6 January 2021).
- World Health Organization. Global Action Plan for the Prevention and Control of NCDs 2013–2020; WHO: Geneva, Switzerland, 2013.
- World Health Organization. Strengthening Health Systems to Accelerate Delivery of Noncommunicable Diseases Services at the Primary Health Care Level—A One-Year Progress Review of the Implementation of the 2016 Colombo Declaration on NCDs; WHO: New Delhi, India, 2017.
- World Health Organization. Implementation Tools—Package of Essential Noncommunicable (PEN) Disease Interventions for Primary Health Care in Low-Resource Settings; WHO: Geneva, Switzerland, 2013.
- Beaglehole, R.; Bonita, R.; Horton, R.; Adams, C.; Alleyne, G.; Asaria, P.; Baugh, V.; Bekedam, H.; Billo, N.; Casswell, S.; et al. Priority Actions for the Non-Communicable Disease Crisis. Lancet 2011, 377, 1438–1447. [Google Scholar] [CrossRef] [PubMed]
- Varghese, C.; Nongkynrih, B.; Onakpoya, I.; McCall, M.; Barkley, S.; Collins, T.E. Better Health and Wellbeing for Billion More People: Integrating Non-Communicable Diseases in Primary Care. BMJ 2019, 364, I327. [Google Scholar] [CrossRef] [PubMed]
- Lê, G.; Morgan, R.; Bestall, J.; Featherstone, I.; Veale, T.; Ensor, T. Can Service Integration Work for Universal Health Coverage? Evidence from around the Globe. Health Policy 2016, 120, 406–419. [Google Scholar] [CrossRef] [PubMed]
- Leslie, H.H.; Spiegelman, D.; Zhoub, X.; Kruka, M.E. Service Readiness of Health Facilities in Bangladesh, Haiti, Kenya, Malawi, Namibia, Nepal, Rwanda, Senegal, Uganda and the United Republic of Tanzania. Bull. World Health Organ. 2017, 95, 738–748. [Google Scholar] [CrossRef] [PubMed]
- Huque, R.; Nasreen, S.; Ahmed, F.; Hicks, J.P.; Walley, J.; Newell, J.N.; Elsey, H. Integrating a Diabetes and Hypertension Case Management Package within Primary Health Care: A Mixed Methods Feasibility Study in Bangladesh. BMC Health Serv. Res. 2018, 18, 811. [Google Scholar] [CrossRef] [PubMed]
- Hyon, C.S.; Nam, K.Y.; Sun, H.C.; Garg, R.; Shrestha, S.M.; Ok, K.U.; Kumar, R. Package of Essential Noncommunicable Disease (PEN) Interventions in Primary Health-Care Settings in the Democratic People’s Republic of Korea: A Feasibility Study. WHO South-East Asia J. Public Health 2017, 6, 69–73. [Google Scholar] [CrossRef]
- Wangchuk, D.; Virdi, N.; Garg, R.; Mendis, S.; Nair, N.; Wangchuk, D.; Kumar, R. Package of Essential Noncommunicable Disease (PEN) Interventions in Primary Health-Care Settings of Bhutan: A Performance Assessment Study. WHO South-East Asia J. Public Health 2014, 3, 154. [Google Scholar] [CrossRef]
- Aye, L.L.; Tripathy, J.P.; Maung Maung, T.; Oo, M.M.; Nwe, M.L.; Thu, H.M.M.; Ko, K.; Kaung, K.K. Experiences from the Pilot Implementation of the Package of Essential Non-Communicable Disease Interventions (PEN) in Myanmar, 2017–2018: A Mixed Methods Study. PLoS ONE 2020, 15, e0229081. [Google Scholar] [CrossRef]
- Kontsevaya, A.; Farrington, J. Implementation of a Package of Essential Noncommunicable (PEN) Disease Interventions in Kyrgyzstan: Evaluation of Effects and Costs in Bishkek after One Year; WHO: Copenhagen, Denmark, 2017.
- Tesema, A.G.; Ajisegiri, W.S.; Abimbola, S.; Balane, C.; Kengne, A.P.; Shiferaw, F.; Dangou, J.-M.; Narasimhan, P.; Joshi, R.; Peiris, D. How Well Are Non-Communicable Disease Services Being Integrated into Primary Health Care in Africa: A Review of Progress against World Health Organization’s African Regional Targets. PLoS ONE 2020, 15, e0240984. [Google Scholar] [CrossRef]
- Mendis, S.; Al Bashir, I.; Dissanayake, L.; Varghese, C.; Fadhil, I.; Marhe, E. Gaps in Capacity in Primary Care in Low-Resource Settings for Implementation of Essential Noncommunicable Disease Interventions. Int. J. Hypertens 2012, 2012, 584041. [Google Scholar] [CrossRef]
- Allen, L.; Nicholson, B.; Yeung, B.; Goiana-da-Silva, F. Implementation of Non-Communicable Disease Policies: A Geopolitical Analysis of 151 Countries. Lancet Glob. Health 2020, 8, e50–e58. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Service Availability and Readiness Assessment (SARA)—An Annual Monitoring System for Service Delivery Reference Manual; Who: Geneva, Switzerland, 2015.
- GBD 2017 DALYs and HALE Collaborators Global, Regional, and National Disability-Adjusted Life-Years (DALYs) for 359 Diseases and Injuries and Healthy Life Expectancy (HALE) for 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1859–1922. [CrossRef]
- Institute for Health Metrics and Evaluation GBD Results Tool|GHDx. Available online: http://ghdx.healthdata.org/gbd-results-tool (accessed on 6 January 2021).
- Mallawaarachchi, D.S.V.; Wickremasinghe, S.C.; Somatunga, L.C.; Siriwardena, V.T.; Gunawardena, N.S. Healthy Lifestyle Centres: A Service for Screening Noncommunicable Diseases through Primary Health-Care Institutions in Sri Lanka. WHO South-East Asia J. Public Health 2016, 5, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Karunaratne, N.P.; Kumara, G.S.P.; Karunathilake, K.T.G.S.; Karunathilake, G.V.K.M.; Kaushalya, P.G.M.; Kavinda, H.W.I.; Keshala, A.A.M.; Ponnamperuma, T. Bypassing Primary Healthcare Institutions: Reasons Identified by Patients’ Attending the out-Patient Department. J. Ruhunu Clin. Soc. 2019, 24, 16. [Google Scholar] [CrossRef]
- Ministry of Health Nutrition and Indigenous Medicine. Road Map for the Primary Healthcare System Strengthening Project (PSSP); Ministry of Health Nutrition and Indigenous Medicine: Colombo, Sri Lanka, 2019.
- The World Bank; Health Nutrition and Population Global Practice. Project Appraisal Document: Primary Health Care System Strengthening Project; The World Bank: Washington, DC, USA, 2018. [Google Scholar]
- Creswell, J.; Plano Clark, V. Designing and Conducting Mixed Methods Research, 2nd ed.; Sage Publications Ltd: London UK, 2010. [Google Scholar]
- The World Bank Sri Lanka|Data. Available online: https://data.worldbank.org/country/sri-lanka?view=chart (accessed on 6 January 2021).
- Department of Census and Statistics; Ministry of Finance and Planning; United Nations Population Fund Census of Population and Housing. 2012 Key Findings Department of Census and Statistics Ministry of Finance and Planning Supported by UNFPA; United Nations Population Fund Sri Lanka: Colombo, Sri Lanka, 2012.
- Government of Sri Lanka List of Codes for the Administrative Divisions of Sri Lanka. 2001. Available online: http://www.statistics.gov.lk/qlink/AdminDivCode/Southern (accessed on 6 January 2021).
- Ministry of Health Summary of Government Hospitals. Available online: http://www.health.gov.lk/moh_final/english/others.php?pid=92 (accessed on 7 January 2021).
- Ministry of Health Nutrition and Indigenous Medicine. Guidelines for Operationalizing Primary Medical Care Services in Sri Lanka; Ministry of Health Nutrition and Indigenous Medicine: Colombo, Sri Lanka, 2019.
- Tong, A.; Sainsbury, P.; Craig, J. Consolidated Criteria for Reporting Qualitative Research (COREQ): A 32-Item Checklist for Interviews and Focus Groups. Int. J. Qual. Health Care 2007, 19, 349–357. [Google Scholar] [CrossRef]
- Kamvura, T.T.; Dambi, J.M.; Chiriseri, E.; Turner, J.; Verhey, R.; Chibanda, D. Barriers to the Provision of Non-Communicable Disease Care in Zimbabwe: A Qualitative Study of Primary Health Care Nurses. BMC Nurs. 2022, 21, 64. [Google Scholar] [CrossRef]
- Bullen, C.; McCormack, J.; Calder, A.; Parag, V.; Subramaniam, K.; Majumdar, A.; Huang, P.H.; Devi, R.; El Bizri, L.; Goodyear-Smith, F. The Impact of COVID-19 on the Care of People Living with Noncommunicable Diseases in Low- and Middle-Income Countries: An Online Survey of Physicians and Pharmacists in Nine Countries. Prim. Health Care Res. Dev. 2021, 22, e30. [Google Scholar] [CrossRef]
- Delobelle, P.A.; Abbas, M.; Datay, I.; De Sa, A.; Levitt, N.; Schouw, D.; Reid, S. Non-Communicable Disease Care and Management in Two Sites of the Cape Town Metro during the First Wave of COVID-19: A Rapid Appraisal. Afr. J. Prim. Health Care Fam. Med. 2022, 14, 3215. [Google Scholar] [CrossRef]
- Yadav, U.N.; Mistry, S.K.; Ghimire, S.; Schneider, C.H.; Rawal, L.B.; Acharya, S.P.; Roxas, B.H.; Harris, M.F. Recognizing the Roles of Primary Health Care in Addressing Non-Communicable Diseases in Low- and Middle-Income Countries: Lesson from COVID-19, Implications for the Future. J. Glob. Health 2021, 11, 03120. [Google Scholar] [CrossRef]
- Basu, P.; Mahajan, M.; Patira, N.; Prasad, S.; Mogri, S.; Muwonge, R.; Lucas, E.; Sankaranarayanan, R.; Iyer, S.; Naik, N.; et al. A Pilot Study to Evaluate Home-Based Screening for the Common Non-Communicable Diseases by a Dedicated Cadre of Community Health Workers in a Rural Setting in India. BMC Public Health 2019, 19, 14. [Google Scholar] [CrossRef]
- Karunaratna, S.; Weerasinghe, M.C.; Ranasinghe, T.; Jayasuriya, R.; Chandraratne, N.; Herath, H.; Quaife, M. Improving Uptake of Non-Communicable Disease Screening in Sri Lanka: Eliciting People’s Preferences Using a Discrete Choice Experiment. Health Policy Plan 2022, 37, 218–231. [Google Scholar] [CrossRef] [PubMed]
- Weigl, B.H.; Neogi, T.; McGuire, H. Point-of-Care Diagnostics in Low-Resource Settings and Their Impact on Care in the Age of the Noncommunicable and Chronic Disease Epidemic. J. Lab. Autom. 2014, 19, 248–257. [Google Scholar] [CrossRef]
- Gialamas, A.; Yelland, L.N.; Ryan, P.; Willson, K.; Laurence, C.O.; Bubner, T.K.; Tideman, P.; Beilby, J.J. Does Point-of-Care Testing Lead to the Same or Better Adherence to Medication? A Randomised Controlled Trial: The PoCT in General Practice Trial. Med. J. Aust. 2009, 191, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Kuupiel, D.; Tlou, B.; Bawontuo, V.; Drain, P.K.; Mashamba-Thompson, T.P. Poor Supply Chain Management and Stock-Outs of Point-of-Care Diagnostic Tests in Upper East Region’s Primary Healthcare Clinics, Ghana. PLoS ONE 2019, 14, e0211498. [Google Scholar] [CrossRef] [PubMed]
- Stevens, P.E.; Levin, A. Evaluation and Management of Chronic Kidney Disease: Synopsis of the Kidney Disease: Improving Global Outcomes 2012 Clinical Practice Guideline. Ann. Intern. Med. 2013, 158, 825–830. [Google Scholar] [CrossRef]
- Sutton, R.T.; Pincock, D.; Baumgart, D.C.; Sadowski, D.C.; Fedorak, R.N.; Kroeker, K.I. An Overview of Clinical Decision Support Systems: Benefits, Risks, and Strategies for Success. NPJ Digit. Med. 2020, 3, 17. [Google Scholar] [CrossRef]
- Heller, D.J.; Kumar, A.; Kishore, S.P.; Horowitz, C.R.; Joshi, R.; Vedanthan, R. Assessment of Barriers and Facilitators to the Delivery of Care for Noncommunicable Diseases by Nonphysician Health Workers in Low- and Middle-Income Countries: A Systematic Review and Qualitative Analysis. JAMA Netw. Open 2019, 2, e1916545. [Google Scholar] [CrossRef]
- Islam, K.; Huque, R.; Saif-Ur-Rahman, K.M.; Ehtesham Kabir, A.N.M.; Enayet Hussain, A.H.M. Implementation Status of Non-Communicable Disease Control Program at Primary Health Care Level in Bangladesh: Findings from a Qualitative Research. Public Health Pract. 2022, 3, 100271. [Google Scholar] [CrossRef]
- Binte, F.R.; Sarker, M.; Yasmin, F.; De Allegri, M.; Sharani, A. Exploring Health-Seeking Behavior for Non-Communicable Chronic Conditions in Northern Bangladesh. PLOS Glob Public Health 2022, 2, e0000497. [Google Scholar] [CrossRef]
- Pati, M.K.; Swaroop, N.; Kar, A.; Aggarwal, P.; Jayanna, K.; Van Damme, W. A Narrative Review of Gaps in the Provision of Integrated Care for Noncommunicable Diseases in India. Public Health Rev. 2020, 41, 8. [Google Scholar] [CrossRef]
- Pruthu, T.K.; Majella, M.G.; Nair, D.; Ramaswamy, G.; Palanivel, C.; Subitha, L.; Kumar, S.G.; Kar, S.S. Does Audit Improve Diabetes Care in a Primary Care Setting? A Management Tool to Address Health System Gaps. J. Nat. Sci. Biol. Med. 2015, 6, S58–S62. [Google Scholar] [CrossRef] [PubMed]
- Ramya, S.; Anand, A.; Bhaskar, S.; Prasad, S. Clinical Audit on Assessment of Non-Glycemic Parameters in Diabetic Patients by Physicians. J. Fam. Med. Prim. Care 2021, 10, 1921. [Google Scholar] [CrossRef]
- Al Mutairi, A.; Al Dheshe, A.; Al Gahtani, A.; Al Mutairi, F.; Al Ghofaili, M.; Al Sawayyed, S.; Heena, H. Audit of Diabetes Mellitus among Patients Attending an Employee Health Clinic at a Tertiary Care Centre in Riyadh, Saudi Arabia. J. Fam. Med. Prim. Care 2019, 8, 975. [Google Scholar] [CrossRef] [PubMed]
- Adams, O.P.; Carter, A. Are Primary Care Practitioners in Barbados Following Diabetes Guidelines?—A Chart Audit with Comparison between Public and Private Care Sectors. BMC Res. Notes 2011, 4, 199. [Google Scholar] [CrossRef]
- Majella, M.G.; Chinnakali, P.; Naik, B.N.; Thekkur, P.; Nag, B.; Ramaswamy, G. How Much Do Persons with Diabetes in a Rural Area of South India Know about Diabetes Management? A Step toward Person-Centered Care. J. Fam. Med. Prim. Care 2017, 6, 605–609. [Google Scholar] [CrossRef]
- Worswick, J.; Wayne, S.C.; Bennett, R.; Fiander, M.; Mayhew, A.; Weir, M.C.; Sullivan, K.J.; Grimshaw, J.M. Improving Quality of Care for Persons with Diabetes: An Overview of Systematic Reviews—What Does the Evidence Tell Us? Syst. Rev. 2013, 2, 26. [Google Scholar] [CrossRef]
- Govender, I.; Ehrlich, R.; van Vuuren, U.; de Vries, E.; Namane, M.; de Sa, A.; Murie, K.; Schlemmer, A.; Govender, S.; Isaacs, A.; et al. Clinical Audit of Diabetes Management Can Improve the Quality of Care in a Resource-Limited Primary Care Setting. Int. J. Qual. Health Care 2012, 24, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Dweakat, R.; Barghouthi, N.; Bouziyeh, Y.; Abu-Hijleh, R.; Ramlawi, A.; Shamasnah, I.; Venter, W. Clinical Audit to Assess Quality of Service in a Newly Implemented NCD Programme: A Cross-Sectional Survey to Review a Pilot Implementation of the WHO PEN Approach in Salfit District, Occupied Palestinian Territory. Lancet 2017, 390, S28. [Google Scholar] [CrossRef]
- Abaynew, Y.; Hussien, M. A Qualitative Study on Barriers to Treatment and Control of Hypertension Among Patients at Dessie Referral Hospital, Northeast Ethiopia, Ethiopia: Healthcare Workers’ Perspective. Integr. Blood Press. Control 2021, 14, 173–178. [Google Scholar] [CrossRef]
- Gupta, S.; Dhamija, J.P.; Mohan, I.; Gupta, R. Qualitative Study of Barriers to Adherence to Antihypertensive Medication among Rural Women in India. Int. J. Hypertens. 2019, 2019, 5749648. [Google Scholar] [CrossRef]

| Primary Medical Care Institution | Individuals Aged ≥ 35 Years in the Identified GN Divisions | Number of Individuals Aged ≥ 35 Years with Documentation Of screening for NCD Risk in the HMIS Database | Number of Individuals Aged ≥ 35 Years Screened for NCD Risk from Assigned GN Division Based on Paper-Based Registers | ||
|---|---|---|---|---|---|
| n | (%) 2 | n | (%) 2 | ||
| PMCI 1 | 2051 | 115 | (5.6) | 436 | (21.3) |
| PMCI 2 | 6819 | 1324 | (19.4) | 1572 | (23.1) |
| PMCI 3 | 3300 | 27 | (0.8) | 1748 | (53.0) |
| PMCI 4 | 17,145 | 448 | (2.6) | 1457 | (8.5) |
| PMCI 5 | 26,034 | 952 | (3.7) | 7624 | (29.3) |
| PMCI 6 | 1700 | 402 | (23.6) | 479 | (28.2) |
| PMCI 7 | 6994 | 3033 | (44.1) | 2953 | (42.2) |
| PMCI 8 | 7294 | 443 | (6.1) | 3075 | (42.2) |
| PMCI 9 | 15,278 | 687 | (4.5) | 871 | (5.7) |
| Total 3 | 86,615 | 7431 | (8.6) | 20215 | (23.3) |
| Characteristics | PMCI 1 | PMCI 2 | PMCI 3 | PMCI 4 | PMCI 5 | PMCI 6 | PMCI 7 | PMCI 8 | PMCI 9 | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| n (%) 1 | n (%) 1 | n (%) 1 | n (%) 1 | n (%) 1 | n (%) 1 | n (%) 1 | n (%) 1 | n (%) 1 | n (%) 1 | |
| Total | 579 | 1688 | 1761 | 2472 | 8214 | 542 | 3043 | 3501 | 897 | 22,697 |
| Age (in years) | ||||||||||
| 18–24 | 0 (0) | 1 (0.1) | 3 (0.2) | 41 (1.7) | 10 (0.1) | 12 (2.2) | 1 (0) | 71 (2.0) | 0 (0) | 139 (0.6) |
| 25–34 | 4 (0.7) | 26 (1.5) | 10 (0.6) | 195 (7.9) | 191 (2.3) | 41 (7.6) | 35 (1.2) | 283 (8.1) | 3 (0.3) | 788 (3.5) |
| 35–44 | 181 (31.3) | 507 (30.0) | 488 (27.7) | 781 (31.6) | 1571 (19.1) | 144 (26.6) | 783 (25.7) | 752 (21.5) | 186 (20.7) | 5393 (23.8) |
| 45–54 | 184 (31.8) | 431 (25.5) | 552 (31.4) | 744 (30.1) | 1918 (23.4) | 133 (24.5) | 772 (25.4) | 821 (23.5) | 256 (28.5) | 5811 (25.6) |
| 55–64 | 161 (27.8) | 418 (24.8) | 458 (26.0) | 468 (18.9) | 1813 (22.1) | 110 (20.3) | 720 (23.7) | 838 (23.9) | 308 (34.3) | 5294 (23.3) |
| ≥65 | 49 (8.5) | 301 (17.8) | 248 (14.1) | 236 (9.6) | 1799 (21.9) | 96 (17.7) | 730 (24.0) | 732 (20.9) | 141 (15.7) | 4332 (19.1) |
| Not recorded | 0 (0) | 4 (0.2) | 2 (0.1) | 7 (0.3) | 912 (11.1) | 6 (1.1) | 2 (0.1) | 4 (0.1) | 3 (0.3) | 940 (4.1) |
| Gender | ||||||||||
| Male | 193 (33.3) | 536 (31.8) | 0 (0) | 778 (31.5) | 2601 (31.7) | 175 (32.3) | 1001 (32.9) | 1123 (32.1) | 322 (35.9) | 6729 (29.7) |
| Female | 386 (66.7) | 1152 (68.3) | 0 (0) | 1693 (68.5) | 5593 (68.1) | 367 (67.7) | 2041 (67.1) | 2378 (67.9) | 575 (64.1) | 14,185 (62.5) |
| Others | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (0) | 0 (0) | 0 (0) | 1 (0) |
| Not recorded | 0 (0) | 0 (0) | 1761 (100) | 1 (0) | 20 (0.2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1782 (7.9) |
| Assigned GN division 2 | ||||||||||
| Yes | 396 (68.4) | 1506 (89.2) | 1716 (97.4) | 1456 (58.9) | 7718 (94) | 404 (74.5) | 2988 (98.2) | 3041 (86.9) | 592 (66.0) | 19,817 (87.3) |
| No | 141 (24.4) | 94 (5.6) | 0 (0) | 884 (35.8) | 423 (5.2) | 14 (2.6) | 55 (1.8) | 75 (2.1) | 23 (2.6) | 1709 (7.5) |
| Not recorded | 42 (7.3) | 88 (5.2) | 45 (2.6) | 132 (5.3) | 73 (0.9) | 124 (22.9) | 0 (0) | 385 (11) | 282 (31.4) | 1171 (5.2) |
| Tobacco Smoking | ||||||||||
| Yes | 46 (7.9) | 169 (10.0) | 78 (4.4) | 205 (8.3) | 554 (6.7) | 58 (10.7) | 128 (4.2) | 278 (7.9) | 130 (14.5) | 1646 (7.3) |
| No | 507 (87.6) | 1299 (77) | 1270 (72.1) | 2261 (91.5) | 6693 (81.5) | 482 (88.9) | 2912 (95.7) | 3195 (91.3) | 728 (81.2) | 19,347 (85.2) |
| Not recorded | 26 (4.5) | 220 (13) | 413 (23.5) | 6 (0.2) | 967 (11.8) | 2 (0.4) | 3 (0.1) | 28 (0.8) | 39 (4.4) | 1704 (7.5) |
| Alcohol use | ||||||||||
| Yes | 71 (12.3) | 312 (18.5) | 107 (6.1) | 443 (17.9) | 824 (10.0) | 98 (18.1) | 310 (10.2) | 497 (14.2) | 139 (15.5) | 2801 (12.3) |
| No | 483 (83.4) | 1189 (70.4) | 1341 (76.2) | 2022 (81.8) | 6411 (78.1) | 435 (80.3) | 2727 (89.6) | 2983 (85.2) | 727 (81.1) | 18,318 (80.7) |
| Not recorded | 25 (4.3) | 187 (11.1) | 313 (17.8) | 7 (0.3) | 979 (11.9) | 9 (1.7) | 6 (0.2) | 21 (0.6) | 31 (3.5) | 1578 (7.0) |
| Body Mass Index | ||||||||||
| <18.5 | 42 (7.3) | 86 (5.1) | 125 (7.1) | 249 (10.1) | 341 (4.2) | 70 (12.9) | 232 (7.6) | 365 (10.4) | 87 (9.7) | 1597 (7) |
| 18.5–24.9 | 282 (48.7) | 963 (57.1) | 869 (49.4) | 1150 (46.5) | 3522 (42.9) | 267 (49.3) | 1506 (49.5) | 1862 (53.2) | 437 (48.7) | 10,858 (47.8) |
| 25.0–29.9 | 183 (31.6) | 503 (29.8) | 523 (29.7) | 792 (32) | 3081 (37.5) | 152 (28) | 980 (32.2) | 973 (27.8) | 274 (30.6) | 7461 (32.9) |
| ≥30.0 | 71 (12.3) | 132 (7.8) | 130 (7.4) | 279 (11.3) | 1270 (15.5) | 52 (9.6) | 319 (10.5) | 299 (8.5) | 98 (10.9) | 2650 (11.7) |
| Not recorded | 1 (0.2) | 4 (0.2) | 114 (6.5) | 2 (0.1) | 0 (0) | 1 (0.2) | 6 (0.2) | 2 (0.1) | 1 (0.1) | 131 (0.6) |
| Waist Circumference 3 | ||||||||||
| Normal | 197 (34) | 932 (55.2) | 0 (0) | 299 (12.1) | 1911 (23.3) | 185 (34.1) | 900 (29.6) | 1788 (51.1) | 248 (27.7) | 6460 (28.5) |
| Abnormal | 380 (65.6) | 737 (43.7) | 0 (0) | 783 (31.7) | 5363 (65.3) | 295 (54.4) | 2094 (68.8) | 1708 (48.8) | 195 (21.7) | 11,555 (50.9) |
| Not recorded | 2 (0.4) | 19 (1.1) | 1761 (100) | 1390 (56.2) | 940 (11.4) | 62 (11.4) | 49 (1.6) | 5 (0.1) | 454 (50.6) | 4682 (20.6) |
| Blood Pressure 4 | ||||||||||
| Normal | 439 (75.8) | 1380 (81.8) | 1166 (66.2) | 2214 (89.6) | 5450 (66.4) | 391 (72.1) | 2085 (68.5) | 2257 (64.5) | 590 (65.8) | 15,972 (70.4) |
| High | 138 (23.8) | 288 (17.1) | 100 (5.7) | 243 (9.8) | 2645 (32.2) | 143 (26.4) | 956 (31.4) | 1200 (34.3) | 307 (34.2) | 6020 (26.5) |
| Not recorded | 2 (0.4) | 20 (1.2) | 495 (28.1) | 15 (0.6) | 119 (1.5) | 8 (1.5) | 2 (0.1) | 44 (1.3) | 0 (0) | 705 (3.1) |
| Blood Glucose 5 | ||||||||||
| Normal | 487 (84.1) | 1405 (83.2) | 1512 (85.9) | 2048 (82.9) | 6894 (83.9) | 406 (74.9) | 2639 (86.7) | 3116 (89) | 757 (84.4) | 19,264 (84.9) |
| High | 82 (14.2) | 265 (15.7) | 132 (7.5) | 412 (16.7) | 1192 (14.5) | 58 (10.7) | 394 (13) | 351 (10) | 138 (15.4) | 3024 (13.3) |
| Not recorded | 10 (1.7) | 18 (1.1) | 117 (6.6) | 12 (0.5) | 128 (1.6) | 78 (14.4) | 10 (0.3) | 34 (1) | 2 (0.2) | 409 (1.8) |
| Total Cholesterol 6 | ||||||||||
| Normal | 395 (68.2) | 609 (36.1) | 982 (55.8) | 153 (6.2) | 948 (11.5) | 109 (20.1) | 1059 (34.8) | 2113 (60.4) | 101 (11.3) | 6469 (28.5) |
| High | 140 (24.2) | 872 (51.7) | 676 (38.4) | 230 (9.3) | 444 (5.4) | 213 (39.3) | 743 (24.4) | 1366 (39) | 213 (23.8) | 4897 (21.6) |
| Not recorded | 44 (7.6) | 207 (12.3) | 103 (5.9) | 2089 (84.5) | 6822 (83.1) | 220 (40.6) | 1241 (40.8) | 22 (0.6) | 583 (65) | 11,331 (49.9) |
| Serum Creatinine 7 | ||||||||||
| Normal | 172 (29.7) | 432 (25.6) | 1534 (87.1) | 315 (12.7) | 38 (0.5) | 0 (0) | 1 (0) | 922 (26.3) | 2 (0.2) | 3416 (15.1) |
| High | 9 (1.6) | 158 (9.4) | 102 (5.8) | 16 (0.7) | 0 (0) | 0 (0) | 0 (0) | 16 (0.5) | 0 (0) | 301 (1.3) |
| Not recorded | 398 (68.7) | 1098 (65.1) | 125 (7.1) | 2141 (86.6) | 8176 (99.5) | 542 (100) | 3042 (100) | 2563 (73.2) | 895 (99.8) | 18,980 (83.6) |
| CVD Risk 8 | ||||||||||
| <10% | 567 (97.9) | 1376 (81.5) | 0 (0) | 2312 (93.5) | 6422 (78.2) | 503 (92.8) | 2710 (89.1) | 2577 (73.6) | 836 (93.2) | 17,303 (76.2) |
| 10–20% | 1 (0.2) | 65 (3.9) | 0 (0) | 32 (1.3) | 371 (4.5) | 22 (4.1) | 121 (4) | 184 (5.3) | 45 (5) | 841 (3.7) |
| 20–30% | 1 (0.2) | 16 (1) | 0 (0) | 12 (0.5) | 137 (1.7) | 7 (1.3) | 58 (1.9) | 65 (1.9) | 6 (0.7) | 302 (1.3) |
| >30% | 1 (0.2) | 4 (0.2) | 0 (0) | 6 (0.2) | 187 (2.3) | 5 (0.9) | 52 (1.7) | 58 (1.7) | 10 (1.1) | 323 (1.4) |
| Not recorded | 9 (1.6) | 227 (13.5) | 1761 (100) | 110 (4.5) | 1097 (13.4) | 5 (0.9) | 102 (3.4) | 617 (17.6) | 0 (0) | 3928 (17.3) |
| Investigation/Examination | Eligible 1 | Tested | Control/Normal 2 | ||
|---|---|---|---|---|---|
| N | n | (%) 3 | N | (%) 4 | |
| Diabetes | |||||
| BP measurement in the last visit | 349 | 199 | (57.0) | 126 | (63.3) |
| Blood glucose test conducted in the last 3 months | 328 | 117 | (35.7) | 46 | (39.3) |
| Lipid profile test in the last year | 229 | 41 | (17.9) | 7 | (17.1) |
| Renal function test in the last year | 229 | 16 | (7.0) | 12 | (75.0) |
| ECG in the last year | 229 | 10 | (4.4) | 10 | (100) |
| Foot examination in the last year | 229 | 4 | (1.8) | 3 | (75.0) |
| Fundus examination | 229 | 2 | (0.9) | 2 | (100) |
| Hypertension | |||||
| BP measurement in the last visit | 326 | 228 | (69.9) | 107 | (46.9) |
| Blood glucose assessment in the last year | 225 | 62 | (27.6) | 49 | (79.0) |
| Lipid profile test in the last year | 225 | 28 | (12.4) | 7 | (25.0) |
| Renal function test in the last year | 225 | 7 | (3.1) | 7 | (100.0) |
| ECG in the last year | 225 | 13 | (5.8) | 12 | (92.3) |
| Fundus examination | 225 | 1 | (0.4) | 1 | (100) |
| Investigation/Examination | Total | Place of Investigation | |||
|---|---|---|---|---|---|
| N | PMCI | Public 1 | Private | Not Recorded | |
| n (%) 2 | n (%) 2 | n (%) 2 | n (%) 2 | ||
| Blood Glucose | 179 | 78 (43.5) | 26 (14.5) | 51 (34.2) | 14 (7.8) |
| Lipid Profile | 69 | 26 (37.7) | 11 (15.9) | 26 (37.8) | 8 (8.7) |
| Renal Function Test | 23 | 5 (21.7) | 8 (34.8) | 7 (30.4) | 3 (13.1) |
| ECG | 23 | 18 (78.3) | 3 (13.0) | 2 (8.7) | 0 (0.0) |
| Foot examination | 4 | 4 (100) | 0 (0) | 0 (0) | 0 (0) |
| Fundus | 3 | 3 (100) | 0 (0) | 0 (0) | 0 (0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nair, D.; Thekkur, P.; Fernando, M.; Kumar, A.M.V.; Satyanarayana, S.; Chandraratne, N.; Chandrasiri, A.; Attygalle, D.E.; Higashi, H.; Bandara, J.; et al. Outcomes and Challenges in Noncommunicable Disease Care Provision in Health Facilities Supported by Primary Health Care System Strengthening Project in Sri Lanka: A Mixed-Methods Study. Healthcare 2023, 11, 202. https://doi.org/10.3390/healthcare11020202
Nair D, Thekkur P, Fernando M, Kumar AMV, Satyanarayana S, Chandraratne N, Chandrasiri A, Attygalle DE, Higashi H, Bandara J, et al. Outcomes and Challenges in Noncommunicable Disease Care Provision in Health Facilities Supported by Primary Health Care System Strengthening Project in Sri Lanka: A Mixed-Methods Study. Healthcare. 2023; 11(2):202. https://doi.org/10.3390/healthcare11020202
Chicago/Turabian StyleNair, Divya, Pruthu Thekkur, Manoj Fernando, Ajay M. V. Kumar, Srinath Satyanarayana, Nadeeka Chandraratne, Amila Chandrasiri, Deepika Eranjanie Attygalle, Hideki Higashi, Jayasundara Bandara, and et al. 2023. "Outcomes and Challenges in Noncommunicable Disease Care Provision in Health Facilities Supported by Primary Health Care System Strengthening Project in Sri Lanka: A Mixed-Methods Study" Healthcare 11, no. 2: 202. https://doi.org/10.3390/healthcare11020202
APA StyleNair, D., Thekkur, P., Fernando, M., Kumar, A. M. V., Satyanarayana, S., Chandraratne, N., Chandrasiri, A., Attygalle, D. E., Higashi, H., Bandara, J., Berger, S. D., & Harries, A. D. (2023). Outcomes and Challenges in Noncommunicable Disease Care Provision in Health Facilities Supported by Primary Health Care System Strengthening Project in Sri Lanka: A Mixed-Methods Study. Healthcare, 11(2), 202. https://doi.org/10.3390/healthcare11020202







