Prevalence of Cardio-Embolic Brain Complications in Permanent and Paroxysmal Atrial Fibrillation Patients
Abstract
1. Background
2. Material and Methods
Statistical Analysis
3. Results
4. Discussion
5. Limitations of the Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2020, 42, 373–498. [Google Scholar] [CrossRef]
- Chugh, S.S.; Havmoeller, R.; Narayanan, K.; Singh, D.; Rienstra, M.; Benjamin, E.J.; Gillum, R.F.; Kim, Y.H.; McAnulty, J.H., Jr.; Zheng, Z.J.; et al. Worldwide epidemiology of atrial fibrillation: A Global Burden of Disease 2010 Study. Circulation 2014, 129, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J.; Hwang, Y.H.; Park, M.S.; Kim, J.T.; Choi, K.H.; Jung, J.M.; Yu, S.; Kim, C.K.; Oh, K.; Song, T.J.; et al. Atrial Fibrillation Related and Unrelated Stroke Recurrence Among Ischemic Stroke Patients With Atrial Fibrillation. Front. Neurol. 2021, 12, 744607. [Google Scholar] [CrossRef] [PubMed]
- Lilli, A.; Di Cori, A.; Zacà, V. Thromboembolic risk and effect of oral anticoagulation according to atrial fibrillation patterns: A systematic review and meta-analysis. Clin. Cardiol. 2017, 40, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Valentin Fuster, J.N.; Robert, A.; Harrington, Z.; Eapen, J. Hurst’s the heart. In Hurst’s the Heart, 14th ed.; Valentin Fuster, J.N., Robert, A.H., Zubin, J.E., Eds.; McGraw-Hill Education: New York, NY, USA, 2017; Volume 1, pp. 19–52. [Google Scholar]
- Bennett, D.A.; Krishnamurthi, R.V.; Barker-Collo, S.; Forouzanfar, M.H.; Naghavi, M.; Connor, M.; Lawes, C.M.; Moran, A.E.; Anderson, L.M.; Roth, G.A.; et al. The global burden of ischemic stroke: Findings of the GBD 2010 study. Glob. Heart 2014, 9, 107–112. [Google Scholar] [CrossRef]
- Barker-Collo, S.; Bennett, D.A.; Krishnamurthi, R.V.; Parmar, P.; Feigin, V.L.; Naghavi, M.; Forouzanfar, M.H.; Johnson, C.O.; Nguyen, G.; Mensah, G.A.; et al. Sex Differences in Stroke Incidence, Prevalence, Mortality and Disability-Adjusted Life Years: Results from the Global Burden of Disease Study 2013. Neuroepidemiology 2015, 45, 203–214. [Google Scholar] [CrossRef]
- Ntaios, G.; Vemmou, A.; Koroboki, E.; Savvari, P.; Makaritsis, K.; Saliaris, M.; Andrikopoulos, G.; Vemmos, K. The type of atrial fibrillation is associated with long-term outcome in patients with acute ischemic stroke. Int. J. Cardiol. 2013, 167, 1519–1523. [Google Scholar] [CrossRef] [PubMed]
- Botto, G.L.; Tortora, G.; Casale, M.C.; Canevese, F.L.; Brasca, F.A.M. Impact of the Pattern of Atrial Fibrillation on Stroke Risk and Mortality. Arrhythm Electrophysiol. Rev. 2021, 10, 68–76. [Google Scholar] [CrossRef]
- Hagii, J.; Metoki, N.; Saito, S.; Shiroto, H.; Sasaki, S.; Takahashi, K.; Hitomi, H.; Baba, Y.; Yamada, N.; Seino, S.; et al. Persistent or permanent atrial fibrillation is associated with severe cardioembolic stroke in patients with non-valvular atrial fibrillation. Thromb. J. 2021, 19, 22. [Google Scholar] [CrossRef]
- Inaba, O.; Yamauchi, Y.; Sekigawa, M.; Miwa, N.; Yamaguchi, J.; Nagata, Y.; Obayashi, T.; Miyamoto, T.; Kamata, T.; Isobe, M.; et al. Atrial fibrillation type matters: Greater infarct volume and worse neurological defects seen in acute cardiogenic cerebral embolism due to persistent or permanent rather than paroxysmal atrial fibrillation. Eur. Eur. Pacing Arrhythm. Card. Electrophysiol. J. Work. Groups Card. Pacing Arrhythm. Card. Cell. Electrophysiol. Eur. Soc. Cardiol. 2018, 20, 1591–1597. [Google Scholar] [CrossRef]
- Ren, J.; Yang, Y.; Zhu, J.; Wu, S.; Wang, J.; Zhang, H.; Shao, X.; Lyu, S. Type of atrial fibrillation and outcomes in patients without oral anticoagulants. Clin. Cardiol. 2021, 44, 168–175. [Google Scholar] [CrossRef]
- Boriani, G.; Laroche, C.; Diemberger, I.; Fantecchi, E.; Popescu, M.I.; Rasmussen, L.H.; Dan, G.A.; Kalarus, Z.; Tavazzi, L.; Maggioni, A.P.; et al. ‘Real-world’ management and outcomes of patients with paroxysmal vs. non-paroxysmal atrial fibrillation in Europe: The EURObservational Research Programme-Atrial Fibrillation (EORP-AF) General Pilot Registry. Eur. Eur. Pacing Arrhythm. Card. Electrophysiol. J. Work. Groups Card. Pacing Arrhythm. Card. Cell. Electrophysiol. Eur. Soc. Cardiol. 2016, 18, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Puccio, D.; Novo, G.; Baiamonte, V.; Nuccio, A.; Fazio, G.; Corrado, E.; Coppola, G.; Muratori, I.; Vernuccio, L.; Novo, S. Atrial fibrillation and mild cognitive impairment: What correlation? Minerva Cardioangiol. 2009, 57, 143–150. [Google Scholar] [PubMed]
- Chen, L.Y.; Norby, F.L.; Gottesman, R.F.; Mosley, T.H.; Soliman, E.Z.; Agarwal, S.K.; Loehr, L.R.; Folsom, A.R.; Coresh, J.; Alonso, A. Association of Atrial Fibrillation With Cognitive Decline and Dementia Over 20 Years: The ARIC-NCS (Atherosclerosis Risk in Communities Neurocognitive Study). J. Am. Heart Assoc. 2018, 7, e007301. [Google Scholar] [CrossRef] [PubMed]
- Falsetti, L.; Viticchi, G.; Buratti, L.; Grigioni, F.; Capucci, A.; Silvestrini, M. Interactions between Atrial Fibrillation, Cardiovascular Risk Factors, and ApoE Genotype in Promoting Cognitive Decline in Patients with Alzheimer’s Disease: A Prospective Cohort Study. J. Alzheimers Dis. 2018, 62, 713–725. [Google Scholar] [CrossRef]
- Bellomo, A.; De Benedetto, G.; Fossati, C.; D’Ottavio, E.; Formosa, V.; Gianturco, V.; Iori, A.; Marigliano, B.; Lo Iacono, C.; Troisi, G.; et al. Atrial fibrillation (AF) and cognitive impairment in the elderly: A case-control study. Arch. Gerontol. Geriatr. 2012, 55, 247–250. [Google Scholar] [CrossRef]
- Manolis, T.A.; Manolis, A.A.; Apostolopoulos, E.J.; Melita, H.; Manolis, A.S. Atrial Fibrillation and Cognitive Impairment: An Associated Burden or Burden by Association? Angiology 2020, 71, 498–519. [Google Scholar] [CrossRef]
- Santangeli, P.; Di Biase, L.; Bai, R.; Mohanty, S.; Pump, A.; Cereceda Brantes, M.; Horton, R.; Burkhardt, J.D.; Lakkireddy, D.; Reddy, Y.M.; et al. Atrial fibrillation and the risk of incident dementia: A meta-analysis. Heart Rhythm 2012, 9, 1761–1768. [Google Scholar] [CrossRef]
- Tsang, T.S.; Gersh, B.J.; Appleton, C.P.; Tajik, A.J.; Barnes, M.E.; Bailey, K.R.; Oh, J.K.; Leibson, C.; Montgomery, S.C.; Seward, J.B. Left ventricular diastolic dysfunction as a predictor of the first diagnosed nonvalvular atrial fibrillation in 840 elderly men and women. J. Am. Coll. Cardiol. 2002, 40, 1636–1644. [Google Scholar] [CrossRef]
- Thacker, E.L.; McKnight, B.; Psaty, B.M.; Longstreth, W.T., Jr.; Sitlani, C.M.; Dublin, S.; Arnold, A.M.; Fitzpatrick, A.L.; Gottesman, R.F.; Heckbert, S.R. Atrial fibrillation and cognitive decline: A longitudinal cohort study. Neurology 2013, 81, 119–125. [Google Scholar] [CrossRef]
- Kummer, B.R.; Diaz, I.; Wu, X.; Aaroe, A.E.; Chen, M.L.; Iadecola, C.; Kamel, H.; Navi, B.B. Associations between cerebrovascular risk factors and parkinson disease. Ann. Neurol. 2019, 86, 572–581. [Google Scholar] [CrossRef]
- Han, S.; Moon, I.; Choi, E.K.; Han, K.D.; Cho, H.C.; Lee, S.Y.; Yang, S.; Kwon, S.; Choi, Y.J.; Lee, H.J.; et al. Increased atrial fibrillation risk in Parkinson’s disease: A nationwide population-based study. Ann. Clin. Transl. Neurol. 2021, 8, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.T.; Chan, L.; Wu, D.; Chen, W.T.; Chien, L.N. Association Between Parkinson’s Disease and Atrial Fibrillation: A Population-Based Study. Front. Neurol. 2019, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Çanga, Y.; Emre, A.; Yüksel, G.A.; Karataş, M.B.; Yelgeç, N.S.; Gürkan, U.; Çalık, A.N.; Tireli, H.; Terzi, S. Assessment of Atrial Conduction Times in Patients with Newly Diagnosed Parkinson’s Disease. Park. Dis. 2018, 2018, 2916905. [Google Scholar] [CrossRef]
- Becker, C.; Jick, S.S.; Meier, C.R. Risk of stroke in patients with idiopathic Parkinson disease. Park. Relat. Disord. 2010, 16, 31–35. [Google Scholar] [CrossRef]
- Struck, L.K.; Rodnitzky, R.L.; Dobson, J.K. Stroke and its modification in Parkinson’s disease. Stroke 1990, 21, 1395–1399. [Google Scholar] [CrossRef]
- Huang, Y.-P.; Chen, L.-S.; Yen, M.-F.; Fann, C.-Y.; Chiu, Y.-H.; Chen, H.-H.; Pan, S.-L. Parkinson’s Disease Is Related to an Increased Risk of Ischemic Stroke—A Population-Based Propensity Score-Matched Follow-Up Study. PLoS ONE 2013, 8, e68314. [Google Scholar] [CrossRef]
- KDIGO 2018 Clinical Practice Guideline for the Prevention, Diagnosis, Evaluation, and Treatment of Hepatitis C in Chronic Kidney Disease. Kidney Int. Suppl. 2018, 8, 91–165. [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.-P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef] [PubMed]
- Ghani, A.; Maas, A.H.E.M.; Delnoy, P.P.H.M.; Ramdat Misier, A.R.; Ottervanger, J.P.; Elvan, A. Sex-Based Differences in Cardiac Arrhythmias, ICD Utilisation and Cardiac Resynchronisation Therapy. Neth. Heart J. 2011, 19, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Andrew, N.E.; Thrift, A.G.; Cadilhac, D.A. The Prevalence, Impact and Economic Implications of Atrial Fibrillation in Stroke: What Progress Has Been Made? Neuroepidemiology 2013, 40, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://data.worldbank.org/indicator/SP.DYN.LE00.IN?locations=RO (accessed on 23 December 2022).
- Chao, T.-F.; Lip, G.Y.H.; Liu, C.-J.; Tuan, T.-C.; Chen, S.-J.; Wang, K.-L.; Lin, Y.-J.; Chang, S.-L.; Lo, L.-W.; Hu, Y.-F.; et al. Validation of a Modified CHA2DS2-VASc Score for Stroke Risk Stratification in Asian Patients With Atrial Fibrillation. Stroke 2016, 47, 2462–2469. [Google Scholar] [CrossRef]
- Eun, M.Y.; Kim, J.Y.; Hwang, Y.H.; Park, M.S.; Kim, J.T.; Choi, K.H.; Jung, J.M.; Yu, S.; Kim, C.K.; Oh, K.; et al. Initiation of Guideline-Matched Oral Anticoagulant in Atrial Fibrillation-Related Stroke. J. Stroke 2021, 23, 113–123. [Google Scholar] [CrossRef]
- Wańkowicz, P.; Staszewski, J.; Dębiec, A.; Nowakowska-Kotas, M.; Szylińska, A.; Rotter, I. Ischemic Stroke Risk Factors in Patients with Atrial Fibrillation Treated with New Oral Anticoagulants. J. Clin. Med. 2021, 10, 1223. [Google Scholar] [CrossRef]
- Borowsky, L.H.; Regan, S.; Chang, Y.; Ayres, A.; Greenberg, S.M.; Singer, D.E. First Diagnosis of Atrial Fibrillation at the Time of Stroke. Cerebrovasc. Dis. 2017, 43, 192–199. [Google Scholar] [CrossRef]
- Zaprutko, T.; Florczak-Wyspiańska, J.; Kopciuch, D.; Paczkowska, A.; Ratajczak, P.; Dorszewska, J.; Nowakowska, E.; Kus, K. Costs of Stroke and Incidence of First Diagnosis of Atrial Fibrillation at Time of Stroke. Neurology Ward Hospital Poznań, Poland 2018. Healthcare 2021, 9, 999. [Google Scholar] [CrossRef]
- Kishore, A.; Vail, A.; Majid, A.; Dawson, J.; Lees, K.R.; Tyrrell, P.J.; Smith, C.J. Detection of atrial fibrillation after ischemic stroke or transient ischemic attack: A systematic review and meta-analysis. Stroke 2014, 45, 520–526. [Google Scholar] [CrossRef]
- Freedman, B.; Potpara, T.S.; Lip, G.Y.H. Stroke prevention in atrial fibrillation. Lancet 2016, 388, 806–817. [Google Scholar] [CrossRef]
- Friberg, L.; Rosenqvist, M.; Lindgren, A.; Terént, A.; Norrving, B.; Asplund, K. High Prevalence of Atrial Fibrillation Among Patients With Ischemic Stroke. Stroke 2014, 45, 2599–2605. [Google Scholar] [CrossRef] [PubMed]
- Averlant, L.; Ficheur, G.; Ferret, L.; Boulé, S.; Puisieux, F.; Luyckx, M.; Soula, J.; Georges, A.; Beuscart, R.; Chazard, E.; et al. Underuse of Oral Anticoagulants and Inappropriate Prescription of Antiplatelet Therapy in Older Inpatients with Atrial Fibrillation. Drugs Aging 2017, 34, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Je, N.K. Underutilization of anticoagulants in patients with nonvalvular atrial fibrillation in the era of non-vitamin K antagonist oral anticoagulants. Int. J. Arrhythmia 2022, 23, 1. [Google Scholar] [CrossRef]
- Kaplan, R.M.; Ziegler, P.D.; Koehler, J.; Landman, S.; Sarkar, S.; Passman, R.S. Use of Oral Anticoagulation in a Real-World Population With Device Detected Atrial Fibrillation. J. Am. Heart Assoc. 2020, 9, e018378. [Google Scholar] [CrossRef]
- Ogilvie, I.M.; Newton, N.; Welner, S.A.; Cowell, W.; Lip, G.Y.H. Underuse of Oral Anticoagulants in Atrial Fibrillation: A Systematic Review. Am. J. Med. 2010, 123, 638–645. [Google Scholar] [CrossRef] [PubMed]

| Group 1 Paroxysmal AF (N = 389) | Group 2 Permanent AF (N = 722) | p-Value | ||
|---|---|---|---|---|
| Age, years, mean, (SD) (*) | 69.07 (10.950) | 74.05 (9.978) | <0.001 | |
| Sex | Female | 193 (49.61%) | 386 (53.46%) | 0.221 |
| Male | 196 (50.39%) | 336 (46.54%) | ||
| Urban vs. rural | Urban (city/town) | 226 (58.10%) | 335 (46.40%) | <0.001 |
| Rural (village) | 163 (41.90%) | 387 (53.60%) | ||
| Overweight | 18 (4.63%) | 53 (7.34%) | 0.078 | |
| Obesity | Overall | 107 (27.51%) | 178 (24.65%) | 0.299 |
| Grade I | 57 (14.7%) | 97 (13.4%) | 0.5640 | |
| Grade II | 34 (8.8%) | 40 (5.5%) | 0.0402 | |
| Grade III | 16 (4.1%) | 41 (5.7%) | 0.2672 | |
| CKD stage | 1 | 41 (10.54%) | 42 (5.82%) | <0.001 |
| 2 | 139 (35.73%) | 204 (28.25%) | ||
| 3a | 104 (26.74%) | 179 (24.79%) | ||
| 3b | 71 (18.25%) | 194 (26.87%) | ||
| 4 | 24 (6.17%) | 71 (9.83%) | ||
| 5 | 8 (2.06%) | 27 (3.74%) | ||
| Missing/Unknown | 2 (0.51%) | 5 (0.69%) | ||
| GFR, (*) mean; (SD) | 58.806; (22.6158) | 52.651; (22.175) | <0.001 | |
| Diabetes mellitus | 128 (32.9%) | 209 (28.95%) | 0.171 | |
| COPD | 81 (20.82%) | 156 (21.61%) | 0.761 | |
| Asthma | 41 (10.54%) | 72 (9.97%) | 0.765 | |
| Hyperuricemia | 47% (12.08%) | 112 (15.51%) | 0.119 | |
| Dyslipidemia | 278 (71.47%) | 446 (61.77%) | 0.001 | |
| High LDLc levels Missing/Unknown | 222 (57.07%) 1 (0.26%) | 379 (52.49%) 1 (0.14%) | 0.138 | |
| Low HDLc levels Missing/Unknown | 122 (31.36%) 1 (0.26%) | 228 (31.58) 1 (0.14%) | 0.951 | |
| Hypercholesterolemia Missing/Unknown | 269 (69.15%) 1 (0.26%) | 440 (60.94%) 1 (0.14%) | 0.006 | |
| Hypertriglyceridemia Missing/Unknown | 189 (48.59%) 1 (0.26) | 325 (45.01%) 1 (0.14%) | 0.247 | |
| Group 1 Paroxysmal AF (N = 389) | Group 2 Permanent AF (N = 722) | p-Value | |
|---|---|---|---|
| Stroke Missing/Unknown | 104 (26.74%) 1 (0.26%) | 351 (48.75%) 1 (0.14%) | <0.001 |
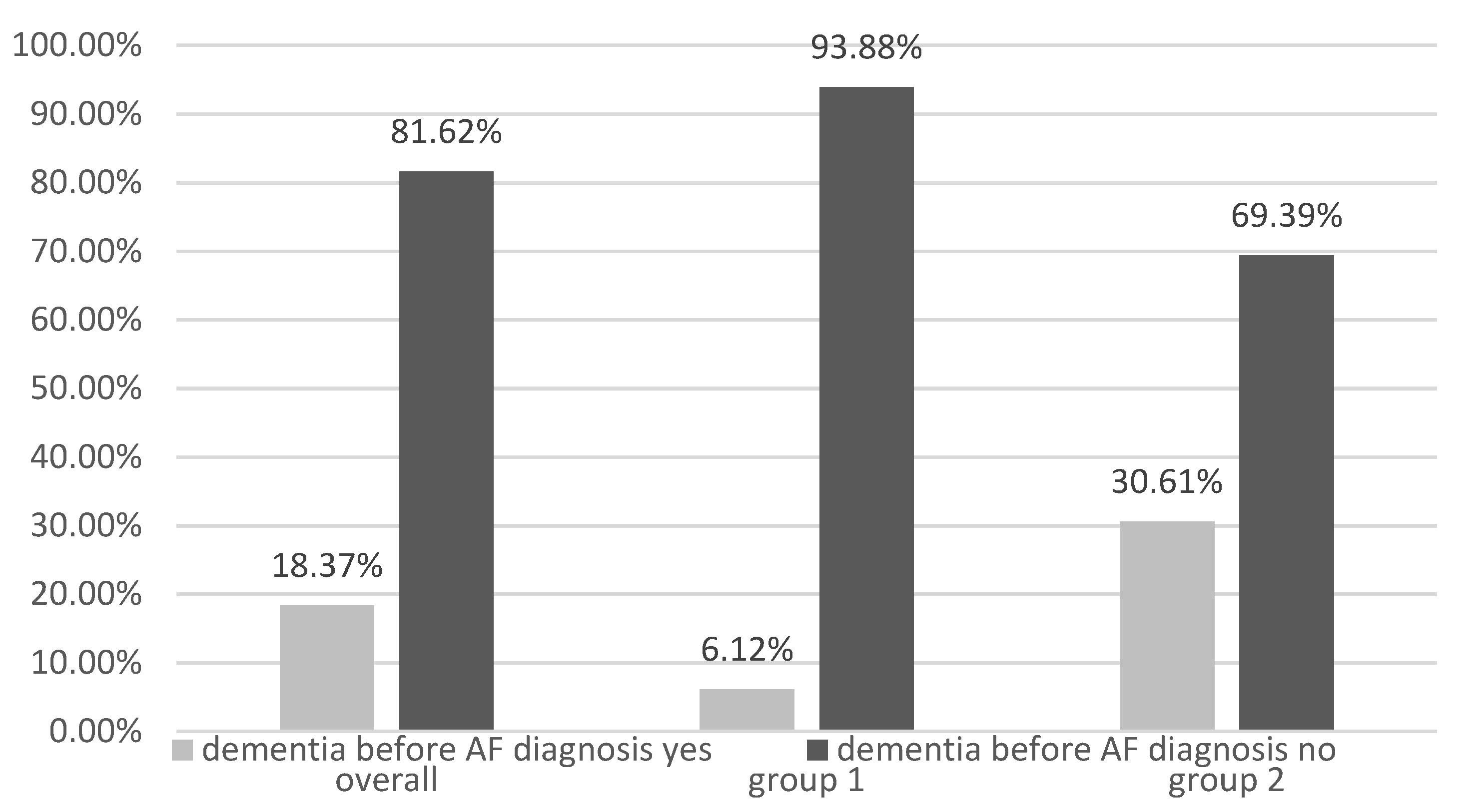
| Dementia | 15 (3.86%) | 74 (10.25%) | <0.001 |
| Parkinson’s disease | 14 (3.6%) | 43 (5.96%) | 0.089 |
| Variables in the Equation | B | S.E. | Wald | df | Sig. | Exp(B) | 95% C.I. for EXP(B) | |
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Overweight | −0.674 | 0.600 | 1.261 | 1 | 0.261 | 0.510 | 0.157 | 1.652 |
| Obesity | 1.219 | 0.635 | 3.684 | 1 | 0.045 | 3.383 | 1.975 | 11.746 |
| Constant | −2.331 | 1.251 | 3.471 | 1 | 0.062 | 0.097 | ||
| Variables in the Equation | B | S.E. | Wald | df | Sig. | Exp(B) | 95% C.I. for EXP(B) | |
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Overweight | 0.144 | 0.308 | 0.217 | 1 | 0.642 | 1.154 | 0.631 | 2.113 |
| Obesity | 0.464 | 0.335 | 1.915 | 1 | 0.166 | 1.590 | 0.825 | 3.065 |
| Constant | −0.237 | 0.762 | 0.096 | 1 | 0.756 | 0.789 | ||
| Group 1 Paroxysmal AF N (%) * | Group 2 Permanent AF N (%) * | OR (95% CI) Group 1 Group 2 | p-Value | |
|---|---|---|---|---|
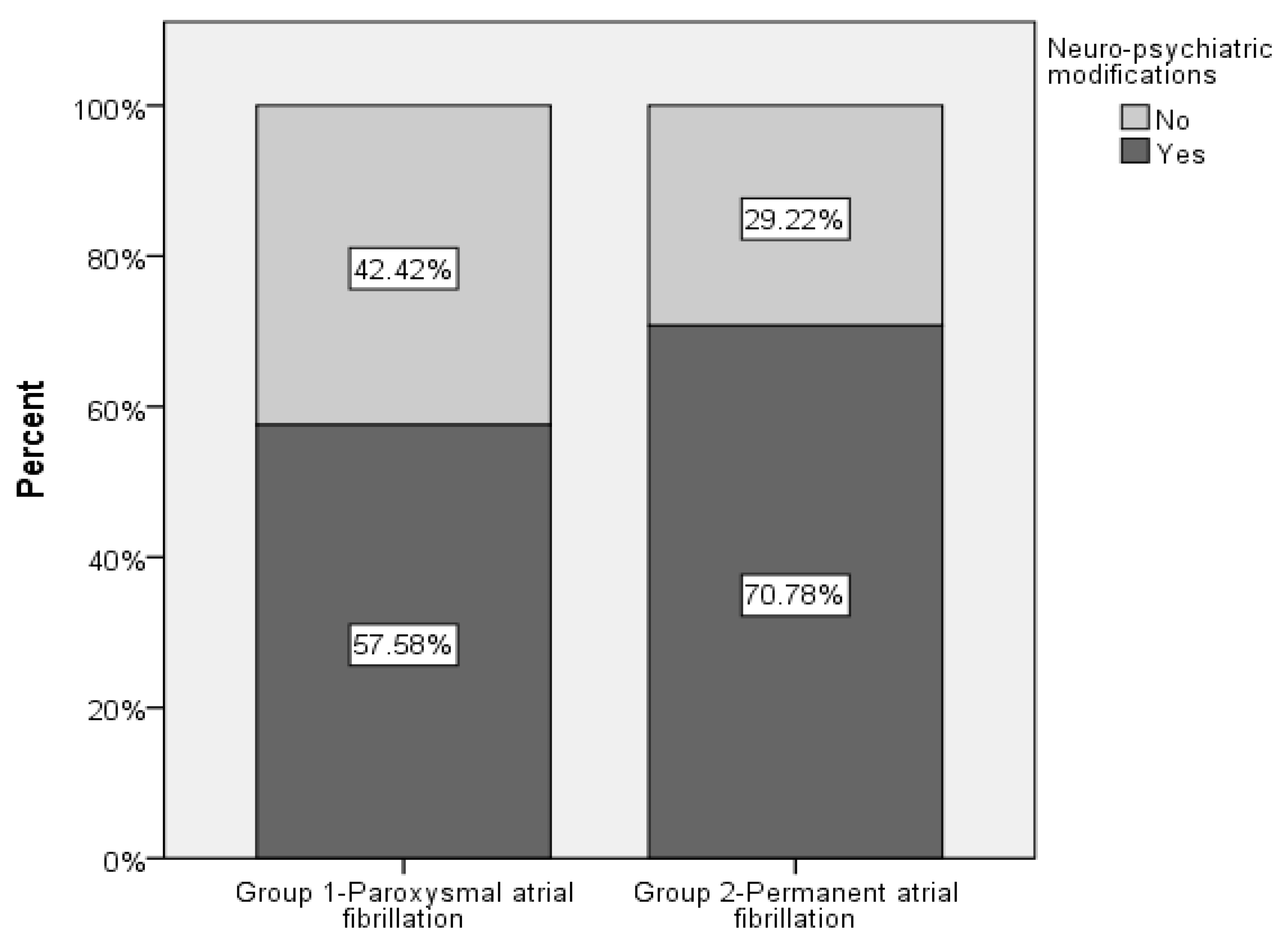
| Neuro-psychiatric changes | 298 (26.82%) | 598 (53.65%) | 1.848 (1.373; 2.486) 1 | <0.001 |
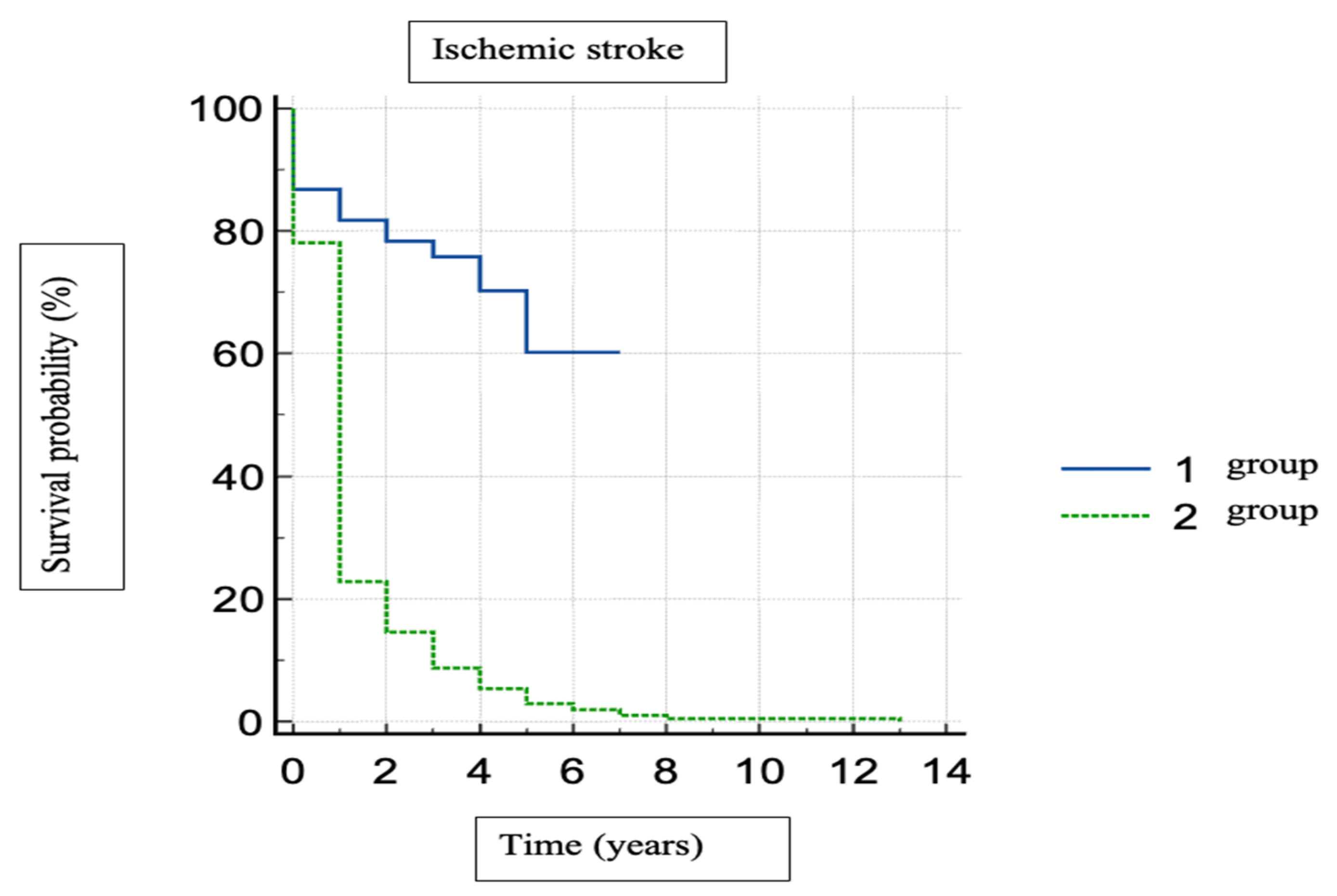
| Stroke | 49 (4.41%) | 266 (23.94%) | 3.773 (2.662; 5.348) 1 | <0.001 |
| Dementia | 12 (1.08%) | 59 (5.31%) | 1.900 (0.992; 3.641) 1 | 0.053 |
| Patients with Neuro-Psychiatric Changes N (%) * | OR (95% CI) | p-Value | |
|---|---|---|---|
| AF type Group 1—Paroxysmal AF Group 2—Permanent AF | 298 (26.82%) 596 (53.65%) | 1.848 (1.373; 2.486) 1 | <0.001 |
| Gender Female Male | 447 (40.23%) 447 (40.23%) | 1 1.657 (1.250; 2.197) | <0.001 |
| Age (years) | 894 (80.47%) | 1.041 (1.027; 1.056) | <0.001 |
| Urban (city/town) Rural (village) | 452 (40,68%) 442 (39.78%) | 0.8371 (0.5194; 1.3492) | 0.4653 |
| CKD | 894 (80.47%) | 1.0544 (0.8460; 1.3141) | 0.6371 |
| Dyslipidemia | 894 (80.47%) | 1.2068 (0.6320; 2.3045) | 0.5690 |
| Hypercholesterolemia | 894 (80.47%) | 0.7352 (0.3820; 1.4149) | 0.3570 |
| Patients with Stroke N (%) * | OR (95% CI) | p-Value | |
|---|---|---|---|
| FIA type Group 1—Paroxysmal AF Group 2—Permanent AF | 49 (4.41%) 266 (23.94%) | 3.773 (2.662; 5.348) 1 | <0.001 |
| Age (years) | 315 (28.35%) | 1.029 (1.014; 1.044) | <0.001 |
| Patients with Stroke N (%) * | OR (95% CI) | p-Value | |
|---|---|---|---|
| FIA type Group 1—Paroxysmal AF Group 2—Permanent AF | 12 (1.08%) 59 (5.31%) | 1.900 (0.992; 3.641) 1 | <0.053 |
| Age (years) | 71 (6.39%) | 1.088 (1.056; 1.122) | <0.001 |
| With OAC | Without OAC | ||||||
|---|---|---|---|---|---|---|---|
| Group 1 | Group 2 | p | Group 1 | Group 2 | p | ||
| NS | no | N = 64(38.68%) | N = 134(29.37%) | p = 0.000088 | N = 100(43.82%) | N = 78(29.23%) | p = 0.308255 |
| yes | N = 85(61.32%) | N = 377(70.63%) | N = 140(56.18%) | N = 133(70.77%) | |||
| Stroke | no | N = 107(75.18%) | N = 259(44.37%) | p = <0.00001 | N = 178(72.11%) | N = 112(53.30%) | p < 0.00001 |
| yes | N = 42(24.82%) | N = 252(55.63%) | N = 62(27.89%) | N = 99(46.70%) | |||
| Dementia | no | N = 147(93.43%) | N = 462(85.62%) | p = 0.014293 | N = 227(97.60%) | N = 186(91.08%) | p = 0.014146 |
| yes | N = 2(6.57%) | N = 33(14.38%) | N = 13(2.30%) | N = 25(8.92%) | |||
| Parkinson’s disease | no | N = 145(95.62%) | N = 478(94.37%) | p = 0.078101 | N = 230(96.81%) | N = 201(93.93%) | p = 0.768168 |
| yes | N = 4(4.38%) | N = 33(5.63%) | N = 10(3.19%) | N = 10(6.07%) | |||
| Group 1 with OAC | Group 1 without OAC | p | Group 2 with OAC | Group 2 without OAC | p | ||
|---|---|---|---|---|---|---|---|
| NS | no | N = 64(38.68%) | N = 100(43.82%) | p = 0.802774 | N = 134(29.37%) | N = 78(29.23%) | p = 0.003941 |
| yes | N = 85(61.32%) | N = 140(56.18%) | N = 377(70.63%) | N = 133(70.77%) | |||
| Stroke | no | N = 107(75.18%) | N = 178(72.11%) | p = 0.609987 | N = 259(44.37%) | N = 112(53.30%) | p = 0.558054 |
| yes | N = 42(24.82%) | N = 62(27.89%) | N = 252(55.63%) | N = 99(46.70%) | |||
| Dementia | no | N = 147(93.43%) | N = 227(97.60%) | p = 0.042472 | N = 462(85.62%) | N = 186(91.08%) | p < 0.00001 |
| yes | N = 2(6.57%) | N = 13(2.30%) | N = 33(14.38%) | N = 25(8.92%) | |||
| Parkinson’s disease | no | N = 145(95.62%) | N = 230(96.81%) | p = 0.445518 | N = 478(94.37%) | N = 201(93.93%) | p = 0.374859 |
| yes | N = 4(4.38%) | N = 10(3.19%) | N = 33(5.63%) | N = 10(6.07%) |
| Total Population (N = 1111) (N * = 167) | Group 1—Paroxysmal AF (N = 389) (N * = 63) | Group 2—Permanent AF (N = 722) (N * = 104) | p-Value | |
|---|---|---|---|---|
| Stroke No Yes Missing/Unknown | 654 (58.87%) 455 (40.95%) 2 (0.18%) | 284 (73.01%) 104 (26.74%) 1 (0.26) | 370 (51.25%) 351 (48.75%) 1 (0.14%) | p < 0.001 |
| Stroke before AF diagnosis No Yes | 27 (16.17%) 140 (83.83%) | 8 (12.70%) 55 (87.30%) | 19 (18.27%) 85 (81.73%) | p = 0.343 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosca, C.I.; Sharma, A.; Nisulescu, D.-D.; Otiman, G.; Duda-Seiman, D.-M.; Morariu, S.I.; Lighezan, D.F.; Kundnani, N.R. Prevalence of Cardio-Embolic Brain Complications in Permanent and Paroxysmal Atrial Fibrillation Patients. Healthcare 2023, 11, 175. https://doi.org/10.3390/healthcare11020175
Rosca CI, Sharma A, Nisulescu D-D, Otiman G, Duda-Seiman D-M, Morariu SI, Lighezan DF, Kundnani NR. Prevalence of Cardio-Embolic Brain Complications in Permanent and Paroxysmal Atrial Fibrillation Patients. Healthcare. 2023; 11(2):175. https://doi.org/10.3390/healthcare11020175
Chicago/Turabian StyleRosca, Ciprian Ilie, Abhinav Sharma, Daniel-Dumitru Nisulescu, Gabriela Otiman, Daniel-Marius Duda-Seiman, Stelian Ioan Morariu, Daniel Florin Lighezan, and Nilima Rajpal Kundnani. 2023. "Prevalence of Cardio-Embolic Brain Complications in Permanent and Paroxysmal Atrial Fibrillation Patients" Healthcare 11, no. 2: 175. https://doi.org/10.3390/healthcare11020175
APA StyleRosca, C. I., Sharma, A., Nisulescu, D.-D., Otiman, G., Duda-Seiman, D.-M., Morariu, S. I., Lighezan, D. F., & Kundnani, N. R. (2023). Prevalence of Cardio-Embolic Brain Complications in Permanent and Paroxysmal Atrial Fibrillation Patients. Healthcare, 11(2), 175. https://doi.org/10.3390/healthcare11020175










