Low Emulsifier Diet in Healthy Female Adults: A Feasibility Study of Nutrition Education and Counseling Intervention
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Study Design
2.3. Baseline Demographic and Dietary Intake Data
2.4. Nutrition Education and Counseling
2.5. Outcome Assessment
2.6. Dietary Emulsifier Intake Assessment
2.7. Ethical Approval
2.8. Statistical Analysis
3. Results
3.1. Characteristics of Study Participants
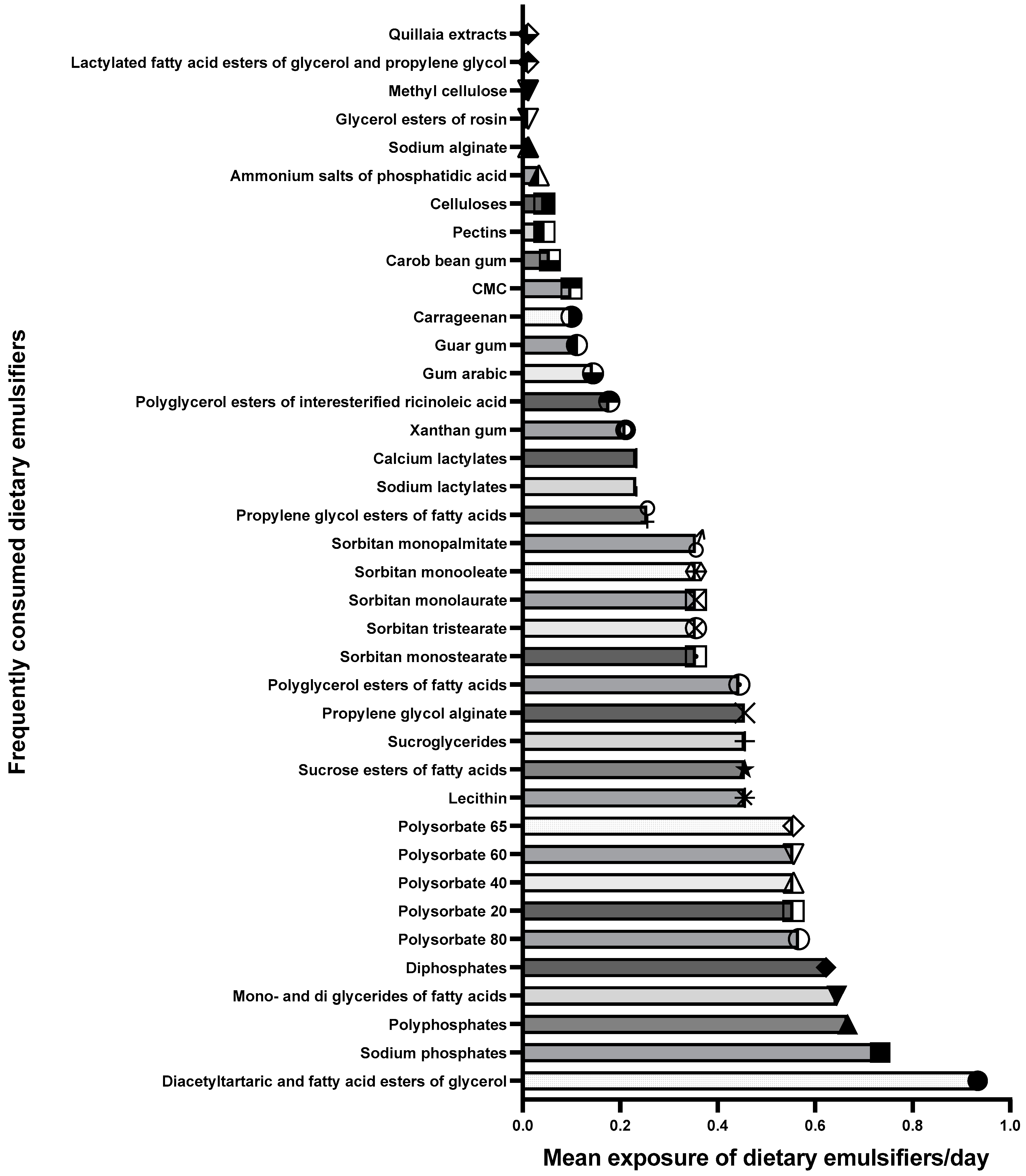
3.2. Dietary Emulsifier Intake at Baseline
3.3. Main Food Groups Contributing to Emulsifier Intake at Baseline
3.4. Effect of the Low-Emulsifier Diet on Dietary Emulsifier Intake
3.5. Effect of the Low-Emulsifier Diet on Nutrient Intake
3.6. Adherence and Acceptability of the Low-Emulsifier Diet
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roberts, C.L.; Rushworth, S.L.; Richman, E.; Rhodes, J.M. Hypothesis: Increased consumption of emulsifiers as an explanation for the rising incidence of Crohn’s disease. J Crohn’s Colitis 2013, 7, 338–341. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.; Sandall, A.; Smith, L.; Rossi, M.; Whelan, K. Food additive emulsifiers: A review of their role in foods, legislation and classifications, presence in food supply, dietary exposure, and safety assessment. Nutr. Rev. 2021, 79, 726–741. [Google Scholar] [CrossRef] [PubMed]
- FAO; WHO. Alimentarius: International Food Standards; Class Names and the International Numbering System for Food Additives, Report No. Cac/gl 36-1989; FAO: Rome, Italy, 2017. [Google Scholar]
- Food Standards Agency. Approved Additives and e Numbers. 2020. Available online: https://www.food.gov.uk/business-guidance/approved-additives-and-e-numbers (accessed on 5 May 2021).
- FDA. Overview of Food Ingredients, Additives, & Colors. U.S. Food & Drug Administration. 2018. Available online: https://www.fda.gov/food/food-ingredients-packaging/overview-food-ingredients-additives-colors#foodadd (accessed on 5 May 2021).
- Laster, J.; Bonnes, S.L.; Rocha, J. Increased use of emulsifiers in processed foods and the links to obesity. Curr. Gastroenterol. Rep. 2019, 21, 61. [Google Scholar] [CrossRef] [PubMed]
- Carocho, M.; Barreiro, M.F.; Morales, P.; Ferreira, I. Adding molecules to food, pros and cons: A review on synthetic and natural food additives. Compr. Rev. Food Sci. Food Saf. 2014, 13, 377–399. [Google Scholar] [CrossRef]
- Chassaing, B.; Koren, O.; Goodrich, J.K.; Poole, A.C.; Srinivasan, S.; Ley, R.E.; Gewirtz, A.T. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 2015, 519, 92–96. [Google Scholar] [CrossRef]
- Shang, Q.; Sun, W.; Shan, X.; Jiang, H.; Cai, C.; Hao, J.; Li, G.; Yu, G. Carrageenan-induced colitis is associated with decreased population of anti-inflammatory bacterium, Akkermansia muciniphila, in the gut microbiota of c57bl/6j mice. Toxicol. Lett. 2017, 279, 87–95. [Google Scholar] [CrossRef]
- Bancil, A.S.; Sandall, A.M.; Rossi, M.; Chassaing, B.; Lindsay, J.O.; Whelan, K. Food additive emulsifiers and their impact on gut microbiome, permeability, and inflammation: Mechanistic insights in inflammatory bowel disease. J. Crohn’s Colitis 2021, 15, 1068–1079. [Google Scholar] [CrossRef]
- De Siena, M.; Raoul, P.; Costantini, L.; Scarpellini, E.; Cintoni, M.; Gasbarrini, A.; Rinninella, E.; Mele, M.C. Food emulsifiers and metabolic syndrome: The role of the gut microbiota. Foods 2022, 11, 2205. [Google Scholar] [CrossRef]
- Olendzki, B.C.; Silverstein, T.D.; Persuitte, G.M.; Ma, Y.; Baldwin, K.R.; Cave, D. An anti-inflammatory diet as treatment for inflammatory bowel disease: A case series report. Nutr. J. 2014, 13, 5. [Google Scholar] [CrossRef]
- Mirmiran, P.; Moslehi, N.; Morshedzadeh, N.; Shivappa, N.; Hébert, J.R.; Farsi, F.; Daryani, N.E. Does the inflammatory potential of diet affect disease activity in patients with inflammatory bowel disease? Nutr. J. 2019, 18, 65. [Google Scholar] [CrossRef]
- Sabino, J.; Lewis, J.D.; Colombel, J.F. Treating inflammatory bowel disease with diet: A taste test. Gastroenterology 2019, 157, 295–297. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Ananthakrishnan, A.N. New approaches along the ibd course: Diet, tight control and stem cells. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 82–84. [Google Scholar] [CrossRef] [PubMed]
- Sandall, A.M.; Cox, S.R.; Lindsay, J.O.; Gewirtz, A.T.; Chassaing, B.; Rossi, M.; Whelan, K. Emulsifiers impact colonic length in mice and emulsifier restriction is feasible in people with crohn’s disease. Nutrients 2020, 12, 2827. [Google Scholar] [CrossRef] [PubMed]
- Staudacher, H.M.; Irving, P.M.; Lomer, M.C.E.; Whelan, K. The challenges of control groups, placebos and blinding in clinical trials of dietary interventions. Proc. Nutr. Soc. 2017, 76, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, G.A.; Dodd, S.; Williamson, P.R. Design and analysis of pilot studies: Recommendations for good practice. J. Eval. Clin. Pract. 2004, 10, 307–312. [Google Scholar] [CrossRef]
- Julious, S.A. Sample size of 12 per group rule of thumb for a pilot study. Pharm. Stat. J. Appl. Stat. Pharm. Ind. 2005, 4, 287–291. [Google Scholar] [CrossRef]
- Fallaize, R.; Franco, R.Z.; Hwang, F.; Lovegrove, J.A. Evaluation of the enutri automated personalised nutrition advice by users and nutrition professionals in the UK. PLoS ONE 2019, 14, e0214931. [Google Scholar] [CrossRef]
- Alvarez, J.; Mellor, D. A critical review of primary school meal provision in the UK and Spain. J. Hum. Nutr. Diet. 2016, 29, 40–63. [Google Scholar]
- Mumena, W.A.; Ateek, A.A.; Alamri, R.K.; Alobaid, S.A.; Alshallali, S.H.; Afifi, S.Y.; Aljohani, G.A.; Kutbi, H.A. Fast-food consumption, dietary quality, and dietary intake of adolescents in Saudi Arabia. Int. J. Environ. Res. Public Health 2022, 19, 5083. [Google Scholar] [CrossRef]
- Lee, D.; Swan, C.K.; Suskind, D.; Wahbeh, G.; Vanamala, J.; Baldassano, R.N.; Leonard, M.B.; Lampe, J.W. Children with crohn’s disease frequently consume select food additives. Dig. Dis. Sci. 2018, 63, 2722–2728. [Google Scholar] [CrossRef]
- Chazelas, E.; Druesne-Pecollo, N.; Esseddik, Y.; de Edelenyi, F.S.; Agaesse, C.; De Sa, A.; Lutchia, R.; Rebouillat, P.; Srour, B.; Debras, C.; et al. Exposure to food additive mixtures in 106,000 french adults from the nutrinet-sante cohort. Sci. Rep. 2021, 11, 19680. [Google Scholar] [CrossRef]
- GSFA. Codex General Standard for Food Additives (gsfa) Online Database. 2019. Available online: https://www.fao.org/gsfaonline/index.html (accessed on 5 May 2021).
- Halmos, E.P.; Mack, A.; Gibson, P.R. Review article: Emulsifiers in the food supply and implications for gastrointestinal disease. Aliment. Pharmacol. Ther. 2019, 49, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Aljobair, M.O. Assessment of the bread consumption habits among the people of Riyadh, Saudi Arabia. Pak. J. Nutr. 2017, 16, 293–298. [Google Scholar]
- Nazarenkov, N.; Seeger, K.; Beeken, L.; Ananthakrishnan, A.N.; Khalili, H.; Lewis, J.D.; Konijeti, G.G. Implementing dietary modifications and assessing nutritional adequacy of diets for inflammatory bowel disease. Gastroenterol. Hepatol. 2019, 15, 133–144. [Google Scholar]
- Kalmpourtzidou, A.; Xinias, I.; Agakidis, C.; Mavroudi, A.; Mouselimis, D.; Tsarouchas, A.; Agakidou, E.; Karagiozoglou-Lampoudi, T. Diet quality: A neglected parameter in children with food allergies. A cross-sectional study. Front. Pediatr. 2021, 9, 658778. [Google Scholar] [CrossRef] [PubMed]
- Weaver, C.M.; Dwyer, J.; Fulgoni, V.L., III; King, J.C.; Leveille, G.A.; MacDonald, R.S.; Ordovas, J.; Schnakenberg, D. Processed foods: Contributions to nutrition. Am. J. Clin. Nutr. 2014, 99, 1525–1542. [Google Scholar] [CrossRef]
- Macdiarmid, J.; Blundell, J. Assessing dietary intake: Who, what and why of under-reporting. Nutr. Res. Rev. 1998, 11, 231–253. [Google Scholar] [CrossRef]
- Walker, J.L.; Ardouin, S.; Burrows, T. The validity of dietary assessment methods to accurately measure energy intake in children and adolescents who are overweight or obese: A systematic review. Eur. J. Clin. Nutr. 2018, 72, 185–197. [Google Scholar] [CrossRef]
- Worsley, A. Nutrition knowledge and food consumption: Can nutrition knowledge change food behaviour? Asia Pac. J. Clin. Nutr. 2002, 11, S579–S585. [Google Scholar] [CrossRef]
- Taylor, R.C. Alternative medicine and the medical encounter in Britain and the united states. In Alternative Medicines; Routledge: London, UK, 2022; pp. 191–228. [Google Scholar]
- Behrman, G.; Tebb, S. The use of complementary and alternative interventions as a holistic approach with older adults. J. Relig. Spiritual. Soc. Work. Soc. Thought 2009, 28, 127–140. [Google Scholar] [CrossRef]
- Frobisher, C.; Maxwell, S. The estimation of food portion sizes: A comparison between using descriptions of portion sizes and a photographic food atlas by children and adults. J. Hum. Nutr. Diet. 2003, 16, 181–188. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | n = 30 |
|---|---|
| Sex, n (%): Female | 30 (100%) |
| Age (year), mean ± SD | 22.9 ± 5.84 |
| Height (cm), mean ± SD | 162.16 ± 6.75 |
| Weight (kg), mean ± SD | 61.53 ± 12.00 |
| Region | |
| Riyadh | 3 (10.00%) |
| Makkah | 27 (90.00%) |
| Occupation | |
| Employed, n (%) | 5 (16.66%) |
| Bachelor student, n (%) | 19 (63.32%) |
| Housewife, n (%) | 3 (10.00%) |
| Unemployed, n (%) | 3 (10.00%) |
| Main Food Group | CODEX Food Category | CODEX Number | Contribution to Baseline Emulsifier Intake, n (%) * | Examples |
|---|---|---|---|---|
| Confectionery (8.9%) | Chocolate-based spreads | 05.1.3 | 1 (0.5%) | Nutella |
| Chocolate sweet products | 05.1.4 | 15 (7%) | Twix | |
| Chewing gum | 5.3 | 3 (1.4%) | Extra gum | |
| Dairy products (28.6%) | Fluid milk | 01.1.1 | 5 (2.3%) | Milk (long-life) |
| Flavored fluid milk drinks | 01.1.4 | 2 (0.9%) | Strawberry milkshake | |
| Condensed milk | 01.3.1 | 2 (0.9%) | Condensed milk | |
| Milk powder | 01.5.1 | 8 (3.8%) | Powdered milk | |
| Processed cream | 01.4.2 | 3 (1.4%) | Whipping cream | |
| Processed cream/clotted cream | 01.4.3 | 2 (0.9%) | Sour cream | |
| Dairy-based desserts | 1.7 | 3 (1.4%) | Greek yogurt berries flavor | |
| Processed cheese | 01.6.4 | 20 (9.4%) | Cheese slices and spreads | |
| Un-ripened cheese | 01.6.1 | 14 (6.6%) | Mozzarella and halloumi | |
| Ripened cheese | 01.6.2.1 | 1 (0.5%) | Cheddar | |
| Butter | 02.2.1 | 1 (0.5%) | Butter | |
| Beverages (8.5%) | Coffee, tea, and their substitutes | 14.1.5 | 4 (1.9%) | 2 in 1 coffee Nescafe |
| Sport and carbonated drinks | 14.1.4 | 9 (4.2%) | Spark drink and Pepsi | |
| Diet drinks or sugar substitutes | 11.6 | 4 (1.9%) | Diet coke/Pepsi | |
| Fruit drink concentrate | 14.1.4.3 | 1 (0.5%) | Sunquick | |
| Processed meats (3.3%) | Processed meat | 08.3.2 | 3 (1.4%) | Deli turkey meat slices |
| Frozen processed meat | 08.3.3 | 4 (1.9%) | Frozen burger patties | |
| Processed vegetables and fruits (3.3%) | Vegetable and nut purees | 04.2.2.5 | 4 (1.9%) | Peanut butter and tomato paste |
| Processed fruits and products | 04.1.2 | 3 (1.4%) | Jam | |
| Bakery products (34.7%) | Breads and rolls | 07.1.1 | 54 (25.4%) | Packaged supermarket bread |
| Sweet bakery products | 07.2.2 | 5 (2.3%) | Doughnut | |
| Cakes, cookies, and pies | 07.2.1 | 15 (7%) | Digestive biscuits | |
| Other products (12.6%) | Store-bought pasta or noodles | 06.4.3 | 3 (1.4%) | Instant noodle |
| Emulsified sauces and dips | 12.6.1 | 15 (7%) | Mayonnaise | |
| Non-emulsified sauces | 12.6.2 | 4 (1.9%) | Hot sauce | |
| Chips | 15.1 | 5 (2.3%) | Lays chips |
| Emulsifiers | Baseline | Post-Intervention | p-Value |
|---|---|---|---|
| Diacetyltartaric and fatty acid esters of glycerol | 0.93 ± 0.49 | 0.44 ± 0.43 | 0.00 *** |
| Sodium phosphate | 0.73 ± 0.89 | 0.32 ± 0.49 | 0.00 ** |
| Polyphosphates | 0.66 ± 0.92 | 0.3 ± 0.50 | 0.01 ** |
| Mono and di glycerides of fatty acids | 0.64 ± 0.45 | 0.35 ± 0.38 | 0.00 ** |
| Diphosphates | 0.62 ± 0.86 | 0.32 ± 0.50 | 0.07 |
| Polyoxyethylene 20 sorbitan monooleate (polysorbate 80) | 0.56 ± 0.62 | 0.27 ± 0.36 | 0.03 * |
| Polyoxyethylene 20 sorbitan monolaurate (polysorbate 20) | 0.55 ± 0.62 | 0.27 ± 0.36 | 0.03 * |
| Polyoxyethylene 20 sorbitan monopalmitate (polysorbate 40) | 0.55 ± 0.62 | 0.27 ± 0.36 | 0.03 * |
| Polyoxyethylene 20 sorbitan monostearate (polysorbate 60) | 0.55 ± 0.62 | 0.27 ± 0.36 | 0.03 * |
| Polyoxyethylene 20 sorbitan tristearate (polysorbate 65) | 0.55 ± 0.62 | 0.27 ± 0.36 | 0.03 * |
| Sucrose esters of fatty acids | 0.45 ± 0.53 | 0.21 ± 0.29 | 0.03 * |
| Sucroglycerides | 0.45 ± 0.53 | 0.21 ± 0.29 | 0.03 * |
| Propylene glycol alginate | 0.45 ± 0.52 | 0.24 ± 0.45 | 0.04 * |
| Lecithin | 0.45 ± 0.38 | 0.15 ± 0.31 | 0.00 ** |
| Polyglycerol esters of fatty acids | 0.44 ± 0.54 | 0.21 ± 0.29 | 0.03 * |
| Sorbitan monostearate | 0.35 ± 0.46 | 0.21 ± 0.29 | 0.15 |
| Sorbitan tristearate | 0.35 ± 0.46 | 0.21 ± 0.29 | 0.15 |
| Sorbitan monolaurate | 0.35 ± 0.46 | 0.21 ± 0.29 | 0.15 |
| Sorbitan monooleate | 0.35 ± 0.46 | 0.21 ± 0.29 | 0.15 |
| Sorbitan monopalmitate | 0.35 ± 0.46 | 0.21 ± 0.29 | 0.15 |
| Propylene glycol esters of fatty acids | 0.25 ± 0.41 | 0.20 ± 0.28 | 0.67 |
| Sodium lactylates | 0.23 ± 0.30 | 0.14 ± 0.27 | 0.25 |
| Calcium lactylates | 0.23 ± 0.30 | 0.14 ± 0.27 | 0.25 |
| Xanthan gum | 0.21 ± 0.39 | 0.13 ± 0.33 | 0.28 |
| Polyglycerol esters of interesterified ricinoleic acid | 0.17 ± 0.28 | 0.07 ± 0.22 | 0.11 |
| Gum arabic | 0.14 ± 0.28 | 0.01 ± 0.06 | 0.01 ** |
| Guar gum | 0.11 ± 0.23 | 0.04 ± 0.19 | 0.22 |
| Carrageenan | 0.10 ± 0.23 | 0.05 ± 0.21 | 0.47 |
| Sodium carboxymethyl cellulose | 0.10 ± 0.21 | 0.13 ± 0.28 | 0.78 |
| Carob bean gum | 0.05 ± 0.15 | 0.06 ± 0.20 | 0.86 |
| Pectins | 0.04 ± 0.11 | 0.01 ± 0.06 | 0.18 |
| Celluloses | 0.04 ± 0.11 | 0.02 ± 0.08 | 0.41 |
| Ammonium salts of phosphatidic acid | 0.03 ± 0.10 | 0.01 ± 0.06 | 0.31 |
| Sodium alginate | 0.01 ± 0.06 | 0.01 ± 0.06 | 1.00 |
| Glycerol esters of rosin | 0.01 ± 0.06 | 0.0 ± 0.0 | 0.31 |
| Methyl cellulose | 0.01 ± 0.06 | 0.01± 0.06 | 1.00 |
| Lactylated fatty acid esters of glycerol and propylene glycol | 0.01 ± 0.06 | 0.0 ± 0.0 | 0.31 |
| Quillaia extracts | 0.01 ± 0.06 | 0.0 ± 0.0 | 0.31 |
| Total number of emulsifiers per day | 12.23 ± 10.07 | 6.30 ± 7.59 | 0.00 ** |
| Nutrient | Baseline | Post-Intervention | p-Value |
|---|---|---|---|
| Energy (kcal) | 1576.08 ± 591.84 | 1128.56 ± 402.18 | 0.01 ** |
| Protein (g) | 76.22 ± 69.76 | 55.08 ± 16.54 | 0.00 *** |
| Fat (g) | 62.88 ± 22.03 | 43.78 ± 19.88 | 0.00 *** |
| Carbohydrates (g) | 167.46 ± 65.42 | 121.42 ± 54.74 | 0.00 *** |
| Sugars (g) | 50.87 ± 23.49 | 36.62 ± 19.95 | 0.00 ** |
| Fiber (g) | 13.43 ± 5.26 | 11.92 ± 5.12 | 0.33 |
| Saturated Fat (g) | 20.35 ± 8.60 | 12.09 ± 5.86 | 0.00 *** |
| Monounsaturated Fat (g) | 18.34 ± 7.87 | 15.82 ± 9.00 | 0.10 |
| Polyunsaturated Fat (g) | 10.13 ± 6.55 | 6.91 ± 3.64 | 0.00 ** |
| Trans Fat (g) | 0.43 ± 0.30 | 0.26 ± 0.25 | 0.02 * |
| Sodium (NA) (mg) | 2141.20 ± 1236.76 | 1405.37 ± 1052.53 | 0.00 ** |
| Potassium (K) (mg) | 1802.52 ± 905.27 | 1465.54 ± 476.56 | 0.05 |
| Calcium (Ca) (mg) | 745.20 ± 728.47 | 544.52 ± 703.18 | 0.06 |
| Magnesium (Mg) (mg) | 176.58 ± 91.67 | 150.39 ± 46.40 | 0.11 |
| Phosphorus (P) (mg) | 841.61 ± 393.76 | 673.19 ± 206.01 | 0.03 * |
| Iron (Fe) (mg) | 10.13 ± 4.45 | 9.11 ± 3.06 | 0.31 |
| Copper (Cu) (mg) | 0.71 ± 0.31 | 0.75 ± 0.57 | 0.95 |
| Zinc (Zn) (mg) | 6.03 ± 3.34 | 5.30 ± 1.95 | 0.39 |
| Chloride (Cl) (mg) | 1099.00 ± 1391.25 | 744.70 ± 1410.56 | 0.02 * |
| Manganese (Mn) (mg) | 1.86 ± 0.78 | 1.64 ± 0.65 | 0.12 |
| Selenium (Se) (μg) | 79.26 ± 35.45 | 73.40 ± 30.17 | 0.44 |
| Iodine (I) (μg) | 8.47 ± 5.13 | 8.61 ± 4.63 | 0.24 |
| Vitamin A (Total RE) (μg) | 347.09 ± 473.88 | 372.97 ± 348.59 | 0.28 |
| Vitamin D (μg) | 1.89 ± 1.93 | 1.63 ± 1.44 | 0.22 |
| Vitamin E (mg) | 4.79 ± 3.74 | 4.99 ± 3.62 | 0.89 |
| Thiamin (B1) (mg) | 0.96 ± 0.45 | 0.99 ± 0.55 | 0.76 |
| Riboflavin (B2) (mg) | 1.10 ± 0.49 | 1.02 ± 0.48 | 0.44 |
| Niacin (preformed) (mg) | 17.90 ± 7.63 | 16.38 ± 6.22 | 0.28 |
| Pantothenate (B5) (mg) | 3.55 ± 1.60 | 3.23 ± 1.17 | 0.28 |
| Vitamin B6 (Pyridoxine) (mg) | 1.31 ± 0.74 | 1.29 ± 0.52 | 0.88 |
| Biotin (B7) (μg) | 5.99 ± 3.91 | 6.93 ± 2.77 | 0.59 |
| Folate (B9) DFE (μg) | 276 ± 164.43 | 283.20 ± 155.52 | 0.86 |
| Vitamin B12 (Cobalamin) (μg) | 2.56 ± 2.56 | 2.53 ± 3.41 | 0.55 |
| Vitamin C (mg) | 56.29 ± 46.10 | 49.35 ± 37.21 | 0.70 |
| Response, n (%) | No | Slightly | Neutral | More | Much More |
|---|---|---|---|---|---|
| Meal preparation was more difficult | 8 (26.66) | 12 (40) | 4 (13.33) | 6 (20) | 0 (0) |
| Longer time spent preparing and cooking meals | 17 (56.66) | 6 (20) | 5 (16.66) | 2 (6.66) | 0 (0) |
| Longer time spent food shopping | 11 (36.66) | 10 (33.33) | 2 (6.66) | 7 (23.33) | 0 (0) |
| Finding suitable foods when shopping was more difficult | 10 (33.33) | 7 (23.33) | 5 (16.66) | 9 (30) | 1 (3.33) |
| Finding suitable foods when eating out was more difficult | 2 (6.66) | 12 (40) | 1 (3.33) | 16 (53.33) | 4 (13.33) |
| The flavor of meals and snacks was less appetizing | 16 (53.33) | 10 (33.33) | 3 (10) | 1 (3.33) | 0 (0) |
| More money spent on food shopping and eating out | 16 (53.33) | 9 (30) | 5 (16.66) | 0 (0) | 0 (0) |
| The diet was more difficult | 11 (36.66) | 8 (26.66) | 5 (16.66) | 7 (23.33) | 0 (0) |
| Following the diet for 6–8 weeks would be more difficult than normal | 9 (30) | 9 (30) | 3 (10) | 10 (33.33) | 3 (10) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khatib, M.A.; Saleemani, H.H.; Kurdi, N.B.; Alhibshi, H.N.; Jastaniah, M.A.; Ajabnoor, S.M. Low Emulsifier Diet in Healthy Female Adults: A Feasibility Study of Nutrition Education and Counseling Intervention. Healthcare 2023, 11, 2644. https://doi.org/10.3390/healthcare11192644
Khatib MA, Saleemani HH, Kurdi NB, Alhibshi HN, Jastaniah MA, Ajabnoor SM. Low Emulsifier Diet in Healthy Female Adults: A Feasibility Study of Nutrition Education and Counseling Intervention. Healthcare. 2023; 11(19):2644. https://doi.org/10.3390/healthcare11192644
Chicago/Turabian StyleKhatib, Mai A., Haneen H. Saleemani, Nersian B. Kurdi, Haya N. Alhibshi, Manar A. Jastaniah, and Sarah M. Ajabnoor. 2023. "Low Emulsifier Diet in Healthy Female Adults: A Feasibility Study of Nutrition Education and Counseling Intervention" Healthcare 11, no. 19: 2644. https://doi.org/10.3390/healthcare11192644
APA StyleKhatib, M. A., Saleemani, H. H., Kurdi, N. B., Alhibshi, H. N., Jastaniah, M. A., & Ajabnoor, S. M. (2023). Low Emulsifier Diet in Healthy Female Adults: A Feasibility Study of Nutrition Education and Counseling Intervention. Healthcare, 11(19), 2644. https://doi.org/10.3390/healthcare11192644





