Identifying Factors to Facilitate the Implementation of Decision-Making Tools to Promote Self-Management of Chronic Diseases into Routine Healthcare Practice: A Qualitative Study
Abstract
:1. Introduction
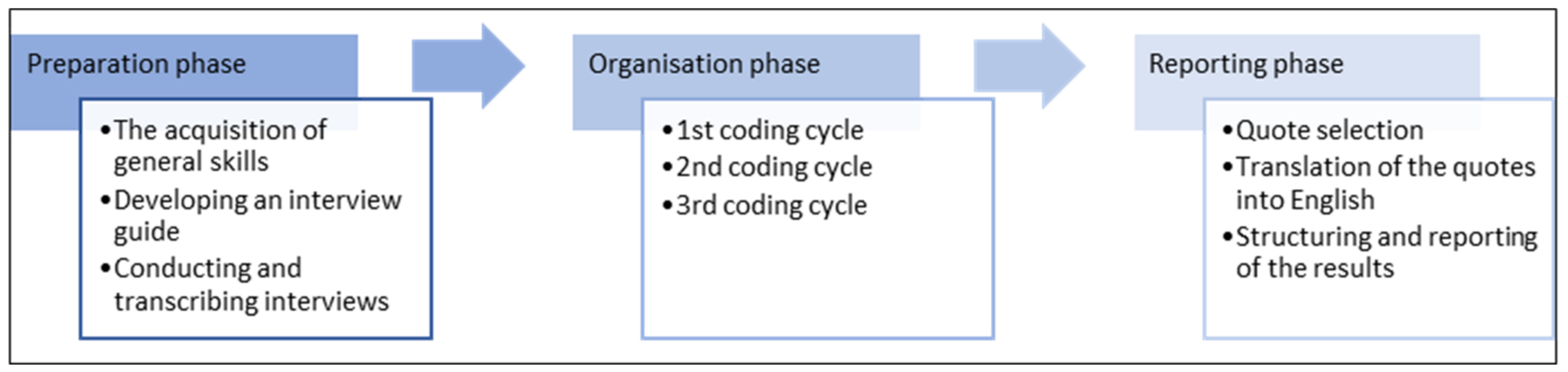
2. Materials and Methods
- Interactive Summary of Findings tables (iSoF): these presentations will provide information in different formats about the quality of evidence and magnitude of relative and absolute effects for each of the core outcomes identified;
- Evidence to Decision frameworks (EtD): using semiautomatic templates, interactive EtD frameworks will be completed for a number of priority questions that will take into account the magnitude of desirable and undesirable effects, stakeholder views on the importance of different outcomes, information on resource use and cost-effectiveness, impact on equity, and other aspects like acceptability or feasibility of the interventions. The frameworks include draft recommendations that could be then applied or adapted to different settings;
- Patient Decision Aids (PtDA) were developed in plain language for all selected situations identified in the previous phases of the study. The aids were produced in six languages (English, French, German, Spanish, Dutch, and Greek) and included evidence to guide decision-making toward patient needs.
2.1. Study Design
2.2. Setting, Sample, and Recruitment Process
2.3. Data Collection and Data Management
2.4. Qualitative Content Analysis
3. Results
3.1. Factors of Decision Tools
3.1.1. Use of Evidence
Well, for me, I think it’s easy, because of my clinical experience and years of work, you discard what you know does not have the strength of evidence and go to the consensus or recommendation system. […]. And well, I know the sources of evidence to use.(HCP 14; hospital; Spain; Row 202)
3.1.2. Existing Patients and Target Group of Patients
3.1.3. Use of Decision Tools
I don’t really know of any decision-making aids from my everyday life that would go in that direction.HCP 19; hospital; Germany; 7)
Prove that ultimately significant improvement in patient care and improvement in goal achievement, that’s point one for me, for ultimately putting that in.(DM 15; primary care; Germany; 99)
“I think leadership must be shared in this moment. I mean, in the hospital you have the head of a service, or the one who knows the most about that disease, which are units, but the patient comes from primary care, and that is, it’s been my mantra for many years. We are here to help primary care and collaborate with them because they are the ones responsible for the patients.”(DM 11; hospital; Spain; 247)
3.2. Individual Healthcare Professional Factors
3.2.1. Knowledge and Skills
“Oh, you know, I’m a chamber chairman in the district and my hobby is continuing education, continuing education of my colleagues […]. So I’m relatively fit, I get a lot of input.”(DM 12; primary care; Germany; 7)
“So first of all, they have to be so confident that they know these decision-making tools and how to use them, whatever. That they can communicate that.”(HCP 14; primary care; Germany; 87)
3.2.2. Cognitions and Attitudes
“I think it would be an innovation. So, it’s nothing that you can’t imagine as a doctor or as a patient. Such a tool is actually obvious, but although it is an obvious measure, I don’t know of any directly comparable one that is in daily use. And in this respect it is something new.”(DM 17; hospital; Germany; 43)
“In other words, feeling supported and having a script for how to do things helps. Because, at the same time, it structures the intervention. And in that way it can serve to evaluate you, to evaluate how things work. I think it’s interesting and well, come on, it’s something I always believe in. It is a methodology in which I like to work like this. Have, well, a process and see how, next step, next step, evaluation and see how it works.”(HCP 4; hospital; Spain; 296)
3.2.3. Professional Behavior
3.3. Factors of Interaction
3.3.1. Interaction with Patients
“[…] of course, people have to be a little bit interested in their own health. And be willing to change something. Because that also means a bit of work for them to register and take care of it. And yes, if they are not motivated, then it will be difficult. But I think that if they realize that they can change something and have a positive influence on the disease, then that is of course motivation enough.”(HCP 20; hospital; Germany; 31)
“[…] it would be great if you could invite them [patients] directly to a small training session, for example. Or simply distribute flyers where videos explaining the procedure can be found.”(HCP 20; hospital; Germany; 66–69)
3.3.2. Professional Interaction
“Our own colleagues from other hospitals, or another region, should explain to us the benefits that the tool brings. I think that that is the strategy we should follow. First, explain the purpose of the tool, then, have the experience of another place where we can see the health results that have been achieved with help of these tools. Show us the experience of patients that are using the tools […].”(DM 8; hospital and primary care, Spain; 270)
“I think medical directors are those who need to know the most. For his medical background, they are in contact with the heads of service, they know all the scientific commissions that depend on the medical direction. […] they can explain to us what they want to do, why, what situation we are in and what we hope to achieve with it.”(DM 11; hospital; Spain; 312)
3.3.3. Roles and Responsibilities
3.4. Organizational Factors
3.4.1. Incentives and Resources
“Often it only takes place between door and door due to time constraints. But if we had a little more time in the clinic to really have another discharge discussion with the patient, so to speak. That would also be a good moment to refer to such a program.”(DM 17; hospital; Germany; 9)
“I’m going to be very sincere; I think we are in a critical moment in primary care in all of Spain. I mean, right now we are time wasting, we have very few tools and very little time to tend to patients. […] It doesn’t have to do, maybe, with what you are asking, but you want to evaluate a strategy, where probably the system starts to break in a few years and we will do what we did 40 years ago, which is visit the patients for a few minutes and not do any of the prevention and health promotion.”(HCP 10; primary care; Spain; 165)
“And I say the other quite brutal key in medicine is a reimbursement, so whether that is paid in some form or whatever. Whether that’s somehow times-, whether that’s reimbursed in some form so to speak yes. That is certainly something that would always be a trigger or a driving effect.“(DM 18; primary care; Germany; 36)
“I would present and do it, for example, as part of cardiology or internal medicine training events, quality circles, local congresses. So that’s how medical innovations get into use.”(DM 18; primary care; Germany; 62)
3.4.2. Capacity of Organizational Change
“The middle management, which we call supervisors, have to always be in the know of anything that is being implemented, which doesn’t mean that they are the ones who take leadership in these tools, because a head of service or a middle manager, do have a very wide vision, and they have a lot of knowledge in management and activities management, and numbers, but that doesn’t always go hand in hand with leadership regarding implementation of new things.”(DM 12; hospital; Spain; 313)
“No, I don’t think it’s a high priority for now. Let’s just say that we have enough to deal with the normal challenges of everyday life. In this respect, it always has to be critically questioned.”(DM 6; primary care; Germany; 39)
3.5. Social, Political, and Legal Factors
3.5.1. Economic Constraints on the Healthcare Budget
“If [implementation of decision tools] demands costs, then that’s ultimately the responsibility of the healthcare system to implement that. In my opinion, the problem is that the healthcare system requests a lot of actions, but it is not accordingly supported.”(DM 15; primary care, Germany, 67)
3.5.2. Contracts
3.5.3. Legislation, Legal Issues, and Data Protection Policy
“The system is learning; artificial intelligence will certainly lead to them getting smarter. The databases will become larger. When we finally have electronic patient records, that will certainly be supported institutionally, perhaps also in our country. I think that evidence is coming from this area.”(DM 9; primary care; Germany, 19)
3.5.4. Influential People and Organizations
3.5.5. Healthcare System
“Based on the introduction of the DIGA [Digital healthcare applications], the interest for digital applications in healthcare will ultimately increase. And I think that in a few years, that’s going to be a help tool, especially for patients with increasing medical needs, and the shortages in medical care […]. The tool means for patients a kind of shared decision which helps them to get their treatment or achieve their goal.”(DM 15; primary care; Germany, 100–102)
3.5.6. Social Changes and Paradigm
“[…] there is still a paternalist attitude from health professionals towards patients. Patients follow physicians and nurses advises. I believe the step forward regarding patients’ participation must be undertaken.”(DM 8; hospital and primary care; Spain; 297)
3.5.7. Perspectives of Managers vs. Health Care Professionals
3.5.8. Contextualization of the Results by the Focus Group
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Birken, S.A.; Lee, S.Y.; Weiner, B.J.; Chin, M.H.; Schaefer, C.T. Improving the effectiveness of health care innovation implementation: Middle managers as change agents. Med. Care Res. Rev. 2013, 70, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Fleuren, M.; Wiefferink, K.; Paulussen, T. Determinants of innovation within health care organisations: Literature review and Delphi study. Int. J. Qual. Health Care 2004, 16, 107–123. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, M.P.; Desmartis, M.; Labrecque, M.; Car, J.; Pagliari, C.; Pluye, P.; Frémont, P.; Gagnon, J.; Tremblay, N.; Légaré, F. Systematic review of factors influencing the adoption of information and communication technologies by healthcare professionals. J. Med. Syst. 2012, 36, 241–277. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, L.; Ferlie, E.; Hawkins, C. Innovation in healthcare: How does credible evidence influence professionals? Health Soc. Care Commun. 2003, 11, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Birken, S.A.; Lee, S.Y.; Weiner, B.J.; Chin, M.H.; Chiu, M.; Schaefer, C.T. From strategy to action: How top managers’ support increases middle managers’ commitment to innovation implementation in health care organisations. Health Care Manag. Rev. 2015, 40, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Brace, C.; Schmocker, S.; Huang, H.; Victor, J.C.; McLeod, R.S.; Kennedy, E.D. Physicians’ Awareness and Attitudes Toward Decision Aids for Patients with Cancer. J. Clin. Oncol. 2010, 28, 2286–2292. [Google Scholar] [CrossRef]
- Scalia, P.; Elwyn, G.; Durand, M.A. “Provoking conversations”: Case studies of organizations where Option Grid™ decision aids have become ‘normalized’. BMC Med. Inform. Decis. Mak. 2017, 17, 124. [Google Scholar] [CrossRef]
- Hsu, C.; Liss, D.T.; Westbrook, E.O.; Arterburn, D. Incorporating Patient Decision Aids into Standard Clinical Practice in an Integrated Delivery System. Med. Decis. Mak. 2013, 33, 85–97. [Google Scholar] [CrossRef]
- Sepucha, K.R.; Simmons, L.H.; Barry, M.J.; Edgman-Levitan, S.; Licurse, A.M.; Chaguturu, S.K. Ten Years, Forty Decision Aids, and Thousands of Patient Uses: Shared Decision Making at Massachusetts General Hospital. Health Aff. 2016, 35, 630–636. [Google Scholar] [CrossRef]
- Tattersall, R.L. The expert patient: A new approach to chronic disease management for the twenty-first century. Clin. Med. 2002, 2, 227–229. [Google Scholar] [CrossRef]
- Orrego, C.; Ballester, M.; Heymans, M.; Camus, E.; Groene, O.; de Guzman, E.N.; Pardo-Hernandez, H.; Sunol, R.; COMPAR-EU Group. Talking the same language on patient empowerment: Development and content validation of a taxonomy of self-management interventions for chronic conditions. Health Expect. 2021, 24, 1626–1638. [Google Scholar] [CrossRef] [PubMed]
- Ballester, M.; Orrego, C.; Heijmans, M.; Alonso-Coello, P.; Versteegh, M.M.; Mavridis, D.; Groene, O.; Immonen, K.; Wagner, C.; Canelo-Aybar, C.; et al. Comparing the effectiveness and cost-effectiveness of self-management interventions in four high-priority chronic conditions in Europe (COMPAR-EU): A research protocol. BMJ Open 2020, 10, e034680. [Google Scholar] [CrossRef] [PubMed]
- Groene, O.; Zietzsch, P.; Krah, N.; Gilmore, K. Identifying Factors to Facilitate the Implementation of Self-Management Decision-Making Tools into Routine Healthcare Practice. Open Science Framework. 22 August 2022. Available online: https://osf.io/3k92x (accessed on 29 June 2023).
- Flottorp, S.A.; Oxman, A.D.; Krause, J.; Musila, N.R.; Wensing, M.; Godycki-Cwirko, M.; Baker, R.; Eccles, M.P. A checklist for identifying determinants of practice: A systematic review and synthesis of frameworks and taxonomies of factors that prevent or enable improvements in healthcare professional practice. Implement. Sci. 2013, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- McGreevy, J.; Orrevall, Y.; Belqaid, K.; Bernhardson, B.M. Reflections on the process of translation and cultural adaptation of an instrument to investigate taste and smell changes in adults with cancer. Scand. J. Caring Sci. 2014, 28, 204–211. [Google Scholar] [CrossRef]
- Tong, A.; Sainsbury, P.; Craig, J. Consolidated criteria for reporting qualitative research (COREQ): A 32-item checklist for interviews and focus groups. Int. J. Qual. Health Care 2007, 19, 349–357. [Google Scholar] [CrossRef]
- Hsieh, H.-F.; Shannon, S.E. Three Approaches to Qualitative Content Analysis. Qual. Health Res. 2005, 15, 1277–1288. [Google Scholar] [CrossRef]
- Gale, R.C.; Wu, J.; Erhardt, T.; Bounthavong, M.; Reardon, C.M.; Damschroder, L.J.; Midboe, A.M. Comparison of rapid vs in-depth qualitative analytic methods from a process evaluation of academic detailing in the Veterans Health Administration. Implement. Sci. 2019, 14, 11. [Google Scholar] [CrossRef]
- Assarroudi, A.; Heshmati Nabavi, F.; Armat, M.R.; Ebadi, A.; Vaismoradi, M. Directed qualitative content analysis: The description and elaboration of its underpinning methods and data analysis process. J. Res. Nurs. 2018, 23, 42–55. [Google Scholar] [CrossRef]
- Elo, S.; Kyngäs, H. The qualitative content analysis. J. Adv. Nurs. 2008, 62, 107–115. [Google Scholar] [CrossRef]
- Zhang, Y.; Wildemuth, B.M. Qualitative analysis of content. In Applications of Social Research Methods to Questions in Information and Library Science; Wildemuth, B., Ed.; Libraries Unlimited: Westport, CT, USA, 2009; pp. 308–319. [Google Scholar]
- Mayring, P.H. Qualitative Content Analysis. A Step-by-Step Guide; London Sage: London, UK, 2021. [Google Scholar]
- Légaré, F.; Witteman, H.O. Shared Decision Making: Examining Key Elements and Barriers to Adoption into Routine Clinical Practice. Health Aff. 2013, 32, 276–284. [Google Scholar] [CrossRef]
- Jackson, T.; Shields, M.D.; Heaney, L.G.; Kendall, M.; Pearce, C.J.; Hui, C.Y.; Pinnock, H. The Impact of Financial Incentives on the Implementation of Asthma or Diabetes Self-Management: A Systematic Review. PLoS ONE 2017, 20, e0187478. [Google Scholar] [CrossRef] [PubMed]
- Elwyn, G.; Scholl, I.; Tietbohl, C.; Mann, M.; Edwards, A.G.; Clay, C.; Légaré, F.; van der Weijden, T.; Lewis, C.L.; Wexler, R.M.; et al. “Many Miles to Go …”: A Systematic Review of the Implementation of Patient Decision Support Interventions into Routine Clinical Practice. BMC Med. Inform. Decis. Mak. 2013, 13, S14. [Google Scholar] [CrossRef]
- Lin, G.A.; Halley, M.; Rendle, K.A.; Tietbohl, C.; May, S.G.; Trujillo, L.; Frosch, D.L. An Effort to Spread Decision Aids In Five California Primary Care Practices Yielded Low Distribution, Highlighting Hurdles. Health Aff. 2013, 32, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Van Tol-Geerdink, J.J.; van Oort, I.M.; Somford, D.M.; Wijburg, C.J.; Geboers, A.; van Uden-Kraan, C.F.; de Vries, M.; Stalmeier, P.F. Implementation of a Decision Aid for Localized Prostate Cancer in Routine Care: A Successful Implementation Strategy. Health Inform. J. 2020, 26, 1194–1207. [Google Scholar] [CrossRef] [PubMed]
- Joseph-Williams, N.; Lloyd, A.; Edwards, A.; Stobbart, L.; Tomson, D.; Macphail, S.; Dodd, C.; Brain, K.; Elwyn, G.; Thomson, R. Implementing Shared Decision Making in the NHS: Lessons from the MAGIC Programme. BMJ 2017, 357, j1744. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. Shared Decision Making. Available online: https://www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/nice-guidelines/shared-decision-making (accessed on 29 June 2023).
- Heidenreich, A.; Bastian, P.J.; Bellmunt, J.; Bolla, M.; Joniau, S.; van der Kwast, T.; Mason, M.; Matveev, V.; Wiegel, T.; Zattoni, F.; et al. EAU Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent—Update 2013. Eur. Urol. 2014, 65, 124–137. [Google Scholar] [CrossRef]
| Demographics | Categories | n (%) |
|---|---|---|
| Country | Germany Spain | 20 (54) 17 (46) |
| Gender | female | 18 (49) |
| male | 19 (51) | |
| Age | 32–45 | 11 (30) |
| 46–55 | 12 (32) | |
| 56–65 | 14 (38) | |
| Role | healthcare professional | 17 (46) |
| decision-maker | 20 (54) | |
| Institution | hospital | 19 (51) |
| special care | 4 (11) | |
| primary care | 14 (38) |
| Factors | Key Topics from the Focus Group Discussion |
|---|---|
| Factors of decision tools |
|
| Factors of interaction | Role of clinical leaders
|
| Individual healthcare professional factors |
|
| Organizational factors | Key: Alignment with organizational priorities
|
| Social, political, and legal factors |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krah, N.S.; Zietzsch, P.; Salrach, C.; Toro, C.A.; Ballester, M.; Orrego, C.; Groene, O. Identifying Factors to Facilitate the Implementation of Decision-Making Tools to Promote Self-Management of Chronic Diseases into Routine Healthcare Practice: A Qualitative Study. Healthcare 2023, 11, 2397. https://doi.org/10.3390/healthcare11172397
Krah NS, Zietzsch P, Salrach C, Toro CA, Ballester M, Orrego C, Groene O. Identifying Factors to Facilitate the Implementation of Decision-Making Tools to Promote Self-Management of Chronic Diseases into Routine Healthcare Practice: A Qualitative Study. Healthcare. 2023; 11(17):2397. https://doi.org/10.3390/healthcare11172397
Chicago/Turabian StyleKrah, Nina Sofie, Paula Zietzsch, Cristina Salrach, Cecilia Alvarez Toro, Marta Ballester, Carola Orrego, and Oliver Groene. 2023. "Identifying Factors to Facilitate the Implementation of Decision-Making Tools to Promote Self-Management of Chronic Diseases into Routine Healthcare Practice: A Qualitative Study" Healthcare 11, no. 17: 2397. https://doi.org/10.3390/healthcare11172397
APA StyleKrah, N. S., Zietzsch, P., Salrach, C., Toro, C. A., Ballester, M., Orrego, C., & Groene, O. (2023). Identifying Factors to Facilitate the Implementation of Decision-Making Tools to Promote Self-Management of Chronic Diseases into Routine Healthcare Practice: A Qualitative Study. Healthcare, 11(17), 2397. https://doi.org/10.3390/healthcare11172397






