Living Alone, Physical Health, and Mortality in Breast Cancer Survivors: A Prospective Observational Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Assessment of Living Alone Status and Physical Health
2.3. Assessment of Study Outcome
2.4. Assessments of Covariates
2.5. Statistical Analyses
3. Results
3.1. Baseline Characteristics
3.2. Baseline Characteristics Stratified by Physical Function Score and Living Arrangement
3.3. Independent Associations of Living Alone, Physical Function, and Other Physical Health Scores with Mortality
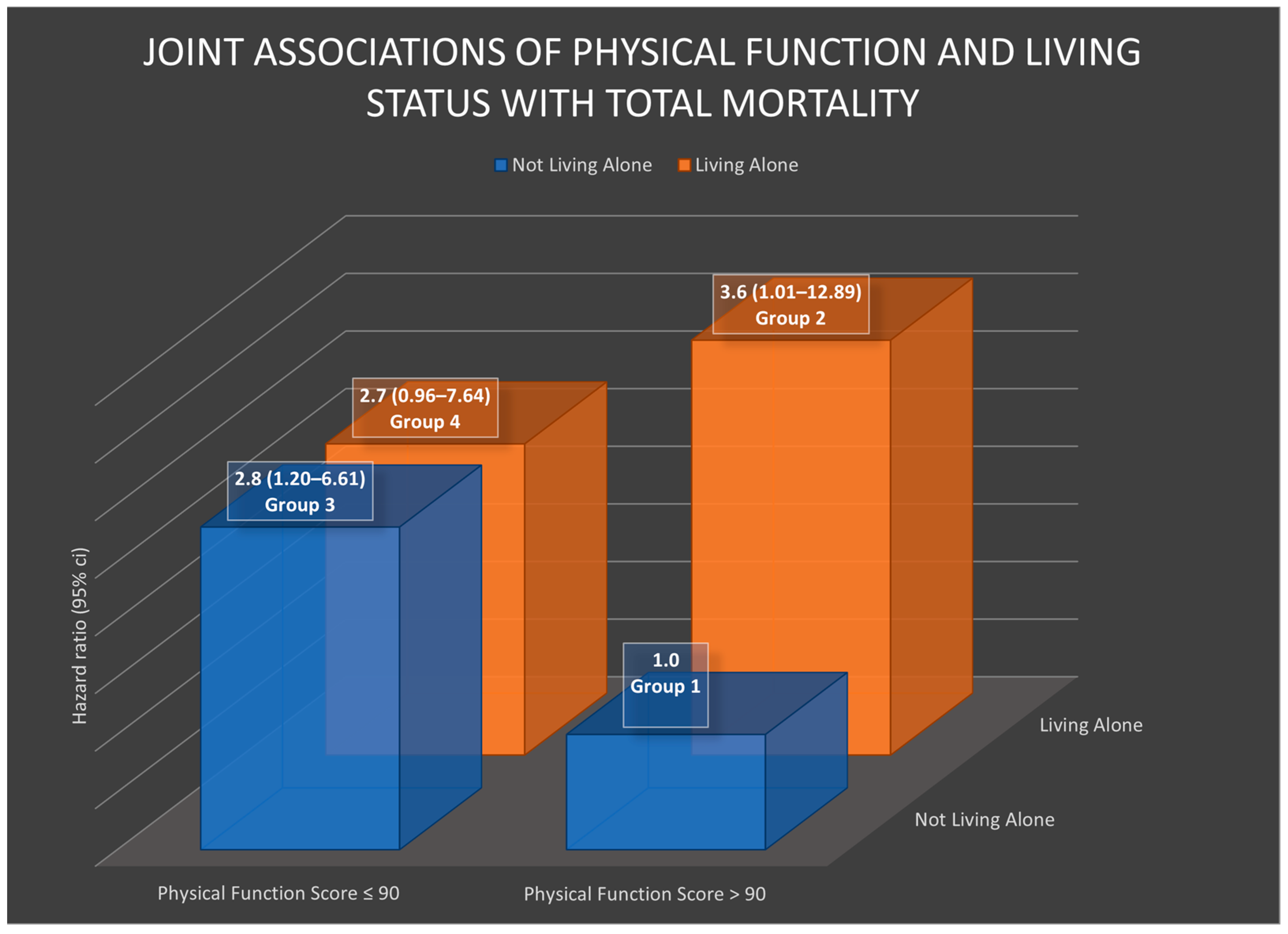
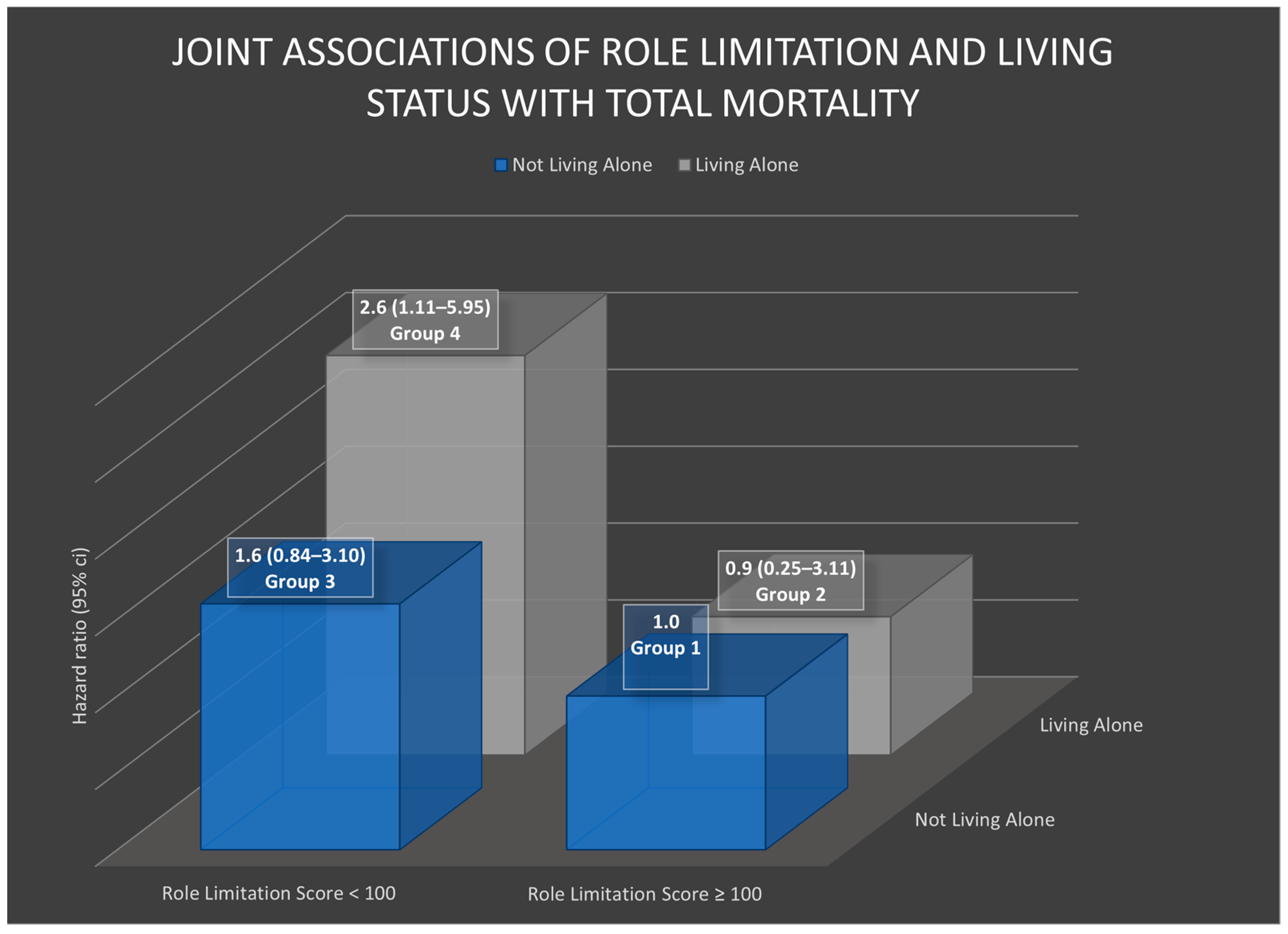
3.4. Joint Associations of Physical Health and Living Alone with Mortality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Survivorship: During and after Treatment. Available online: https://www.cancer.org/cancer/survivorship.html (accessed on 18 August 2023).
- Key Statistics for Breast Cancer. Available online: https://www.cancer.org/cancer/types/breast-cancer/about/how-common-is-breast-cancer.html (accessed on 18 August 2023).
- Lee, I.; Park, C. The mediating effect of social support on uncertainty in illness and quality of life of female cancer survivors: A cross-sectional study. Health Qual. Life Outcomes 2020, 18, 143. [Google Scholar] [CrossRef]
- Nicolaus, T. Building social connectedness vital for public health. Nation’s Health 2023, 53, 1–14. [Google Scholar]
- Hodgson, S.; Watts, I.; Fraser, S.; Roderick, P.; Dambha-Miller, H. Loneliness, social isolation, cardiovascular disease and mortality: A synthesis of the literature and conceptual framework. J. R. Soc. Med. 2020, 113, 185–192. [Google Scholar] [CrossRef]
- Holt-Lunstad, J.; Smith, T.B.; Baker, M.; Harris, T.; Stephenson, D. Loneliness and social isolation as risk factors for mortality: A meta-analytic review. Perspect. Psychol. Sci. 2015, 10, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Paul, E.; Bu, F.; Fancourt, D. Loneliness and Risk for Cardiovascular Disease: Mechanisms and Future Directions. Curr. Cardiol. Rep. 2021, 23, 68. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.C.; Harhay, M.O.; Harhay, M.N. Physical function as a prognostic biomarker among cancer survivors. Br. J. Cancer 2015, 112, 194–198. [Google Scholar] [CrossRef]
- Kroenke, C.H.; Kubzansky, L.D.; Schernhammer, E.S.; Holmes, M.D.; Kawachi, I. Social Networks, Social Support, and Survival After Breast Cancer Diagnosis. J. Clin. Oncol. 2006, 24, 1105–1111. [Google Scholar] [CrossRef]
- Census Bureau Releases New Estimates on America’s Families and Living Arrangements. 2021. Available online: https://www.census.gov/newsroom/press-releases/2021/families-and-living-arrangements.html (accessed on 18 April 2023).
- Hinzey, A.; Gaudier-Diaz, M.M.; Lustberg, M.B.; Devries, A.C. Breast cancer and social environment: Getting by with a little help from our friends. Breast Cancer Res. 2016, 18, 54. [Google Scholar] [CrossRef]
- Abell, J.G.; Steptoe, A. Living alone and mortality: More complicated than it seems. Eur. Heart J. Qual. Care Clin. Outcomes 2019, 5, 187–188. [Google Scholar] [CrossRef]
- Elovainio, M.; Lumme, S.; Arffman, M.; Manderbacka, K.; Pukkala, E.; Hakulinen, C. Living alone as a risk factor for cancer incidence, case-fatality and all-cause mortality: A nationwide registry study. SSM Popul. Health 2021, 15, 100826. [Google Scholar] [CrossRef]
- Kroenke, C.H.; Paskett, E.D.; Cené, C.W.; Caan, B.J.; Luo, J.; Shadyab, A.H.; Robinson, J.R.; Nassir, R.; Lane, D.S.; Anderson, G.L. Prediagnosis social support, social integration, living status, and colorectal cancer mortality in postmenopausal women from the women’s health initiative. Cancer 2020, 126, 1766–1775. [Google Scholar] [CrossRef] [PubMed]
- Nakano, J.; Fukushima, T.; Tanaka, T.; Fu, J.B.; Morishita, S. Physical function predicts mortality in patients with cancer: A systematic review and meta-analysis of observational studies. Support. Care Cancer 2021, 29, 5623–5634. [Google Scholar] [CrossRef]
- Pierce, J.P.; Faerber, S.; Wright, F.A.; Rock, C.L.; Newman, V.; Flatt, S.W.; Kealey, S.; Jones, V.E.; Caan, B.J.; Gold, E.B.; et al. A randomized trial of the effect of a plant-based dietary pattern on additional breast cancer events and survival: The Women’s Healthy Eating and Living (WHEL) Study. Control Clin. Trials 2002, 23, 728–756. [Google Scholar] [CrossRef]
- Hays, R.D.; Morales, L.S. The RAND-36 measure of health-related quality of life. Ann. Med. 2001, 33, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Ware, J.E., Jr.; Sherbourne, C.D. The MOS 36-Item Short-Form Health Survey (SF-36): I. Conceptual Framework and Item Selection. Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef]
- Item Short Form Survey (SF-36) Scoring Instructions. Available online: https://www.rand.org/health-care/surveys_tools/mos/36-item-short-form/scoring.html (accessed on 18 April 2023).
- Tessou, K.D.; Lemus, H.; Hsu, F.-C.; Pierce, J.; Hong, S.; Brown, L.; Wu, T. Independent and Joint Impacts of Acid-Producing Diets and Depression on Physical Health among Breast Cancer Survivors. Nutrients 2021, 13, 2422. [Google Scholar] [CrossRef]
- Pierce, J.P.; Natarajan, L.; Caan, B.J.; Parker, B.A.; Greenberg, E.R.; Flatt, S.W.; Rock, C.L.; Kealey, S.; Al-Delaimy, W.; Bardwell, W.A.; et al. Influence of a Diet Very High in Vegetables, Fruit, and Fiber and Low in Fat on Prognosis Following Treatment for Breast Cancer: The Women’s Healthy Eating and Living (WHEL) randomized trial. JAMA 2007, 298, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Johnson-Kozlow, M.; Rock, C.L.; Gilpin, E.A.; Hollenbach, K.A.; Pierce, J.P. Validation of the WHI brief physical activity questionnaire among women diagnosed with breast cancer. Am. J. Health Behav. 2007, 31, 193–202. [Google Scholar] [CrossRef]
- Hong, S.; Bardwell, W.A.; Natarajan, L.; Flatt, S.W.; Rock, C.L.; Newman, V.A.; Madlensky, L.; Mills, P.J.; Dimsdale, J.E.; Thomson, C.A.; et al. Correlates of physical activity level in breast cancer survivors participating in the Women’s Healthy Eating and Living (WHEL) Study. Breast Cancer Res. Treat. 2007, 101, 225–232. [Google Scholar] [CrossRef]
- Wei, M.Y.; Kabeto, M.U.; Galecki, A.T.; Langa, K.M. Physical Functioning Decline and Mortality in Older Adults With Multimorbidity: Joint Modeling of Longitudinal and Survival Data. J. Gerontol. Ser. A 2019, 74, 226–232. [Google Scholar] [CrossRef]
- Saquib, N.; Pierce, J.P.; Saquib, J.; Flatt, S.W.; Natarajan, L.; Bardwell, W.A.; Patterson, R.E.; Stefanick, M.L.; Thomson, C.A.; Rock, C.L.; et al. Poor physical health predicts time to additional breast cancer events and mortality in breast cancer survivors. Psycho-Oncology 2011, 20, 252–259. [Google Scholar] [CrossRef]
- Liang, Y.; Hao, G.; Wu, M.; Hou, L. Social isolation in adults with cancer: An evolutionary concept analysis. Front. Psychol. 2022, 13, 973640. [Google Scholar] [CrossRef] [PubMed]
- National Academies of Sciences Engineering and Medicine (U.S.). Committee on the Health and Medical Dimensions of Social Isolation and Loneliness in Older Adults. In Social Isolation and Loneliness in Older Adults: Opportunities for the Health Care System, in Consensus Study Report; National Academies Press: Washington, DC, USA, 2020. [Google Scholar]
- Knobf, M.T.; Thompson, A.S.; Fennie, K.; Erdos, D.M. The Effect of a Community-Based Exercise Intervention on Symptoms and Quality of Life. Cancer Nurs. 2014, 37, E43–E50. [Google Scholar] [CrossRef] [PubMed]
- Leach, H.; Danyluk, J.; Culos–Reed, S. Design and Implementation of a Community-Based Exercise Program for Breast Cancer Patients. Curr. Oncol. 2014, 21, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Rief, W.; Bardwell, W.A.; Dimsdale, J.E.; Natarajan, L.; Flatt, S.W.; Pierce, J.P. Long-term course of pain in breast cancer survivors: A 4-year longitudinal study. Breast Cancer Res. Treat. 2011, 130, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Shirazipour, C.H.; Raines, C.; Liu, E.; Ruggieri, R.M.; Capaldi, J.M.; Luna-Lupercio, B.; Diniz, M.A.; Gresham, G.; Bhowmick, N.; Haile, R.W.; et al. Benefits of nature-based walking for breast cancer survivors. BMJ Open 2023, 13, e071041. [Google Scholar] [CrossRef]
- Dawe, J.C. How Public Spaces Can Help Combat Urban Loneliness. 2021. Available online: https://www.salzburgglobal.org/news/latest-news/article/how-public-spaces-can-help-combat-urban-loneliness#:~:text=Access%20to%20green%20and%20public,social%20connection%20and%20support%20networks (accessed on 18 April 2023).
- Rodriguez-Loureiro, L.; Verdoodt, F.; Lefebvre, W.; Vanpoucke, C.; Casas, L.; Gadeyne, S. Long-term exposure to residential green spaces and site-specific cancer mortality in urban Belgium: A 13-year follow-up cohort study. Environ. Int. 2022, 170, 107571. [Google Scholar] [CrossRef]
| Overall | Mortality Status | p-Value | ||
|---|---|---|---|---|
| No (n = 2581) | Yes (n = 288) | |||
| Live alone | 0.016 | |||
| No | 2404 (83.8) | 2177 (84.4) | 227 (78.8) | |
| Yes | 465 (16.2) | 404 (15.7) | 61 (21.2) | |
| Physical function score | 0.0002 | |||
| ≤90 | 1615 (56.3) | 1423 (55.1) | 192 (66.7) | |
| >90 | 1254 (43.7) | 1158 (44.8) | 96 (33.3) | |
| Role limitation score | <0.0001 | |||
| <100 | 1317 (45.9) | 1150 (44.6) | 167 (58.0) | |
| ≥100 | 1550 (54.0) | 1429 (55.4) | 121 (42.0) | |
| Pain score | ||||
| <87.5 | 1433 (50.0) | 1270 (49.2) | 163 (56.6) | 0.0027 |
| ≥87.5 | 1435 (50.0) | 1310 (50.8) | 125 (43.4) | |
| General health score | 0.0025 | |||
| <75 | 1322 (46.1) | 1165 (45.1) | 157 (54.5) | |
| ≥75 | 1547 (53.9) | 1416 (54.9) | 131 (45.5) | |
| Overall physical health score | 0.034 | |||
| <43.125 | 2090 (72.9) | 1865 (72.3) | 225 (78.1) | |
| ≥43.125 | 779 (27.2) | 716 (27.7) | 63 (21.9) | |
| Age at diagnosis (years) | 50.8 ± 8.8 | 50.8 ± 8.7 | 51.4 ± 10.1 | 0.24 |
| Randomization group | 0.78 | |||
| Intervention | 1427 (49.7) | 1286 (49.8) | 141 (49.0) | |
| Comparison | 1442 (50.3) | 1295 (50.2) | 147 (51.0) | |
| Cancer Stage | <0.0001 | |||
| I | 1107 (38.6) | 1054 (40.8) | 53 (18.4) | |
| II | 1618 (56.4) | 1422 (55.1) | 196 (68.1) | |
| IIIA | 144 (5.0) | 105 (4.1) | 39 (13.5) | |
| Chemotherapy | 0.0016 | |||
| Yes | 1997 (69.7) | 1773 (68.8) | 224 (77.8) | |
| No | 870 (30.4) | 806 (31.3) | 64 (22.2) | |
| Radiation therapy | 0.89 | |||
| Yes | 1760 (61.4) | 1582 (61.34) | 178 (61.8) | |
| No | 1105 (38.6) | 995 (38.6) | 110 (38.2) | |
| Hormone status | <0.0001 | |||
| ER+/PR+ | 1778 (62.0) | 1631 (63.2) | 147 (51.0) | |
| Other | 1091 (38.0) | 950 (36.8) | 141 (49.0) | |
| Ethnicity | 0.18 | |||
| White | 2457 (85.6) | 2218 (85.9) | 239 (83.0) | |
| Non-white | 412 (14.4) | 363 (14.1) | 49 (17.0) | |
| Social support summary score (quartile) | 0.50 | |||
| 2–42 | 142 (5.0) | 122 (4.7) | 20 (6.9) | |
| 42–67 | 522 (18.2) | 475 (18.4) | 47 (16.3) | |
| 67–89 | 1126 (39.3) | 1015 (39.3) | 111 (38.5) | |
| 89–100 | 1078 (37.6) | 968 (37.5) | 110 (38.2) | |
| BMI (kg/m2) | 0.0063 | |||
| Underweight (0–18.5) | 28 (1.0) | 23 (0.9) | 5 (1.7) | |
| Healthy weight (18.5–25) | 1209 (42.1) | 1108 (42.9) | 101 (35.1) | |
| Overweight (25–30) | 885 (30.9) | 799 (31.0) | 86 (29.9) | |
| Obese (≥30) | 747 (26.0) | 651 (25.2) | 96 (33.3) | |
| Alcohol consumption (g) | 0.13 | |||
| 0 | 902 (31.4) | 799 (31.0) | 103 (35.8) | |
| ≤0.14 | 492 (17.2) | 434 (16.8) | 58 (20.1) | |
| 0.14–5.95 | 709 (24.7) | 646 (25.0) | 63 (21.9) | |
| 5.95–16.17 | 448 (15.6) | 410 (15.9) | 38 (13.2) | |
| >16.17 | 318 (11.1) | 292 (11.3) | 26 (9.0) | |
| Physical activity (METs/week) | 0.0072 | |||
| ≤225 | 805 (28.1) | 713 (27.6) | 92 (31.9) | |
| 225–675 | 710 (24.8) | 625 (24.2) | 85 (29.5) | |
| 675–1350 | 715 (24.9) | 648 (25.1) | 67 (23.3) | |
| >1350 | 639 (22.3) | 595 (23.1) | 44 (15.3) | |
| Smoking status | <0.0001 | |||
| Never smoker | 1553 (54.1) | 1409 (54.6) | 144 (50.0) | |
| Past smoker with less than 15 pack years | 750 (26.1) | 692 (26.8) | 58 (20.1) | |
| Past smoker with 15 or more pack years | 421 (14.7) | 357 (13.8) | 64 (22.2) | |
| Current smoker | 126 (4.4) | 110 (4.3) | 16 (5.6) | |
| Menopausal status | 0.049 | |||
| Premenopausal | 313 (10.9) | 271 (10.5) | 42 (14.6) | |
| Postmenopausal | 2283 (79.7) | 2057 (79.8) | 226 (78.5) | |
| Perimenopausal | 269 (9.4) | 249 (9.7) | 20 (6.9) | |
| Tumor Size (centimeters) | <0.0001 | |||
| ≤2 | 1667 (58.3) | 1563 (60.8) | 104 (36.1) | |
| >2 | 1194 (41.7) | 1010 (39.3) | 184 (63.9) | |
| Medical conditions that require medications | 0.036 | |||
| None | 1670 (58.2) | 1507 (58.4) | 163 (56.6) | |
| 1 | 691 (24.1) | 615 (23.8) | 76 (26.4) | |
| 2 | 349 (12.2) | 324 (12.6) | 25 (8.7) | |
| 3 or more | 159 (5.5) | 135 (5.2) | 24 (8.3) | |
| Physical Function Score | Live Alone | |||||
|---|---|---|---|---|---|---|
| >90 | ≤90 | p-Value | No | Yes | p-Value | |
| Age at diagnosis (years) | 49.2 ± 8.8 | 52.1 ± 8.7 | <0.0001 | 50.3 ± 8.8 | 53.7 ± 8.7 | <0.0001 |
| Randomization group | 0.96 | 0.46 | ||||
| Intervention | 623 (49.7) | 804 (49.8) | 1203 (50.0) | 224 (48.2) | ||
| Comparison | 631 (50.3) | 811 (50.2) | 1201 (50.0) | 241 (51.8) | ||
| Cancer Stage | 0.081 | 0.95 | ||||
| I | 492 (39.2) | 615 (38.1) | 927 (38.6) | 180 (38.7) | ||
| II | 712 (56.8) | 906 (56.1) | 1355 (56.4) | 263 (56.6) | ||
| IIIA | 50 (4.0) | 94 (5.8) | 122 (5.1) | 22 (4.7) | ||
| Chemotherapy | 0.48 | <0.0001 | ||||
| Yes | 882 (70.3) | 1115 (69.1) | 1718 (71.5) | 279 (60.0) | ||
| No | 372 (29.7) | 498 (30.9) | 684 (28.5) | 186 (40.0) | ||
| Radiation therapy | 0.85 | 0.60 | ||||
| Yes | 771 (61.6) | 989 (61.3) | 1480 (61.6) | 280 (60.3) | ||
| No | 480 (38.4) | 625 (38.7) | 921 (38.4) | 184 (39.7) | ||
| Hormone status | 0.49 | 0.77 | ||||
| ER+/PR+ | 786 (62.7) | 992 (61.4) | 1487 (61.9) | 291 (62.6) | ||
| Other | 468 (37.3) | 623 (38.6) | 917 (38.1) | 174 (37.4) | ||
| Social support summary score (Quartile) | <0.0001 | <0.0001 | ||||
| 2–42 | 37 (2.9) | 105 (6.5) | 86 (3.6) | 56 (12.0) | ||
| 42–67 | 179 (14.2) | 350 (21.5) | 380 (15.7) | 149 (32.0) | ||
| 67–89 | 498 (39.5) | 635 (39.0) | 949 (39.2) | 184 (39.5) | ||
| 89–100 | 547 (43.4) | 537 (33.0) | 1007 (41.6) | 77 (16.5) | ||
| BMI (kg/m2) | <0.0001 | 0.046 | ||||
| Underweight (0–18.5) | 14 (1.1) | 14 (0.9) | 19 (0.8) | 9 (1.9) | ||
| Healthy weight (18.5–25) | 695 (55.1) | 514 (31.6) | 1000 (41.3) | 209 (44.9) | ||
| Overweight (25–30) | 378 (30.0) | 507 (31.1) | 754 (31.1) | 131 (28.1) | ||
| Obese (≥30) | 167 (13.2) | 580 (35.6) | 631 (26.0) | 116 (24.9) | ||
| Alcohol consumption (g) | <0.0001 | 0.18 | ||||
| 0 | 354 (28.1) | 548 (33.7) | 746 (30.8) | 156 (33.5) | ||
| ≤0.14 | 183 (14.5) | 329 (20.2) | 432 (17.8) | 80 (17.2) | ||
| 0.14–5.95 | 308 (24.4) | 401 (24.6) | 612 (25.3) | 97 (20.8) | ||
| 5.95–16.17 | 236 (18.7) | 212 (13.0) | 376 (15.5) | 72 (15.5) | ||
| >16.17 | 180 (14.3) | 138 (8.5) | 257 (10.6) | 61 (13.1) | ||
| Physical activity (METs/week) | <0.0001 | 0.21 | ||||
| 0–225 | 215 (17.1) | 590 (36.2) | 690 (28.5) | 115 (24.7) | ||
| 225–675 | 286 (22.7) | 424 (26.0) | 587 (24.2) | 123 (26.4) | ||
| 675–1350 | 349 (27.7) | 366 (22.5) | 589 (24.3) | 126 (27.0) | ||
| >1350 | 404 (32.0) | 235 (14.4) | 538 (22.2) | 101 (21.7) | ||
| Smoking status | 0.15 | <0.0001 | ||||
| Never smoker | 693 (55.0) | 860 (52.8) | 1355 (55.9) | 198 (42.5) | ||
| Past smoker with less than 15 pack years | 338 (26.8) | 412 (25.3) | 618 (25.5) | 132 (28.3) | ||
| Past smoker with 15 or more pack years | 162 (12.9) | 259 (15.9) | 320 (13.2) | 101 (21.7) | ||
| Current smoker | 54 (4.3) | 72 (4.4) | 95 (3.9) | 31 (6.7) | ||
| Menopausal status | <0.0001 | <0.0001 | ||||
| Premenopausal | 191 (15.3) | 122 (7.6) | 288 (12.0) | 25 (5.4) | ||
| Postmenopausal | 951 (76.0) | 1332 (82.6) | 1884 (78.4) | 399 (86.2) | ||
| Medical conditions that require medications | <0.0001 | 0.15 | ||||
| None | 875 (69.8) | 795 (49.2) | 1421 (59.1) | 249 (53.6) | ||
| 1 | 267 (21.3) | 424 (26.3) | 569 (23.7) | 122 (26.2) | ||
| 2 | 91 (7.3) | 258 (16.0) | 283 (11.8) | 66 (14.2) | ||
| 3 or more | 21 (1.7) | 138 (8.5) | 131 (5.5) | 28 (6.0) | ||
| Category | Age-Adjusted HR (95% CI) | Multivariable-Adjusted HR (95% CI) | |
|---|---|---|---|
| Living alone | No | Ref | Ref |
| Yes | 1.3 (0.72–2.53) | 1.4 (0.75–2.78) | |
| Physical function | >90 | Ref | Ref |
| ≤90 | 2.7 (1.36–5.19) * | 2.1 (1.02–4.23) * | |
| Role limitation | ≥100 | Ref | Ref |
| <100 | 2.0 (1.16–3.59) * | 1.8 (1.03–3.32) * | |
| Pain | ≥87.5 | Ref | Ref |
| <87.5 | 1.3 (0.77–2.28) | 1.1 (0.63–1.97) | |
| General health | ≥75 | Ref | Ref |
| <75 | 2.6 (1.47–4.56) * | 2.6 (1.41–4.67) * | |
| Overall physical health | ≥43.125 | Ref | Ref |
| <43.125 | 2.5 (1.20–5.10) * | 1.9 (0.86–4.03) |
| Living Alone | ||
|---|---|---|
| No HR (95% CI) | Yes HR (95% CI) | |
| Physical function | ||
| >90 | Ref | 3.6 (1.01–12.89) * |
| ≤90 | 2.8 (1.20–6.61) * | 2.7 (0.96–7.64) |
| Role limitation | ||
| ≥100 | Ref | 0.9 (0.25–3.11) |
| <100 | 1.6 (0.84–3.10) | 2.6 (1.11–5.95) * |
| Pain | ||
| ≥87.5 | Ref | 2.6 (1.05–6.55) * |
| <87.5 | 1.5 (0.78–2.90) | 1.2 (0.45–3.14) |
| General health | ||
| ≥75 | Ref | 1.0 (0.33–3.24) |
| <75 | 2.3 (1.16–4.48) * | 4.0 (1.66–9.82) * |
| Overall physical health | ||
| ≥43.125 | Ref | 1.6 (0.78–3.09) |
| <43.125 | 2.2 (0.97–5.10) | 1.1 (0.14–8.60) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doyle, C.; Ko, E.; Lemus, H.; Hsu, F.-C.; Pierce, J.P.; Wu, T. Living Alone, Physical Health, and Mortality in Breast Cancer Survivors: A Prospective Observational Cohort Study. Healthcare 2023, 11, 2379. https://doi.org/10.3390/healthcare11172379
Doyle C, Ko E, Lemus H, Hsu F-C, Pierce JP, Wu T. Living Alone, Physical Health, and Mortality in Breast Cancer Survivors: A Prospective Observational Cohort Study. Healthcare. 2023; 11(17):2379. https://doi.org/10.3390/healthcare11172379
Chicago/Turabian StyleDoyle, Cassie, Eunjeong Ko, Hector Lemus, Fang-Chi Hsu, John P. Pierce, and Tianying Wu. 2023. "Living Alone, Physical Health, and Mortality in Breast Cancer Survivors: A Prospective Observational Cohort Study" Healthcare 11, no. 17: 2379. https://doi.org/10.3390/healthcare11172379
APA StyleDoyle, C., Ko, E., Lemus, H., Hsu, F.-C., Pierce, J. P., & Wu, T. (2023). Living Alone, Physical Health, and Mortality in Breast Cancer Survivors: A Prospective Observational Cohort Study. Healthcare, 11(17), 2379. https://doi.org/10.3390/healthcare11172379






