Analysis of Reports Sent to the Portuguese Pharmacovigilance System and Published Literature Regarding the Safety of Metformin in the Elderly
Abstract
1. Introduction
2. Materials and Methods
2.1. Comprehensive Review
2.2. Analysis of ADRs Reports Sent to the Portuguese Pharmacovigilance System
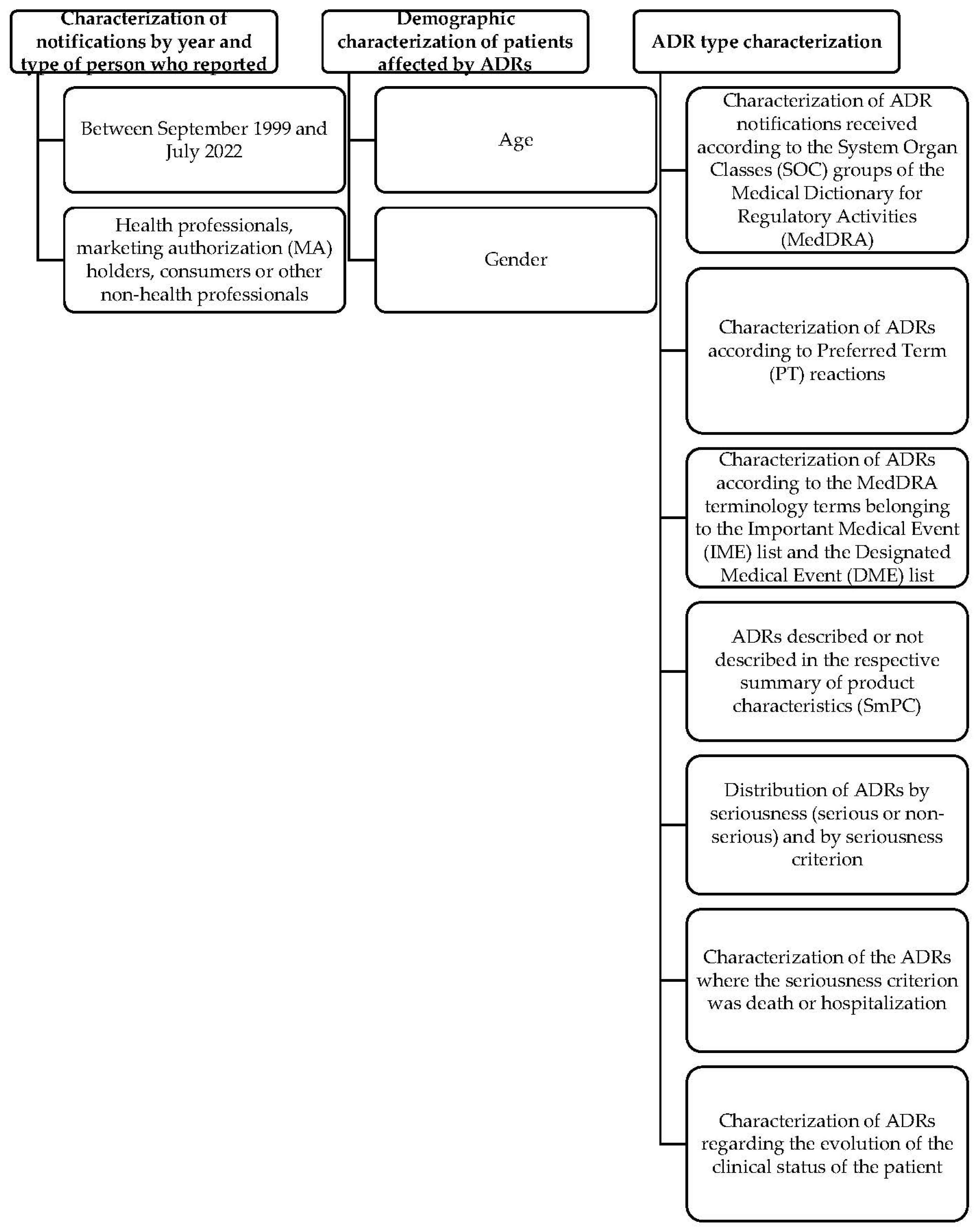
3. Results
3.1. Comprehensive Review
| References | Type of Study and Duration of the Study | Study Population | Number of Patients Aged ≥ 65 Years | Number of Patients Aged < 65 Years | Drugs Compared/Route of Administration | Outcomes | Study Limitations |
|---|---|---|---|---|---|---|---|
| Guo et al., 2021 [11] | Randomized, open and parallel controlled clinical trial. -2 years and 7 months | Patients with T2DM and taking metformin. | n = 150 | n = 736 | Prolonged-release metformin tablets, immediate-release metformin tablets; oral | -Long-release metformin tablets and immediate-release metformin tablets; similar therapeutic efficacy in the treatment of T2DM. -Long-release tablets: lower incidence of adverse effects. | -Questionnaire used does not have sufficient sensitivity to assess the quality of life of users. -Failure to obtain circulating GLP-1 levels of 60 blood samples. |
| Oh et al., 2019 [12] | Multicenter, randomized, double-blind, and parallel group study. -24 weeks | Patients with T2DM and inadequate glycemic control | n = 38 | n = 149 | Metformin alone and metformin in combination with voglibose (vogmet); oral | -Adverse effects hypoglycemia: minors in the vogmet-treated group compared to the metformin-treated group alone. -More significant weight loss when using vogmet treatment. | -Absence of a group alone with voglibose. |
| Hooda et al., 2018 [13] | Case report | A 70-year-old man with T2DM, without microvascular or macrovascular complications, with class 2 obesity, hypertension, dyslipidemia, and hypothyroidism | n = 1 | n = 0 | Metformin and liraglutide; oral and subcutaneous. | -Dehydration and development of acute kidney injury after the use of liraglutide (the main side effects of which are vomiting and nausea). -Development of lactic acidosis associated with metformin. | -Rapid titration of the liraglutide dose without adequate follow-up. |
| Lim et al., 2017 [14] | Multinational, multicenter, randomized, active-controlled, double-blind, phase III trial. -1 year and 8 months | Patients with T2DM who do not take any antidiabetic | n = 77 | n = 356 | Metformin in combination with gemigliptin, metformin monotherapy or gemigliptin; oral. | -Metformin in combination with gemigliptin: efficacy superior to monotherapy with each drug. | -Reduced number of patients using metformin alone. -No safety concerns. |
| Schlender at al., 2017 [15] | Systematic review | Elderly individuals aged ≥65 with T2DM | n = 230229 | n = 0 | Metformin (alone or in combination), placebo or other antidiabetics (gliburide, glimepiride, thiazolidinediones, tolbutamide, biguanides, vildagliptin, rosiglitazone and pioglitazone); oral. | -Safety and efficacy profiles of metformin appear to be better than those of other treatments for the control of T2DM in the elderly. | -Reduced quality and amount of evidence due to lack of information related to adverse effects, including gastrointestinal changes or kidney failure. |
| Becquemont et al., 2016 [16] | Prospective cohort study -3 years | Non-institutionalized patients aged ≥ 65 years and with chronic pain, T2DM or AF. | n = 3434 | n = 0 | Metformin or digoxin or spironolactone; oral. | -Approximately 25% of patients taking metformin receive a dose that is not adapted to renal function, but there is no increase in mortality after a 3-year follow-up. -Renal failure increases the risk of developing lactic acidosis associated with metformin therapy, however none of the deaths were associated with lactic acidosis. | -Small number of patients receiving metformin. -Risk of residual confounding factors and lack of diversity of the group of patients included in this study. |
| Margiani et al., 2014 [17] | Case report | A 70-year-old man with T2DM, prostate hypertrophy, hypertension and who was subjected to temporary ileostomy. | n = 1 | n = 0 | Metformin; oral. | -After ileostomy, dehydration and electrolyte imbalances were observed. -These changes led to the development of a pre-kidney injury. -Consequently, there was an accumulation of metformin which resulted in serious lactic acidosis. | -The patient was also undergoing treatment with diuretics which contributed to the worsening of dehydration and electrolyte imbalances resulting from ileostomy. |
| Hung, 2013 [18] | Retrospective cohort study based on the study population. | Patients with T2DM, with no history of cardiovascular disease and aged ≥ 30 years. | n = 231 | n = 928 | Monotherapy with metformin, glimepiride or gliburide; oral | -Lower risk of developing non-fatal cardiovascular events in the group taking metformin or glimepiride compared to the gliburide therapy group. | -Insufficient information regarding patient comorbidities. |
| Moore et al., 2013 [20] | Cross-sectional study | Patients with T2DM and Alzheimer’s or moderate cognitive dysfunction or individuals with intact cognitive function. | n = 1164 | n = 190 | Metformin alone and metformin in combination with calcium and vitamin B12 supplements; oral | -Use of metformin associated with altered cognitive performance. -Vitamin B12 and calcium supplements may improve metformin-induced vitamin B12 deficiency and contribute to better cognitive results. | -Insufficient information regarding the duration of metformin treatment, severity of diabetes and the use of other antidiabetics. -Reduced sample. |
| Roumie, 2012 [19] | Retrospective cohort study | Patients with T2DM and AMI or stroke | n = 118,014 | n = 135,626 | Monotherapy with metformin or sulphonylureas; oral | -Initial treatment of T2DM with sulphonylureas associated with a higher risk of cardiovascular events and death than with metformin. | -Inaccuracies in measurements associated with results not coming from the central laboratory. |
| Roussel et al., 2010 [21] | -Prospective observational study -2 years | Patients with T2DM and atherothrombosis | n = 12,649 | n = 6.904 | Treatment with or without metformin; oral | -Use of metformin as secondary prevention may decrease mortality. | -Ignorance of the duration of diabetes and metformin use. -Lack of information on the level of glycated hemoglobin. |
| MacDonald, 2010 [22] | Case-control study | Patients with T2DM and HF | n = 3102 | n = 164 | Monotherapy with metformin, metformin in combination; oral | -Lower mortality in patients taking metformin alone or in combination with users who were not taking antidiabetics. | -Study was based on medical diagnoses and existing documents of heart failure, comorbidity and risk factors. -There was no independent confirmation of the diagnoses. |
| Evans, 2010 [23] | Population-based prospective cohort study | Patients with T2DM and CHF | n = 365 | n = 57 | Monotherapy with metformin or sulphonylureas and association of both drugs; oral | -Fewer deaths in patients taking metformin alone or in combination with sulphonylureas compared to sulphonylureas therapy alone. | -Confounding variables that may have contributed to the creation of bias in the differences observed between the groups considered. |
3.2. Portuguese Pharmacovigilance System-Adverse Drug Reaction Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coll, A.P.; Chen, M.; Taskar, P.; Rimmington, D.; Patel, S.; Tadross, J.A.; Cimino, I.; Yang, M.; Welsh, P.; Virtue, S.; et al. GDF15 mediates the effects of metformin on body weight and energy balance. Nature 2020, 578, 444. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Standards of Medical Care in Diabetes-2022; Diabetes Care: Arlington County, VA, USA, 2022; Volume 45. [Google Scholar]
- Nunes, J.S. Practical Aspects in Metformin Therapeutics. Port. J. Diabetes 2014, 9, 127–132. [Google Scholar]
- Metformin: Summary of Product Characteristics. 2017. Available online: https://extranet.infarmed.pt/INFOMED-fo/pesquisa-avancada.xhtml (accessed on 3 August 2022).
- FDA Drug Safety Communication. FDA Revises Warnings Regarding Use of the Diabetes Medicine Metformin in Certain Patients with Reduced Kidney Function. 4 August 2016. Available online: https://www.fda.gov/files/drugs/published/Drug-Safety-Communication-FDA-revises-warnings-regarding-use-of-the-diabetes-medicine-metformin-in-certain-patients-with-reduced-kidney-function-%28PDF%29.pdf (accessed on 8 April 2023).
- Spinewine, A.; Schmader, K.E.; Barber, N.; Hughes, C.; Lapane, K.L.; Swine, C.; Hanlon, J.T. Appropriate prescribing in elderly people: How well can it be measured and optimised? Lancet 2007, 370, 173–184. [Google Scholar] [PubMed]
- World Health Organization. The Importance of Pharmacovigilance; World Health Organization: Geneva, Switzerland, 2002; 48p. [Google Scholar]
- Introductory Guide MedDRA Version 22.1. September 2019. Available online: https://admin.meddra.org/sites/default/files/guidance/file/000354_intguide_22.1.pdf (accessed on 24 August 2022).
- The Uppsala Monitoring Centre. The Use of the WHO-UMC System for Standardised Case Causality Assessment; The Uppsala Monitoring Centre: Uppsala, Sweden, 2013. [Google Scholar]
- Medicines Agency, E. Guideline on Good Pharmacovigilance Practices (GVP)—Module VI—Collection, Management and Submission of Reports of Suspected Adverse Reactions to Medicinal Products (Rev 2). 2017. Available online: ema.europa.eu/en/documents/regulatory-procedural-guideline/guideline-good-pharmacovigilance-practices-gvp-module-vi-collection-management-submission-reports_en.pdf (accessed on 8 April 2023).
- Guo, L.X.; Liu, G.E.; Chen, L.; Wang, H.F.; Guo, J.; Zheng, X.L.; Duan, B.H.; Wang, D.Z.; Zhu, W.; Wang, K.; et al. Comparison of Clinical Efficacy and Safety of Metformin Sustained-Release Tablet (II) (Dulening) and Metformin Tablet (Glucophage) in Treatment of Type 2 Diabetes Mellitus. Front. Endocrinol. 2021, 12, 712200. [Google Scholar] [CrossRef]
- Oh, T.J.; Yu, J.M.; Min, K.W.; Son, H.S.; Lee, M.K.; Yoon, K.H.; Song, Y.D.; Park, J.Y.; Jeong, I.K.; Cha, B.S.; et al. Efficacy and Safety of Voglibose Plus Metformin in Patients with Type 2 Diabetes Mellitus: A Randomized Controlled Trial. Diabetes Metab. J. 2019, 43, 286. [Google Scholar] [CrossRef]
- Hooda, A.; Mehta, A.; Hannallah, F. Metformin-associated lactic acidosis precipitated by liraglutide use: Adverse effects of aggressive antihyperglycaemic therapy. BMJ Case Rep. 2018, 11, e227102. [Google Scholar] [CrossRef]
- Lim, S.; Han, K.A.; Yu, J.; Chamnan, P.; Kim, E.S.; Yoon, K.H.; Kwon, S.; Moon, M.K.; Lee, K.W.; Kim, D.J.; et al. Efficacy and safety of initial combination therapy with gemigliptin and metformin compared with monotherapy with either drug in patients with type 2 diabetes: A double-blind randomized controlled trial (INICOM study). Diabetes Obes. Metab. 2017, 19, 97. [Google Scholar] [CrossRef]
- Schlender, L.; Martinez, Y.V.; Adeniji, C.; Reeves, D.; Faller, B.; Sommerauer, C.; Qur’an, A.; Woodham, A.; Kunnamo, I.; Sönnichsen, A.; et al. Efficacy and safety of metformin in the management of type 2 diabetes mellitus in older adults: A systematic review for the development of recommendations to reduce potentially inappropriate prescribing. BMC Geriatr. 2017, 17 (Suppl. 1), 227. [Google Scholar] [CrossRef] [PubMed]
- Becquemont, L.; Bauduceau, B.; Benattar-Zibi, L.; Al-Salameh, A.; Berrut, G.; Bertin, P.; Bucher, S.; Corruble, E.; Danchin, N.; Derumeaux, G.; et al. Cardiovascular Drugs and Metformin Drug Dosage According to Renal Function in Non-Institutionalized Elderly Patients. Basic Clin. Pharmacol. Toxicol. 2016, 118, 468–473. [Google Scholar] [CrossRef]
- Margiani, C.; Zorcolo, L.; Mura, P.; Saba, M.; Restivo, A.; Scintu, F. Metformin-associated lactic acidosis and temporary ileostomy: A case report. J. Med. Case Rep. 2014, 8, 449. [Google Scholar] [CrossRef]
- Hung, Y.C.; Lin, C.C.; Wang, T.Y.; Chang, M.P.; Sung, F.C.; Chen, C.C. Oral hypoglycaemic agents and the development of non-fatal cardiovascular events in patients with type 2 diabetes mellitus. Diabetes Metab. Res. Rev. 2013, 29, 673–679. [Google Scholar] [CrossRef]
- Roumie, C.L.; Hung, A.M.; Greevy, R.A.; Grijalva, C.G.; Liu, X.; Murff, H.J.; Elasy, T.A.; Griffin, M.R. Comparative effectiveness of sulfonylurea and metformin monotherapy on cardiovascular events in type 2 diabetes mellitus: A cohort study. Ann. Intern. Med. 2012, 157, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Moore, E.M.; Mander, A.G.; Ames, D.; Kotowicz, M.A.; Carne, R.P.; Brodaty, H.; Woodward, M.; Boundy, K.; Ellis, K.A.; Bush, A.I.; et al. Increased Risk of Cognitive Impairment in Patients with Diabetes Is Associated with Metformin. Diabetes Care 2013, 36, 2981–2987. [Google Scholar]
- Roussel, R.; Travert, F.; Pasquet, B.; Wilson, P.W.; Smith, S.C.; Goto, S.; Ravaud, P.; Marre, M.; Porath, A.; Bhatt, D.L.; et al. Metformin Use and Mortality Among Patients With Diabetes and Atherothrombosis. Arch. Intern. Med. 2010, 170, 1892–1899. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, M.R.; Eurich, D.T.; Majumdar, S.R.; Lewsey, J.D.; Bhagra, S.; Jhund, P.S.; Petrie, M.C.; McMurray, J.J.; Petrie, J.R.; McAlister, F.A. Treatment of Type 2 Diabetes and Outcomes in Patients with Heart Failure: A Nested Case–Control Study from the U.K. Gen. Pract. Res. Database Diabetes Care 2010, 33, 1218. [Google Scholar]
- Evans, J.M.; Doney, A.S.; AlZadjali, M.A.; Ogston, S.A.; Petrie, J.R.; Morris, A.D.; Struthers, A.D.; Wong, A.K.; Lang, C.C. Effect of metformin on mortality in patients with heart failure and type 2 diabetes mellitus. Am. J. Cardiol. 2010, 106, 1006–1010. [Google Scholar] [CrossRef]
- McCreight, L.J.; Bailey, C.J.; Pearson, E.R. Metformin and the gastrointestinal tract. Diabetologia 2016, 59, 435. [Google Scholar]
- Mariano, F.; Pozzato, M.; Inguaggiato, P.; Guarena, C.; Turello, E.; Manes, M.; David, P.; Berutti, S.; Consiglio, V.; Amore, A.; et al. Metformin-Associated Lactic Acidosis Undergoing Renal Replacement Therapy in Intensive Care Units: A Five-Million Population-Based Study in the North-West of Italy. Blood Purif. 2017, 44, 198–205. [Google Scholar] [CrossRef]
- INFARMED. How Many Notifications Are Made Annually? Available online: https://www.infarmed.pt/web/infarmed/perguntas-frequentes-area-transversal/medicamentos_uso_humano/notificacao-de-reacoes-adversas/efeitos-indesejaveis-de-medicamentos-ram (accessed on 25 September 2022).
- INFARMED. Evolution of ADR Reports Received in SNF, 1992–2021. Available online: https://www.infarmed.pt/documents/15786/2297404/Notifica%C3%A7%C3%B5es+RAM+1992-2020/db879ca4-1cf8-f21f-672c-43d1296d8841 (accessed on 25 September 2022).
- Comissão Europeia. Chronic Disease 27 July 2017. Available online: https://op.europa.eu/pt/publication-detail/-/publication/ed838bf9-a5e7-4e04-b8f7-3706eed3a354 (accessed on 25 September 2022).
- Hajjar, E.R.; Gray, S.L.; Slattum, P.W., Jr.; Hersh, L.R.; Naples, J.G.; Hanlon, J.T. Geriatrics|Pharmacotherapy: A Pathophysiologic Approach 10e; McGraw Hill: New York, NY, USA, 2017. [Google Scholar]
- PORDATA, Fundação Francisco Manuel Santos. População Residente: Total e Por Grupo Etário. Available online: https://www.pordata.pt/portugal/populacao+residente+total+e+por+grupo+etario-10 (accessed on 9 April 2023).
- Soldin, O.P.; Chung, S.H.; Mattison, D.R. Sex differences in drug disposition. J. Biomed. Biotechnol. 2011, 2011, 187103. [Google Scholar] [CrossRef]
- van den Anker, J.; Reed, M.D.; Allegaert, K.; Kearns, G.L. Developmental Changes in Pharmacokinetics and Pharmacodynamics. J. Clin. Pharmacol. 2018, 58 (Suppl. 10), S10–S25. [Google Scholar]
- Batel-Marques, F.; Mendes, D.; Alves, C.; Penedones, A.; Dias, P.; Martins, A.; Santiago, L.M.; Fontes-Ribeiro, C.; Caramona, M.; Macedo, T. Pharmacovigilance in Portugal: Activity of the Central Pharmacovigilance Unit. Acta Med. Port. 2015, 28, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Lagneau, A.; Vigier, C.; Marianna, A.; Serfaty, R.; Rocher, F.; Spreux, A.; Drici, M.-D. Comparative relevance of declaration of side effects by patients and health professionals. Therapie 2017, 72, 625–633. Available online: https://pubmed.ncbi.nlm.nih.gov/28780021/ (accessed on 9 April 2023). [CrossRef] [PubMed]
- Salvador, M.R.; Monteiro, C.; Pereira, L.; Duarte, A.P. Quality of Spontaneous Reports of Adverse Drug Reactions Sent to a Regional Pharmacovigilance Unit. Int. J. Environ. Res. Public Health 2022, 19, 3754. [Google Scholar] [CrossRef] [PubMed]
- Oscanoa, T.J.; Lizaraso, F.; Carvajal, A. Hospital admissions due to adverse drug reactions in the elderly. A meta-analysis. Eur. J. Clin. Pharmacol. 2017, 73, 759–770. [Google Scholar]
- Sandberg, A.; Salminen, V.; Heinonen, S.; Sivén, M. Under-Reporting of Adverse Drug Reactions in Finland and Healthcare Professionals’ Perspectives on How to Improve Reporting. Healthcare 2022, 10, 1015. [Google Scholar] [CrossRef]
| System Organ Classes (SOC) | Metformin | Metformin in Fixed Combination | Metformin and Other Drugs |
|---|---|---|---|
| Blood and lymphatic system disorders | 6 | 1 | 7 |
| Cardiac disorders | 7 | 10 | 8 |
| Musculoskeletal and connective tissue disorders | 1 | 10 | 2 |
| Eye disorders | 0 | 4 | 0 |
| Gastrointestinal disorders | 54 | 59 | 17 |
| General disorders and administration site conditions | 28 | 28 | 18 |
| Nervous system disorders | 20 | 18 | 14 |
| Metabolism and nutrition disorders | 82 | 57 | 47 |
| Skin and subcutaneous tissue disorders | 13 | 18 | 8 |
| Respiratory, thoracic, and mediastinal diseases | 4 | 15 | 4 |
| Psychiatric disorders | 2 | 8 | 3 |
| Infections and infestations | 1 | 7 | 5 |
| Investigations | 13 | 21 | 17 |
| Immune system disorders | 0 | 0 | 3 |
| Neoplasms benign, malignant, and unspecified (including cysts and polyps) | 1 | 3 | 1 |
| Renal and urinary disorders | 20 | 23 | 14 |
| Vascular disorders | 13 | 3 | 7 |
| Injury, poisoning, and procedural complications | 10 | 12 | 6 |
| Hepatobiliary disorders | 0 | 0 | 6 |
| Congenital, familial, and genetic disorders | 0 | 0 | 1 |
| Surgical and medical procedures | 0 | 2 | 1 |
| Reproductive system and breast disorders | 0 | 8 | 0 |
| PT Reaction | Metformin | Metformin in Combination | Metformin and Other Drugs |
|---|---|---|---|
| Diarrhea | 29 | 24 | 4 |
| Nausea | 8 | 12 | 7 |
| Abdominal pain | 13 | 6 | 3 |
| Vomiting | 14 | 17 | 7 |
| Acute renal injury | 12 | 6 | 8 |
| Hyperlactacidemia | 8 | 7 | 0 |
| Lactic acidosis | 54 | 22 | 5 |
| Metabolic acidosis | 14 | 3 | 7 |
| Diabetic ketoacidosis/Euglycemic diabetic ketoacidosis | 5 | 4 | 7 |
| Hypoglycemia | 12 | 10 | 19 |
| Terms of the IME List (n) | Suspected Drugs (n) * |
|---|---|
| Inflammatory myofibroblastic tumor (1) |
|
| Hemolytic anemia (1) |
|
| Lactic acidosis (71) |
|
| Hyperkalemia (5) |
|
| Sweat gland tumor (1) |
|
| Pulmonary hypertension (1) |
|
| Acute renal injury (14) |
|
| Apnea (1) |
|
| Cardiorespiratory arrest (3) |
|
| Tubulointerstitial nephritis (1) |
|
| Rheumatic polymyalgia (1) |
|
| Altered state of consciousness (7) |
|
| Hemiparesis (1) |
|
| Pulmonary embolism (1) |
|
| Anuria (2) |
|
| Renal failure (4) |
|
| Hematochezia (1) |
|
| Cardiac arrest (2) |
|
| Prerenal insufficiency (1) |
|
| Renal injury (4) |
|
| Lupus-like syndrome (1) |
|
| Multiform Erythema (1) |
|
| Respiratory failure (2) |
|
| Ischemic stroke (1) |
|
| Inadequate diabetes control (5) |
|
| Acute pancreatitis (1) |
|
| Cholangitis (1) |
|
| Cholestasis (1) |
|
| Bacteriemia (1) |
|
| Autoimmune hepatitis (1) |
|
| Duodenal ulcer (1) |
|
| Hypoglycemic encephalopathy (1) |
|
| Metastatic pancreatic carcinoma (1) |
|
| Renal ischemia (1) |
|
| Stroke (1) |
|
| Atrial fibrillation (1) |
|
| Basal cell carcinoma (1) |
|
| Toxic skin rash (4) |
|
| Metabolic decompensation of diabetes (1) |
|
| Hypoglycemic coma (1) |
|
| Microscopic colitis (1) |
|
| Craniocerebral injury (1) |
|
| Erectile dysfunction (1) |
|
| Acute cholecystitis (1) |
|
| Multiple organ dysfunction syndrome (1) |
|
| Autoimmune disorder (1) |
|
| Syncope (1) |
|
| Myocardial infarction (1) |
|
| Euglycemic diabetic ketoacidosis (2) |
|
| Parkinson’s disease (1) |
|
| Pemphigoid (3) |
|
| Coma (1) |
|
| Upper gastrointestinal bleeding (1) |
|
| Chronic renal disease (1) |
|
| Brash syndrome (1) |
|
| Necrotizing esophagitis (2) |
|
| Diabetic ketoacidosis (4) |
|
| Metabolic acidosis (1) |
|
| Troponin I increased (1) |
|
| Cerebral artery occlusion (1) |
|
| Aortic thrombosis (1) |
|
| Renal artery thrombosis (1) |
|
| Bradycardia (4) |
|
| Myelopathy (1) |
|
| Hematemesis (1) |
|
| Hypothermia (2) |
|
| Shock (4) |
|
| Bullous dermatitis (1) |
|
| Heart failure (1) |
|
| Death (1) |
|
| Terms of the DME List (n) | Drugs (n) * |
|---|---|
| Hemolytic anemia (1) Acute kidney injury (9) Renal failure (3) Multiform erythema (1) |
|
| Autoimmune hepatitis (1) |
|
| Autoimmune pancreatitis (2) |
|
| Acute renal injury (10) |
|
| Renal failure (2) |
|
| Acute pancreatitis (1) |
|
| Pulmonary hypertension (2) |
|
| Dehydration (1) |
|
| Pancytopenia (1) |
|
| Drug-induced liver injury (1) |
|
| Adverse Drug Reactions Preferred Term (PT) (n) | Drugs (n) * |
|---|---|
| Heart failure (2) |
|
| Palpitations (2) |
|
| Bradycardia (2) |
|
| Atrioventricular block, palpitations and bradycardia (1) |
|
| Atrial fibrillation (1) |
|
| Myocardial infarction (1) |
|
| Tachycardia (2) |
|
| Brash syndrome (1) |
|
| Adverse Drug Reactions-Preferred Term (PT) (n) | Drugs (n) * |
|---|---|
| Lactic acidosis (25) |
|
| Vitamin B12 deficiency (4) |
|
| Hypoglycemia (12) |
|
| Decreased appetite (1) |
|
| Metabolic acidosis (9) |
|
| Inadequate control of diabetes mellitus (3) |
|
| Ketosis (1) |
|
| Dehydration (3) |
|
| Hyperlactacidemia (2) |
|
| Hyperglycemia (1) |
|
| Metabolic decompensation of diabetes (2) |
|
| Diabetic ketoacidosis (4) |
|
| Euglycemic diabetic ketoacidosis (1) |
|
| Adverse Drug Reaction-Preferred Term (PT) (n) | Drugs (n) * |
|---|---|
| Diarrhea (4) |
|
| Abdominal pain (3) |
|
| Vomiting (9) |
|
| Oral disorders (1) |
|
| Epigastric discomfort (1) |
|
| Nausea (3) |
|
| Hematemesis (1) |
|
| Duodenal ulcer (1) |
|
| Sprue-like enteropathy (1) |
|
| Gastrointestinal disorder (1) |
|
| Autoimmune pancreatitis (1) |
|
| Necrotizing esophagitis (2) |
|
| Ischemic colitis (2) |
|
| Retroperitoneal hematoma (2) |
|
| Mesenteric vein thrombosis (2) |
|
| Constipation (1) |
|
| ADR Preferred Term (PT) (n) | Drugs (n) * |
|---|---|
| Metabolic acidosis (4); Lactic acidosis (5); Renal failure (1); shock (3); Blood pH decrease (1); Respiratory failure (1); Fatigue (1); Neurological Symptoms (1); Toxicity to various agents (1); Renal injury (1); Hyperlactacidemia (1); Acute kidney injury (3); Hyperkalemia (1); Multiple organ dysfunction syndrome (1); Euglycemic diabetic ketoacidosis (2); Aortic thrombosis (1) |
|
| Hypoglycemic encephalopathy (1) |
|
| Hypotension; Amyloidosis (1) |
|
| Speech dysfunction (1); Chest pain (1); Hemiparesis (1); Increased blood pressure (1); Nervous system disfunction (1); Cerebrovascular accident (1); Quadriparesis (1); Aphasia(1); Quadriplegia (1) |
|
| Euglycemic diabetic ketoacidosis (4); acute kidney injury (2) Metabolic acidosis (2) |
|
| Euglycemic diabetic ketoacidosis (1); Lactic acidosis (1) |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esteves, B.; Monteiro, C.; Duarte, A.P.C. Analysis of Reports Sent to the Portuguese Pharmacovigilance System and Published Literature Regarding the Safety of Metformin in the Elderly. Healthcare 2023, 11, 2197. https://doi.org/10.3390/healthcare11152197
Esteves B, Monteiro C, Duarte APC. Analysis of Reports Sent to the Portuguese Pharmacovigilance System and Published Literature Regarding the Safety of Metformin in the Elderly. Healthcare. 2023; 11(15):2197. https://doi.org/10.3390/healthcare11152197
Chicago/Turabian StyleEsteves, Beatriz, Cristina Monteiro, and Ana Paula Coelho Duarte. 2023. "Analysis of Reports Sent to the Portuguese Pharmacovigilance System and Published Literature Regarding the Safety of Metformin in the Elderly" Healthcare 11, no. 15: 2197. https://doi.org/10.3390/healthcare11152197
APA StyleEsteves, B., Monteiro, C., & Duarte, A. P. C. (2023). Analysis of Reports Sent to the Portuguese Pharmacovigilance System and Published Literature Regarding the Safety of Metformin in the Elderly. Healthcare, 11(15), 2197. https://doi.org/10.3390/healthcare11152197







