Association between Sexual Activity during Pregnancy, Pre- and Early-Term Birth, and Vaginal Cytokine Inflammation: A Prospective Study of Black Women
Abstract
1. Introduction
2. Methods
3. Results
3.1. Sample Characteristics
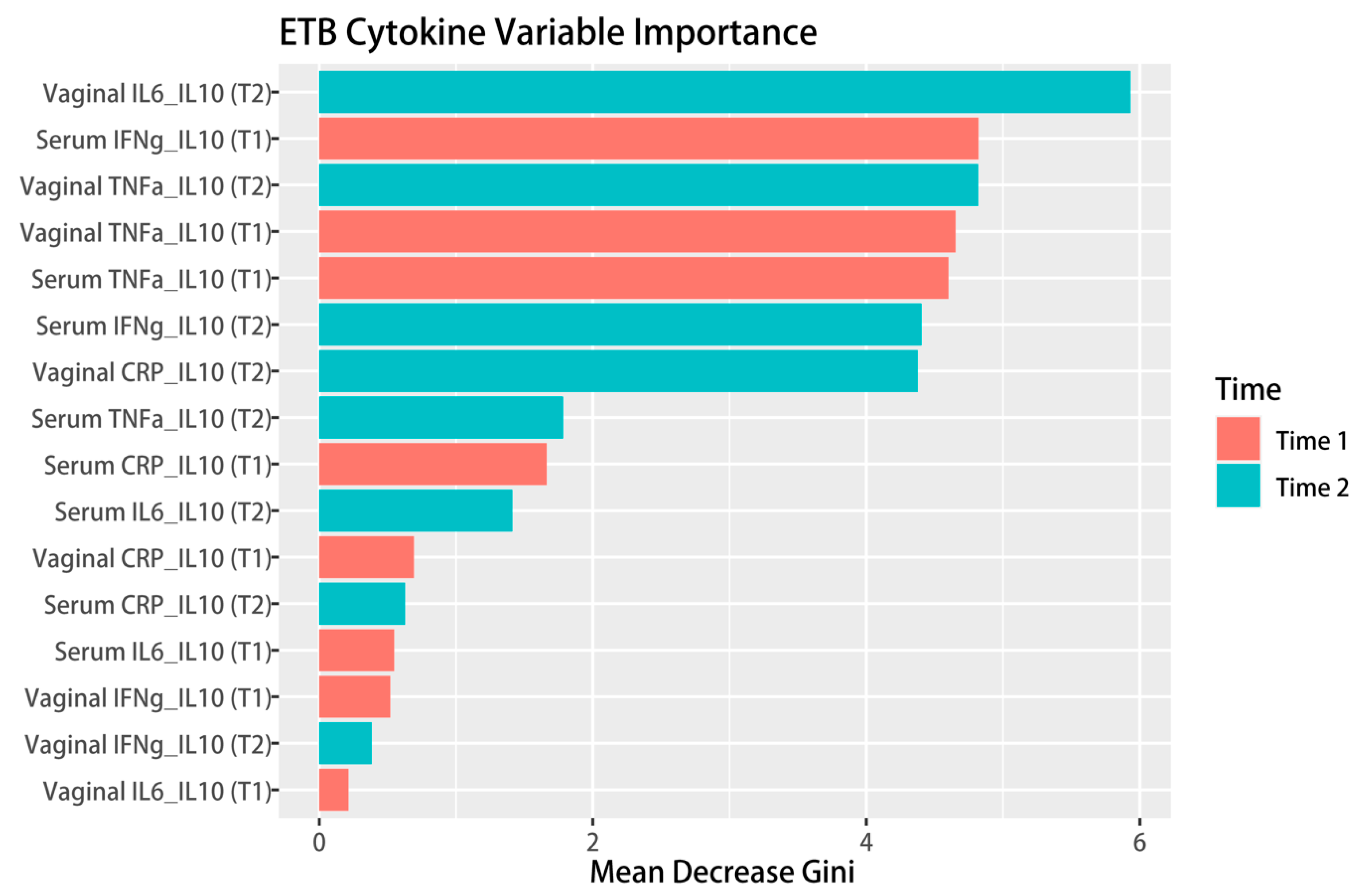
3.2. Linking Sexual Behavior and Inflammation with Birth Outcomes
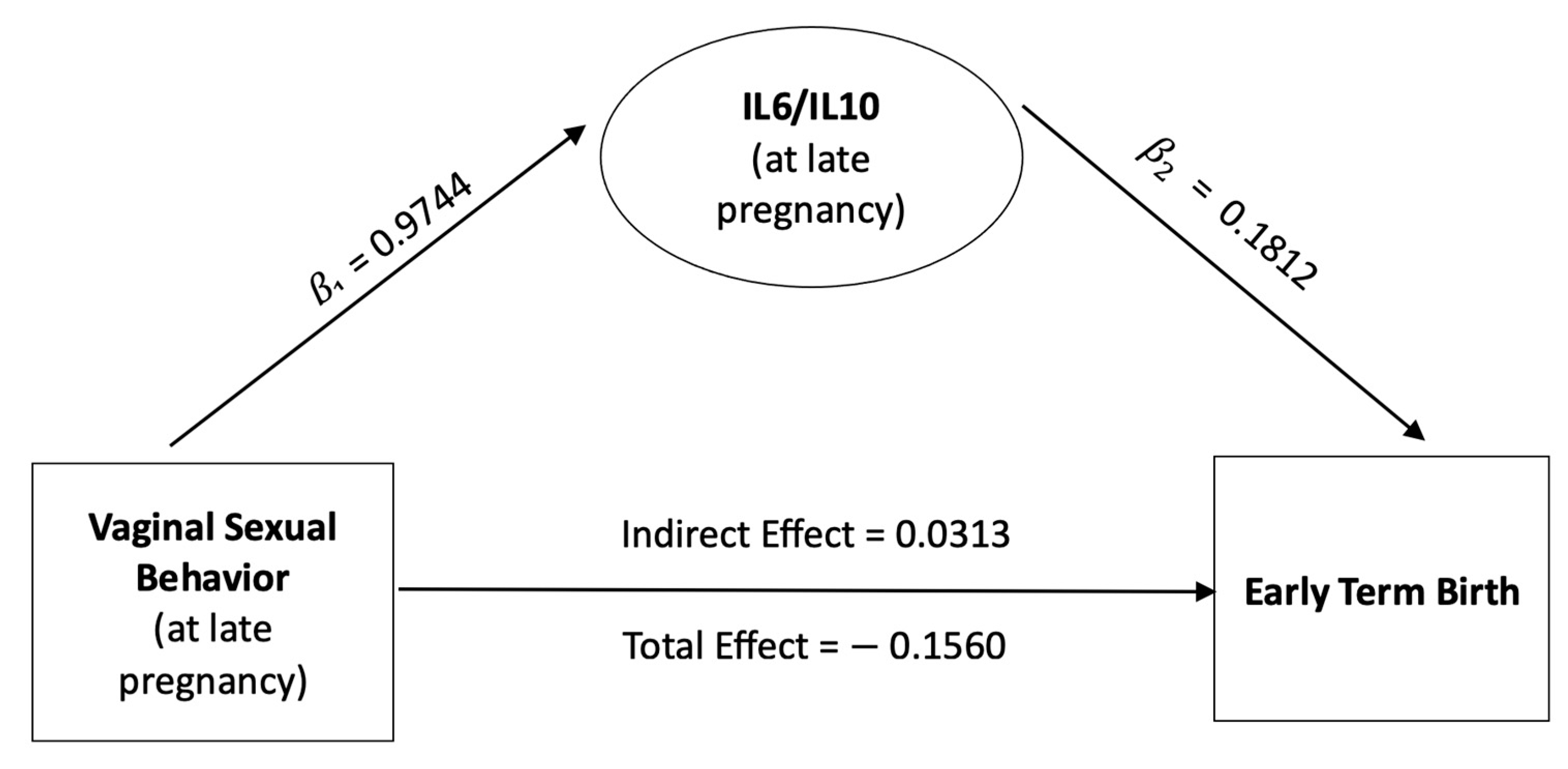
3.3. Mediating Role of the Vaginal IL-6/IL-10 Ratio
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goldenberg, R.L.; Culhane, J.F.; Iams, J.D.; Romero, R. Epidemiology and causes of preterm birth. Lancet 2008, 371, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.A.; Hamilton, B.E.; Osterman, M.J.; Driscoll, A.K.; Mathews, T.J. Births: Final Data for 2015. Natl. Vital Stat. Rep. 2017, 66, 1. [Google Scholar] [PubMed]
- Center for Disease Control and Prevention. Preterm Birth. Available online: https://www.cdc.gov/reproductivehealth/MaternalInfantHealth/PretermBirth.htm (accessed on 1 August 2022).
- Delnord, M.; Blondel, B.; Prunet, C.; Zeitlin, J. Are risk factors for preterm and early-term live singleton birth the same? A population-based study in France. BMJ Open 2018, 8, e018745. [Google Scholar] [CrossRef] [PubMed]
- Blencowe, H.; Lee, A.C.; Cousens, S.; Bahalim, A.; Narwal, R.; Zhong, N.; Chou, D.; Say, L.; Modi, N.; Katz, J.; et al. Preterm birth-associated neurodevelopmental impairment estimates at regional and global levels for 2010. Pediatr. Res. 2013, 74 (Suppl. S1), 17–34. [Google Scholar] [CrossRef] [PubMed]
- D’Onofrio, B.M.; Class, Q.A.; Rickert, M.E.; Larsson, H.; Langstrom, N.; Lichtenstein, P. Preterm birth and mortality and morbidity: A population-based quasi-experimental study. JAMA Psychiatry 2013, 70, 1231–1240. [Google Scholar] [CrossRef] [PubMed]
- Marret, S.; Ancel, P.Y.; Marpeau, L.; Marchand, L.; Pierrat, V.; Larroque, B.; Foix-L’Helias, L.; Thiriez, G.; Fresson, J.; Alberge, C.; et al. Neonatal and 5-year outcomes after birth at 30–34 weeks of gestation. Obstet. Gynecol. 2007, 110, 72–80. [Google Scholar] [CrossRef]
- Moreira, R.S.; Magalhaes, L.C.; Alves, C.R. Effect of preterm birth on motor development, behavior, and school performance of school-age children: A systematic review. J. Pediatr. 2014, 90, 119–134. [Google Scholar] [CrossRef]
- Mwaniki, M.K.; Atieno, M.; Lawn, J.E.; Newton, C.R. Long-term neurodevelopmental outcomes after intrauterine and neonatal insults: A systematic review. Lancet 2012, 379, 445–452. [Google Scholar] [CrossRef]
- Schieve, L.A.; Tian, L.H.; Baio, J.; Rankin, K.; Rosenberg, D.; Wiggins, L.; Maenner, M.J.; Yeargin-Allsopp, M.; Durkin, M.; Rice, C.; et al. Population attributable fractions for three perinatal risk factors for autism spectrum disorders, 2002 and 2008 autism and developmental disabilities monitoring network. Ann. Epidemiol. 2014, 24, 260–266. [Google Scholar] [CrossRef]
- Williams, B.L.; Dunlop, A.L.; Kramer, M.; Dever, B.V.; Hogue, C.; Jain, L. Perinatal origins of first-grade academic failure: Role of prematurity and maternal factors. Pediatrics 2013, 131, 693–700. [Google Scholar] [CrossRef]
- Wocadlo, C.; Rieger, I. Motor impairment and low achievement in very preterm children at eight years of age. Early Hum. Dev. 2008, 84, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Wolke, D.; Eryigit-Madzwamuse, S.; Gutbrod, T. Very preterm/very low birthweight infants’ attachment: Infant and maternal characteristics. Arch. Dis. Child. Fetal Neonatal Ed. 2014, 99, F70–F75. [Google Scholar] [CrossRef] [PubMed]
- Buckles, K.; Guldi, M. Worth the Wait? The Effect of Early Term Birth on Maternal and Infant Health. J. Policy Anal. Manag. 2017, 36, 748–772. [Google Scholar] [CrossRef] [PubMed]
- Darcy-Mahoney, A.; Minter, B.; Higgins, M.; Guo, Y.; Williams, B.; Head Zauche, L.M.; Birth, K. Probability of an Autism Diagnosis by Gestational Age. Newborn Infant Nurs. Rev. 2016, 16, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Leal, M.D.C.; Esteves-Pereira, A.P.; Nakamura-Pereira, M.; Domingues, R.; Dias, M.A.B.; Moreira, M.E.; Theme-Filha, M.; da Gama, S.G.N. Burden of early-term birth on adverse infant outcomes: A population-based cohort study in Brazil. BMJ Open 2017, 7, e017789. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, R.L.; Hauth, J.C.; Andrews, W.W. Intrauterine infection and preterm delivery. N. Engl. J. Med. 2000, 342, 1500–1507. [Google Scholar] [CrossRef]
- Martin, J.A.; Hamilton, B.E.; Osterman, M.J. Births in the United States, 2021. NCHS Data Brief 2022, 1–8. [Google Scholar]
- Martin, J.A.; Hamilton, B.E.; Osterman, M.J.K.; Driscoll, A.K. Births: Final Data for 2018. Natl. Vital Stat. Rep. 2019, 68, 1–47. [Google Scholar]
- McGrady, G.A.; Sung, J.F.; Rowley, D.L.; Hogue, C.J. Preterm delivery and low birth weight among first-born infants of black and white college graduates. Am. J. Epidemiol. 1992, 136, 266–276. [Google Scholar] [CrossRef]
- MacPhedran, S.E. Sexual Activity Recommendations in High-Risk Pregnancies: What is the Evidence? Sex Med. Rev. 2018, 6, 343–357. [Google Scholar] [CrossRef]
- Petridou, E.; Salvanos, H.; Skalkidou, A.; Dessypris, N.; Moustaki, M.; Trichopoulos, D. Are there common triggers of preterm deliveries? BJOG 2001, 108, 598–604. [Google Scholar] [CrossRef]
- Ekwo, E.E.; Gosselink, C.A.; Woolson, R.; Moawad, A.; Long, C.R. Coitus late in pregnancy: Risk of preterm rupture of amniotic sac membranes. Am. J. Obstet. Gynecol. 1993, 168 Pt 1, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.P.; Liu, X.H.; Gao, S.H.; Wang, J.M.; Gu, Y.S.; Zhang, J.Y.; Zhou, X.; Li, Q.X. Risk factors for preterm birth in five Maternal and Child Health hospitals in Beijing. PLoS ONE 2012, 7, e52780. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.C.; Yow, C.M.; Omar, S.Z. Effect of coital activity on onset of labor in women scheduled for labor induction: A randomized controlled trial. Obstet. Gynecol. 2007, 110, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Diaz, S.; Boeke, C.E.; Romans, A.T.; Young, B.; Margulis, A.V.; McElrath, T.F.; Ecker, J.L.; Bateman, B.T. Triggers of spontaneous preterm delivery—Why today? Paediatr. Perinat. Epidemiol. 2014, 28, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Read, J.S.; Klebanoff, M.A. Sexual intercourse during pregnancy and preterm delivery: Effects of vaginal microorganisms. The Vaginal Infections and Prematurity Study Group. Am. J. Obstet. Gynecol. 1993, 168, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Sayle, A.E.; Savitz, D.A.; Thorp, J.M., Jr.; Hertz-Picciotto, I.; Wilcox, A.J. Sexual activity during late pregnancy and risk of preterm delivery. Obstet. Gynecol. 2001, 97, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Di Renzo, G.C.; Tosto, V.; Giardina, I. The biological basis and prevention of preterm birth. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 52, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Lucaroni, F.; Morciano, L.; Rizzo, G.; D’Antonio, F.; Buonuomo, E.; Palombi, L.; Arduini, D. Biomarkers for predicting spontaneous preterm birth: An umbrella systematic review. J. Matern. Fetal Neonatal Med. 2018, 31, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.; Chauhan, M.; Awasthi, S. Interplay of cytokines in preterm birth. Indian J. Med. Res. 2017, 146, 316–327. [Google Scholar] [CrossRef]
- Fettweis, J.M.; Serrano, M.G.; Brooks, J.P.; Edwards, D.J.; Girerd, P.H.; Parikh, H.I.; Huang, B.; Arodz, T.J.; Edupuganti, L.; Glascock, A.L.; et al. The vaginal microbiome and preterm birth. Nat. Med. 2019, 25, 1012–1021. [Google Scholar] [CrossRef] [PubMed]
- Dunn, A.B.; Dunlop, A.L.; Miller, A.H.; Hogue, C.J.; Crofton, J.M.; Corwin, E.J. Complement Activation during Early Pregnancy and Clinical Predictors of Preterm Birth in African American Women. J. Perinat. Neonatal Nurs. 2019, 33, E15–E26. [Google Scholar] [CrossRef] [PubMed]
- Krasnyi, A.M.; Sadekova, A.A.; Vtorushina, V.V.; Kan, N.E.; Tyutyunnik, V.L.; Krechetova, L.V. Extracellular DNA levels and cytokine profiles in preterm birth: A cohort study. Arch. Gynecol. Obstet. 2022, 306, 1495–1502. [Google Scholar] [CrossRef]
- Zhang, J.M.; An, J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Corwin, E.J. Understanding cytokines. Part I: Physiology and mechanism of action. Biol. Res. Nurs. 2000, 2, 30–40. [Google Scholar] [CrossRef]
- Cappelletti, M.; Della Bella, S.; Ferrazzi, E.; Mavilio, D.; Divanovic, S. Inflammation and preterm birth. J. Leukoc. Biol. 2016, 99, 67–78. [Google Scholar] [CrossRef]
- Corwin, E.J.; Hogue, C.J.; Pearce, B.; Hill, C.C.; Read, T.D.; Mulle, J.; Dunlop, A.L. Protocol for the Emory University African American Vaginal, Oral, and Gut Microbiome in Pregnancy Cohort Study. BMC Pregnancy Childbirth 2017, 17, 161. [Google Scholar] [CrossRef]
- Committee on Obstetric Practice; The American Institute of Ultrasound in Medicine; The Society for Maternal-Fetal Medicine. Committee Opinion No 700: Methods for Estimating the Due Date. Obstet. Gynecol. 2017, 129, e150–e154. [Google Scholar] [CrossRef]
- Diagnostics, M. Available online: https://www.mesoscale.com/ (accessed on 1 August 2022).
- Systems, R.D. ELISA Kits. Available online: https://www.rndsystems.com/products/elisas (accessed on 1 August 2022).
- Garcia-Juarez, M.; Camacho-Morales, A. Defining the Role of Anti- and Pro-inflammatory Outcomes of Interleukin-6 in Mental Health. Neuroscience 2022, 492, 32–46. [Google Scholar] [CrossRef]
- Ridker, P.M.; Rane, M. Interleukin-6 Signaling and Anti-Interleukin-6 Therapeutics in Cardiovascular Disease. Circ. Res. 2021, 128, 1728–1746. [Google Scholar] [CrossRef]
- Saraiva, M.; Vieira, P.; O’Garra, A. Biology and therapeutic potential of interleukin-10. J. Exp. Med. 2020, 217, e20190418. [Google Scholar] [CrossRef] [PubMed]
- Cronkite, D.A.; Strutt, T.M. The Regulation of Inflammation by Innate and Adaptive Lymphocytes. J. Immunol. Res. 2018, 2018, 1467538. [Google Scholar] [CrossRef] [PubMed]
- Carson, W.; Kunkel, S. Type I and II Cytokine Superfamilies in Inflammatory Responses. In Inflammation: From Molecular and Cellular Mechanisms to the Clinic; John Wiley & Sons, Inc.: New York, NY, USA, 2017; pp. 587–618. [Google Scholar] [CrossRef]
- Committee opinion no 611: Method for estimating due date. Obstet. Gynecol. 2014, 124, 863–866. [CrossRef] [PubMed]
- Spence, T.; Allsopp, P.J.; Yeates, A.J.; Mulhern, M.S.; Strain, J.J.; McSorley, E.M. Maternal Serum Cytokine Concentrations in Healthy Pregnancy and Preeclampsia. J. Pregnancy 2021, 2021, 6649608. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling The False Discovery Rate—A Practical And Powerful Approach To Multiple Testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Zhao, Y.; Castellanos, F.X. Annual Research Review: Discovery science strategies in studies of the pathophysiology of child and adolescent psychiatric disorders–promises and limitations. J. Child Psychol. Psychiatry 2016, 57, 421–439. [Google Scholar] [CrossRef]
- Basu, S.; Kumbier, K.; Brown, J.B.; Yu, B. Iterative random forests to discover predictive and stable high-order interactions. Proc. Natl. Acad. Sci. USA 2018, 115, 1943–1948. [Google Scholar] [CrossRef]
- Imai, K.; Keele, L.; Tingley, D. A general approach to causal mediation analysis. Psychol. Methods 2010, 15, 309–334. [Google Scholar] [CrossRef]
- Tingley, D.; Yamamoto, T.; Hirose, K.; Keele, L.; Imai, K. Mediation: R package for causal mediation analysis. J. Stat. Softw. 2014, 59, 1–38. [Google Scholar] [CrossRef]
- Yost, N.P.; Owen, J.; Berghella, V.; Thom, E.; Swain, M.; Dildy, G.A., 3rd; Miodovnik, M.; Langer, O.; Sibai, B.; National Institute of Child, H.; et al. Effect of coitus on recurrent preterm birth. Obstet. Gynecol. 2006, 107, 793–797. [Google Scholar] [CrossRef]
- Prairie, E.; Cote, F.; Tsakpinoglou, M.; Mina, M.; Quiniou, C.; Leimert, K.; Olson, D.; Chemtob, S. The determinant role of IL-6 in the establishment of inflammation leading to spontaneous preterm birth. Cytokine Growth Factor Rev. 2021, 59, 118–130. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.A.; Christiaens, I.; Dorian, C.L.; Zaragoza, D.B.; Care, A.S.; Banks, A.M.; Olson, D.M. Interleukin-6 is an essential determinant of on-time parturition in the mouse. Endocrinology 2010, 151, 3996–4006. [Google Scholar] [CrossRef] [PubMed]
- Amabebe, E.; Reynolds, S.; He, X.; Wood, R.; Stern, V.; Anumba, D.O.C. Infection/inflammation-associated preterm delivery within 14 days of presentation with symptoms of preterm labour: A multivariate predictive model. PLoS ONE 2019, 14, e0222455. [Google Scholar] [CrossRef] [PubMed]
- MacKinnon, D.P.; Fairchild, A.J.; Fritz, M.S. Mediation analysis. Annu. Rev. Psychol. 2007, 58, 593–614. [Google Scholar] [CrossRef]
- O’Hara, S.; Zelesco, M.; Sun, Z. Cervical length for predicting preterm birth and a comparison of ultrasonic measurement techniques. Australas J. Ultrasound Med. 2013, 16, 124–134. [Google Scholar] [CrossRef]
- Bortoletto, T.G.; Silva, T.V.; Borovac-Pinheiro, A.; Pereira, C.M.; Silva, A.D.; Franca, M.S.; Hatanaka, A.R.; Argenton, J.P.; Passini, R., Jr.; Mol, B.W.; et al. Cervical length varies considering different populations and gestational outcomes: Results from a systematic review and meta-analysis. PLoS ONE 2021, 16, e0245746. [Google Scholar] [CrossRef]
- Annan, R.A.; Gyimah, L.A.; Apprey, C.; Asamoah-Boakye, O.; Aduku, L.N.E.; Azanu, W.; Luterodt, H.E.; Edusei, A.K. Predictors of adverse birth outcomes among pregnant adolescents in Ashanti Region, Ghana. J. Nutr. Sci. 2021, 10, e67. [Google Scholar] [CrossRef]
- Aliyu, M.H.; Jolly, P.E.; Ehiri, J.E.; Salihu, H.M. High parity and adverse birth outcomes: Exploring the maze. Birth 2005, 32, 45–59. [Google Scholar] [CrossRef]
- Schempf, A.H.; Branum, A.M.; Lukacs, S.L.; Schoendorf, K.C. Maternal age and parity-associated risks of preterm birth: Differences by race/ethnicity. Paediatr. Perinat. Epidemiol. 2007, 21, 34–43. [Google Scholar] [CrossRef]
- Clayborne, Z.M.; Giesbrecht, G.F.; Bell, R.C.; Tomfohr-Madsen, L.M. Relations between neighbourhood socioeconomic status and birth outcomes are mediated by maternal weight. Soc. Sci. Med. 2017, 175, 143–151. [Google Scholar] [CrossRef]
- Brody, S. The relative health benefits of different sexual activities. J. Sex Med. 2010, 7, 1336–1361. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Fernandez, S.; Brown, H.R.; Zhao, Y.; Raithel, J.A.; Bishop, S.L.; Kern, S.B.; Lord, C.; Petkova, E.; Di Martino, A. Perceived social support in adults with autism spectrum disorder and attention-deficit/hyperactivity disorder. Autism Res. 2017, 10, 866–877. [Google Scholar] [CrossRef]
- Shemkus, M.; Zhao, Y.; Mehra, P.; Figueroa, R. Opioid prescribing patterns of oral and maxillofacial surgery residents. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2020, 129, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Castro, T.; Zhao, Y.; Fitzpatrick, S.; Ruglass, L.M.; Hien, D.A. Seeing the forest for the trees: Predicting attendance in trials for co-occurring PTSD and substance use disorders with a machine learning approach. J. Consult. Clin. Psychol. 2021, 89, 869–884. [Google Scholar] [CrossRef]
- Jespers, V.; Kyongo, J.; Joseph, S.; Hardy, L.; Cools, P.; Crucitti, T.; Mwaura, M.; Ndayisaba, G.; Delany-Moretlwe, S.; Buyze, J.; et al. A longitudinal analysis of the vaginal microbiota and vaginal immune mediators in women from sub-Saharan Africa. Sci. Rep. 2017, 7, 11974. [Google Scholar] [CrossRef] [PubMed]
- Lajoie, J.; Kimani, M.; Plummer, F.A.; Nyamiobo, F.; Kaul, R.; Kimani, J.; Fowke, K.R. Association of sex work with reduced activation of the mucosal immune system. J. Infect. Dis. 2014, 210, 319–329. [Google Scholar] [CrossRef]
- Sivro, A.; Mwatelah, R.; Kambaran, C.; Gebrebrhan, H.; Becker, M.G.; Ma, H.; Klatt, N.R.; Zevin, A.S.; King’ola, N.; Wambua, S.; et al. Sex Work Is Associated With Increased Vaginal Microbiome Diversity in Young Women From Mombasa, Kenya. J. Acquir. Immune Defic. Syndr. 2020, 85, 79–87. [Google Scholar] [CrossRef]
| Variables | P sPTB | sETB | FTB |
|---|---|---|---|
| Sample Size | 49 | 93 | 255 (referent) |
| Age (years) | 24.51 ± 4.796 | 25.31 ± 4.83 | 24.75 ± 4.65 |
| Education (High School or less) | 32 (65.3%) | 55 (59.1%) | 125 (49%) |
| BMI at 1st prenatal visit | 26.24 ± 6.29 | 28.08 ± 7.86 | 28.93 ± 7.68 |
| Married or Cohabitating | 32 (65.3%) | 46 (49.5%) | 134 (52.5%) |
| Prior Birth 1 | 30 (61.2%) | 62 (66.7%) | 123 (48.2%) |
| Baby’s sex (Female) 2 | 14 (28.6%) | 52 (55.9%) | 135 (52.9%) |
| Model 1 | Model 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Estimate | SE | z.Value | p-Value | Estimate | SE | z.Value | p-Value |
| (Intercept) | −0.91 | 1.12 | −0.81 | 0.416 | −1.38 | 1.27 | −1.08 | 0.278 |
| age | 0.03 | 0.03 | 0.98 | 0.329 | 0.04 | 0.04 | 1.05 | 0.296 |
| Baby Sex (Female) | 0.22 | 0.29 | 0.75 | 0.450 | 0.19 | 0.32 | 0.59 | 0.553 |
| Prenatal BMI * | −0.04 | 0.02 | −1.78 | 0.074 | −0.03 | 0.02 | −1.48 | 0.138 |
| Parity | 0.76 | 0.32 | 2.36 | 0.018 | 0.67 | 0.36 | 1.87 | 0.061 |
| ≤High School | 0.61 | 0.32 | 1.92 | 0.054 | 0.43 | 0.35 | 1.23 | 0.218 |
| Married and Cohab (Yes) | −0.66 | 0.31 | −2.15 | 0.031 | −0.63 | 0.33 | −1.88 | 0.060 |
| Vaginal Sex | −0.94 | 0.31 | −3.00 | 0.003 | −1.03 | 0.35 | −2.92 | 0.003 |
| IL6_IL10 | 0.18 | 0.08 | 2.32 | 0.020 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dougherty, K.; Zhao, Y.; Dunlop, A.L.; Corwin, E. Association between Sexual Activity during Pregnancy, Pre- and Early-Term Birth, and Vaginal Cytokine Inflammation: A Prospective Study of Black Women. Healthcare 2023, 11, 1995. https://doi.org/10.3390/healthcare11141995
Dougherty K, Zhao Y, Dunlop AL, Corwin E. Association between Sexual Activity during Pregnancy, Pre- and Early-Term Birth, and Vaginal Cytokine Inflammation: A Prospective Study of Black Women. Healthcare. 2023; 11(14):1995. https://doi.org/10.3390/healthcare11141995
Chicago/Turabian StyleDougherty, Kylie, Yihong Zhao, Anne L. Dunlop, and Elizabeth Corwin. 2023. "Association between Sexual Activity during Pregnancy, Pre- and Early-Term Birth, and Vaginal Cytokine Inflammation: A Prospective Study of Black Women" Healthcare 11, no. 14: 1995. https://doi.org/10.3390/healthcare11141995
APA StyleDougherty, K., Zhao, Y., Dunlop, A. L., & Corwin, E. (2023). Association between Sexual Activity during Pregnancy, Pre- and Early-Term Birth, and Vaginal Cytokine Inflammation: A Prospective Study of Black Women. Healthcare, 11(14), 1995. https://doi.org/10.3390/healthcare11141995








