The Impact of Patient Infection Rate on Emergency Department Patient Flow: Hybrid Simulation Study in a Norwegian Case
Abstract
1. Introduction
- What is the likely impact of increased patient infection rate on patient flow parameters in an emergency department?
- What is the disparate effect of the increased patient infection rate on different COVID-19 intervention configurations in the emergency department?
2. Materials and Methods
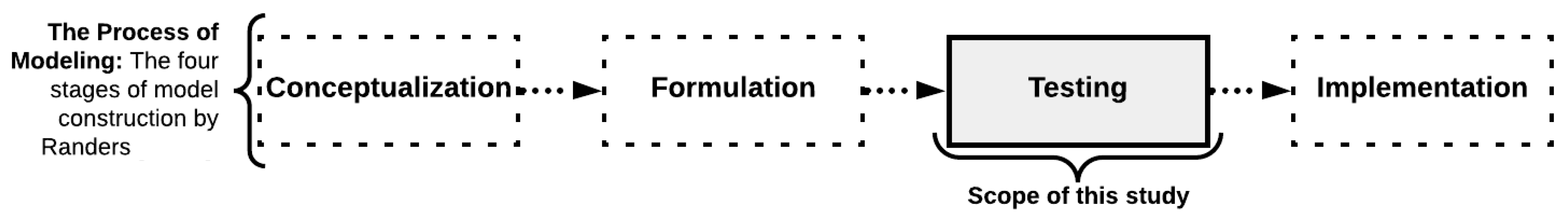
2.1. Theoretical Framework of the Simulation Modeling Method
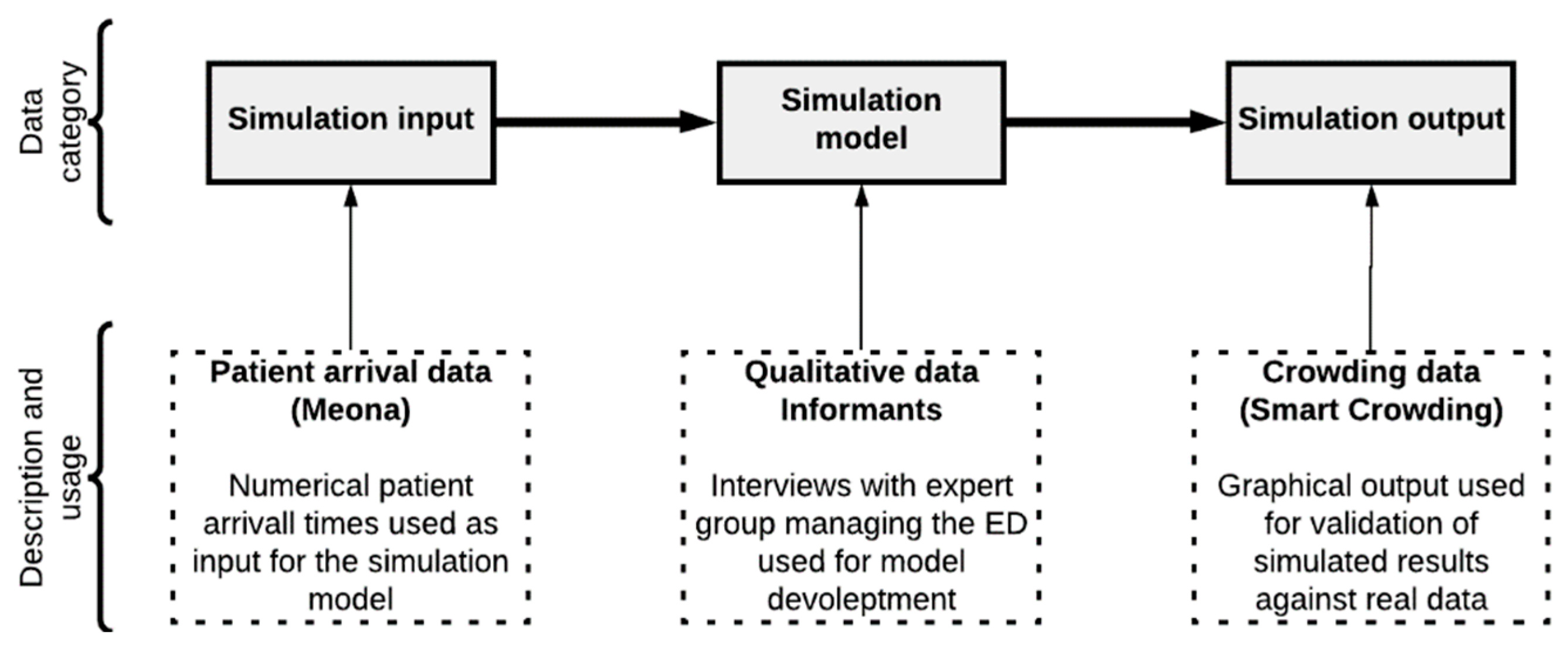
2.2. Types of Data Utilized in This Study
2.2.1. Patient Influx Data
2.2.2. Qualitative Data
2.2.3. Crowding Data
2.3. Case Emergency Department, Interventions, and Resources
2.4. Patient Flow Performance Indicators
2.4.1. Patient Flow Performance Indicators at Focus
2.4.2. Explanation and Model Implementation of Average Length of Stay (ALOS)
2.4.3. Explanation and Model Implementation of Crowding
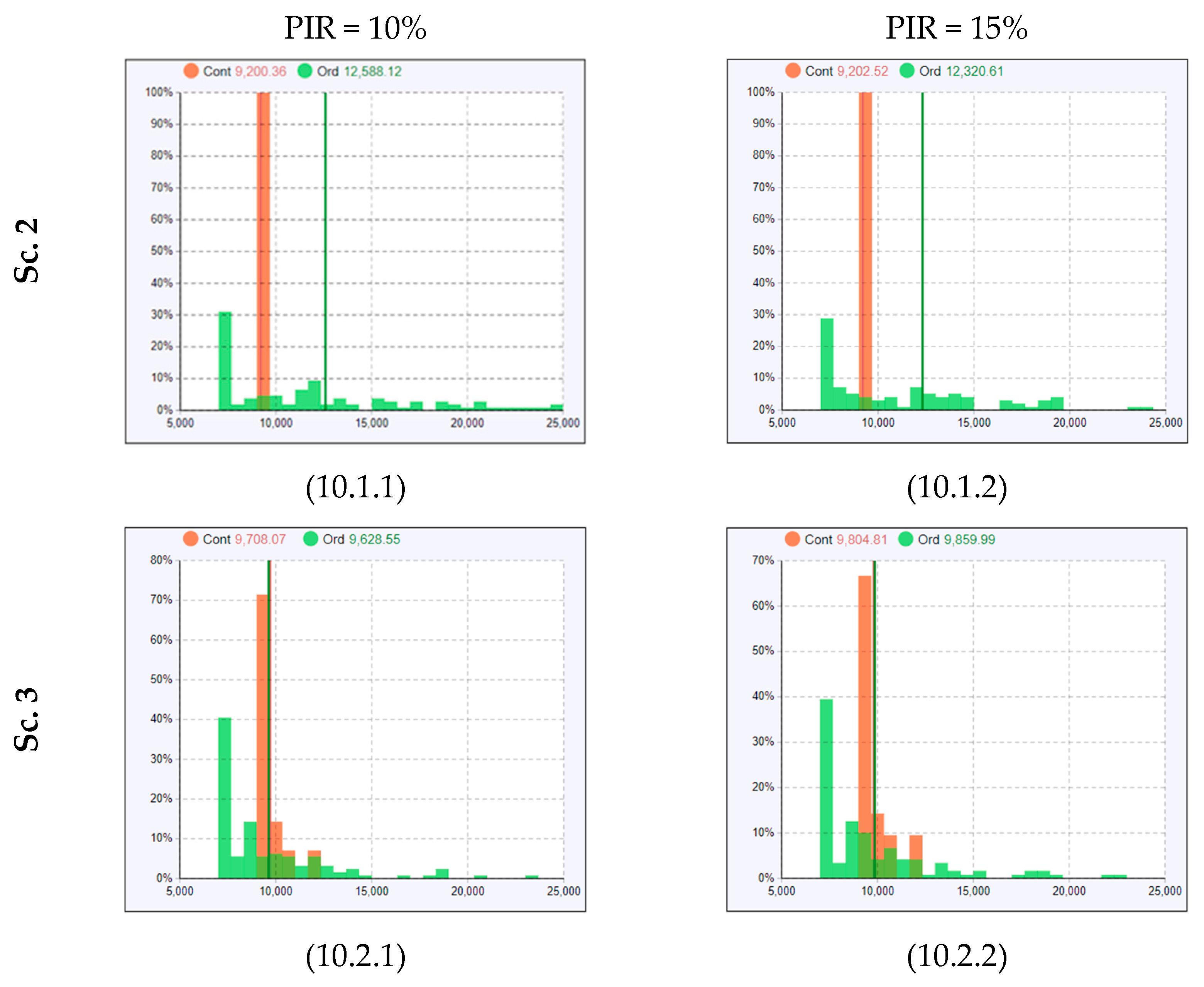
2.5. Experimental Design and Simulation Model Scenarios
3. Results and Discussions
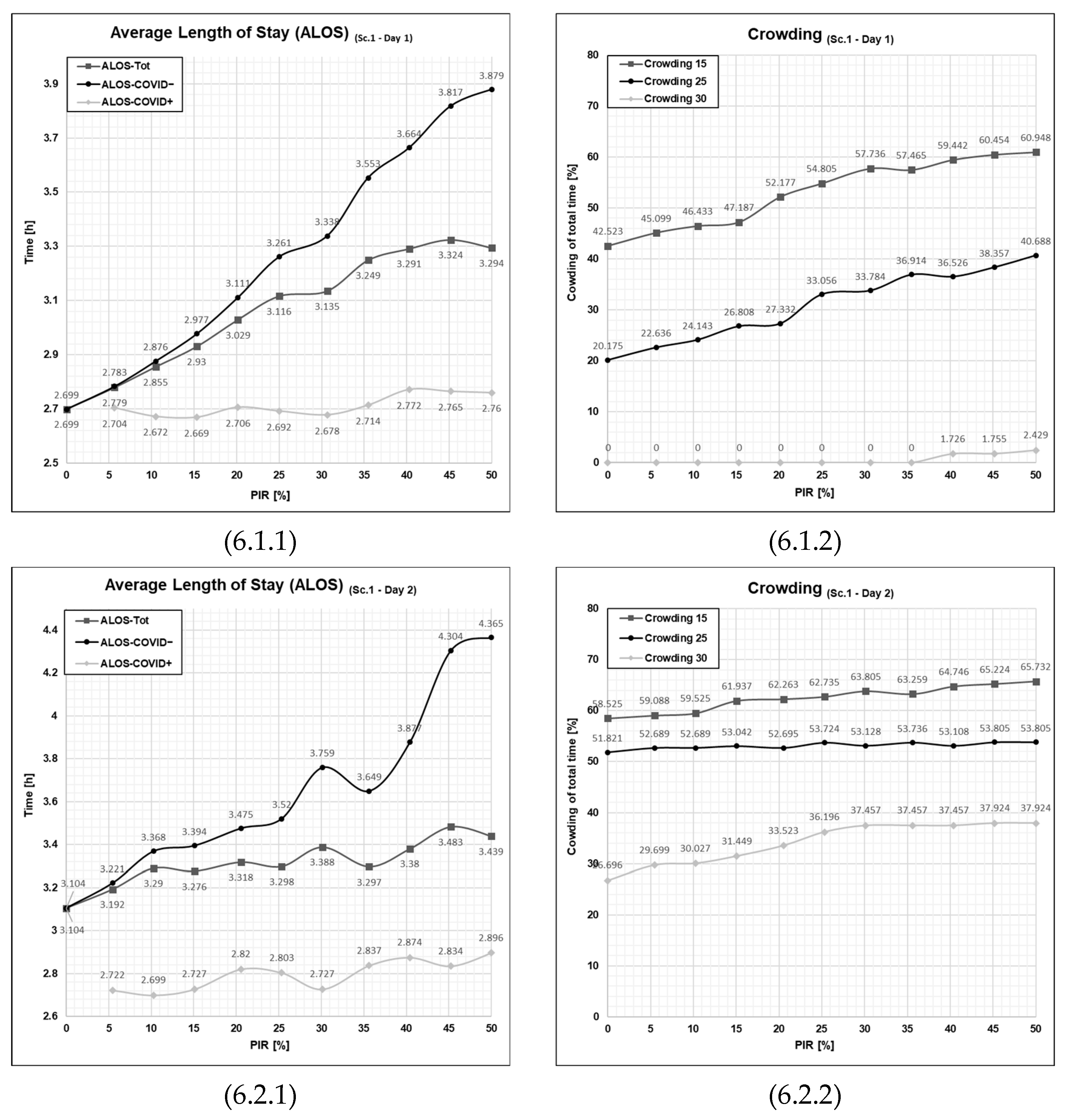
3.1. Results from Running Scenario 1—No Added Resources
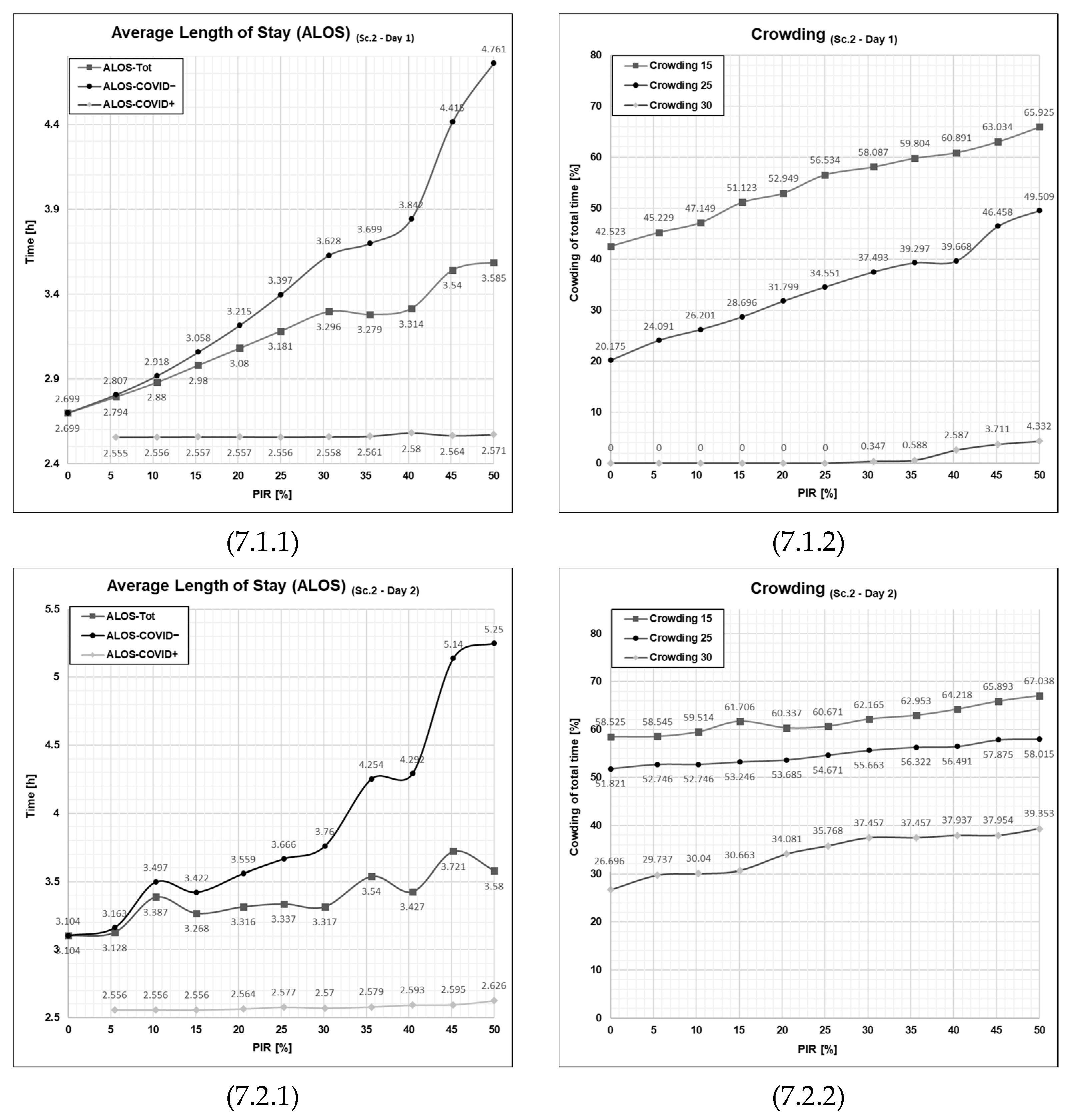
3.2. Results from Running Scenario 2—Added Waiting Zone
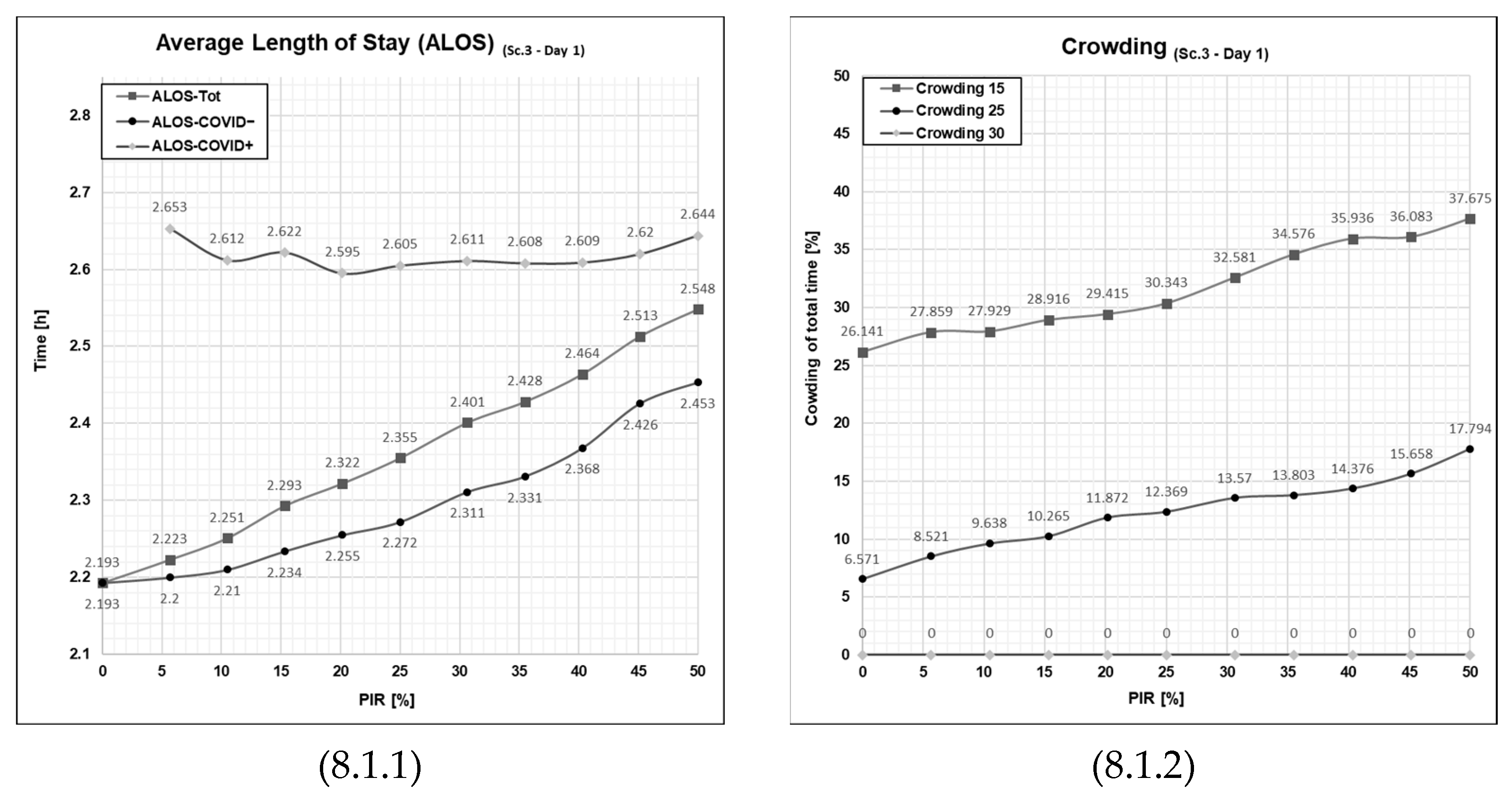
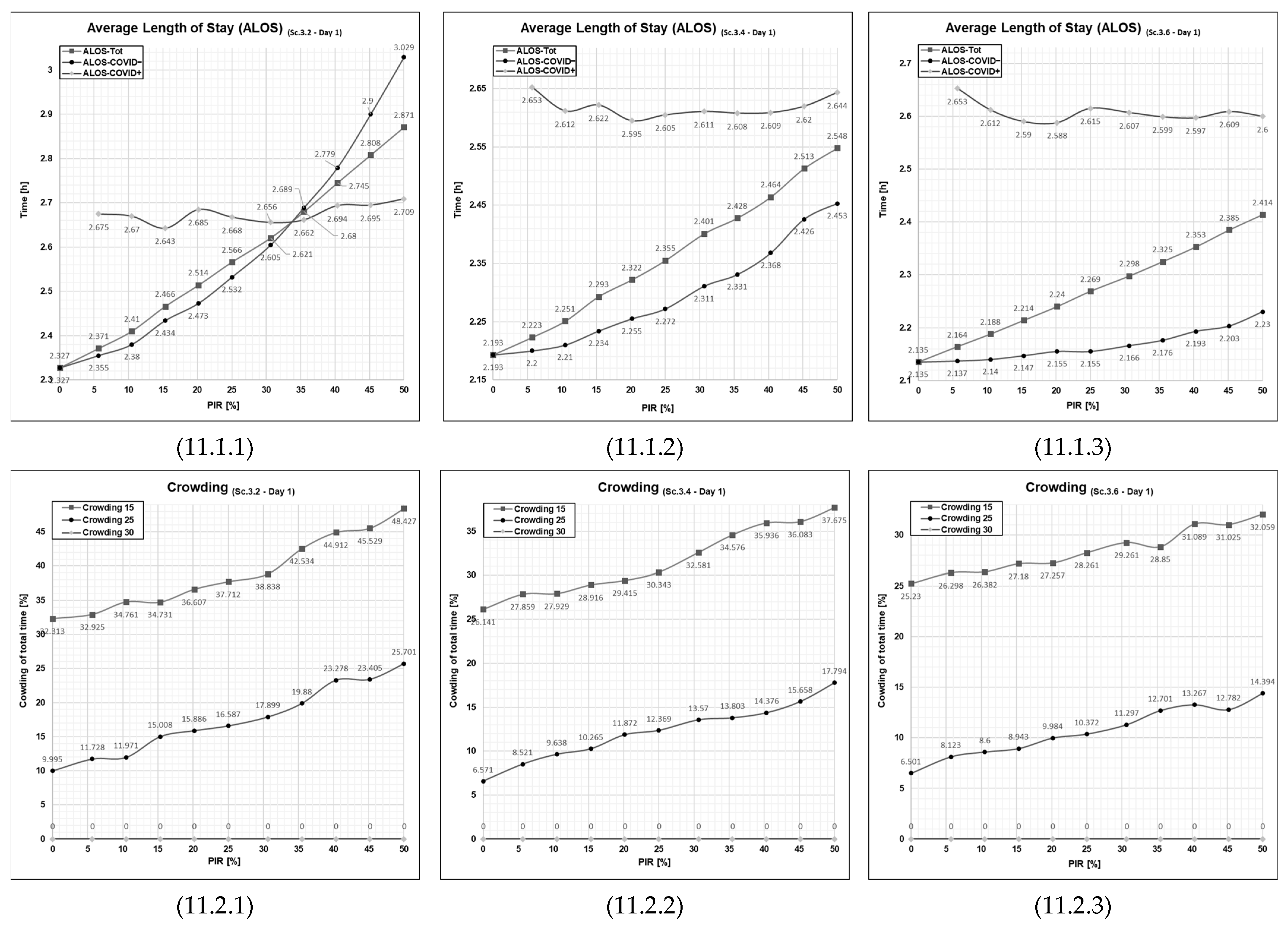
3.3. Results from Running Scenario 3—Added Extra Treatment Rooms
3.4. Results from Running Scenario 4—Added Waiting Zone and Extra Treatment
Discussing the Observed Non-Linearities in Sc. 1–4
3.5. The Effect of Extra Treatment Rooms on the ALOS and Crowding
3.5.1. Patient Flows at an Average Patient Influx Day (Day 1)
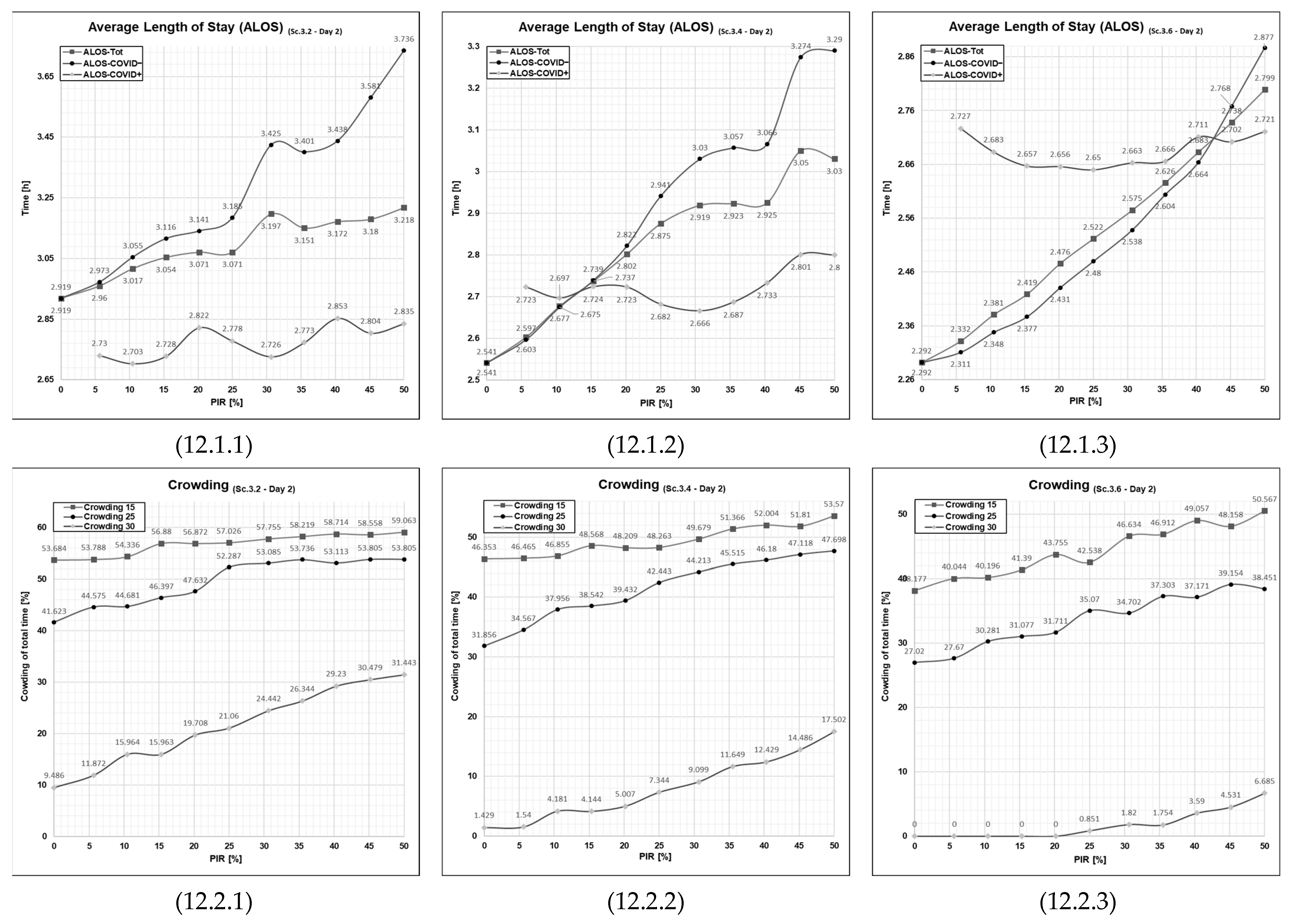
3.5.2. Patient Flows at a High Patient Influx Day (Day 2)
3.6. Discussion of Collateral Impacts of the COVID-19 Pandemic
3.7. Limitations
3.8. Suggestions for Further Research Work
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
Appendix A
| Day 1 | Time to Treatment [h/pt] | ALOS [h/pt] | Crowding [%] | Peak Crowding ED [#] (Time of Peak [When]) | Time Start Use [When] (Time in Use [%]) | Time Full [When] (Time Full [%]) | Time in WR (h/pt) | Times TR Blocked for cont. (#) | Times TR Seized [#] (Times WZ Seized [#]) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PCR | Tot. | Ord. | Con. | Tot. | Ord. | Con. | >15 | >25 | >30 | ED | E. TR | Tri. | WZ | E. TR | Tri. | WZ | |||
| 0% | 0.674 | 0.674 | null | 2.699 | 2.699 | null | 42.379 (11:00) | 20.182 (13:30) | 0.0 (null) | 26 (14:45) | null (0.0) | 11:00 (52.876) | null (0.0) | null (0.0) | 14:19 (4.697) | null (0.0) | 0.034 | 0 | 124 (0) |
| 5% | 0.716 | 0.76 | 0.114 | 2.775 | 2.785 | 2.645 | 44.537 (11:00) | 20.759 (12:15) | 0.0 (null) | 26 (14:45) | null (0.0) | 11:00 (55.295) | null (0.0) | null (0.0) | 14:18 (5.162) | null (0.0) | 0.035 | 4 | 124 (0) |
| 10% | 0.776 | 0.858 | 0.169 | 2.861 | 2.883 | 2.698 | 46.383 (11:00) | 24.992 (12:15) | 0.0 (null) | 27 (14:45) | null (0.0) | 11:00 (56.572) | null (0.0) | null (0.0) | 14:18 (6.327) | null (0.0) | 0.037 | 10 | 124 (0) |
| 15% | 0.824 | 0.916 | 0.141 | 2.909 | 2.941 | 2.67 | 46.907 (11:00) | 25.776 (13:30) | 0.0 (null) | 27 (14:45) | null (0.0) | 11:00 (55.911) | null (0.0) | null (0.0) | 14:18 (9.719) | null (0.0) | 0.051 | 10 | 124 (0) |
| 20% | 0.896 | 1.038 | 0.178 | 3.004 | 3.063 | 2.708 | 47.207 (11:00) | 27.954 (13:30) | 0.0 (null) | 29 (14:45) | null (0.0) | 11:00 (58.642) | null (0.0) | null (0.0) | 14:18 (10.745) | null (0.0) | 0.069 | 13 | 124 (0) |
| 25% | 0.984 | 1.257 | 0.22 | 3.141 | 3.281 | 2.75 | 57.214 (11:00) | 33.357 (12:00) | 0.0 (null) | 30 (15:30) | null (0.0) | 11:00 (61.842) | null (0.0) | null (0.0) | 13:33 (17.229) | null (0.0) | 0.122 | 22 | 124 (0) |
| 30% | 0.999 | 1.407 | 0.255 | 3.202 | 3.432 | 2.784 | 54.681 (11:00) | 33.621 (12:15) | 0.0 (null) | 30 (14:45) | null (0.0) | 11:00 (64.57) | null (0.0) | null (0.0) | 14:18 (14.185) | null (0.0) | 0.099 | 28 | 124 (0) |
| 35% | 1.057 | 1.591 | 0.275 | 3.287 | 3.617 | 2.803 | 56.765 (11:00) | 35.211 (13:30) | 1.749 (15:45) | 31 (15:45) | null (0.0) | 11:00 (65.214) | null (0.0) | null (0.0) | 14:18 (19.983) | null (0.0) | 0.109 | 33 | 124 (0) |
| 40% | 1.024 | 1.587 | 0.265 | 3.264 | 3.612 | 2.794 | 57.004 (11:00) | 38.161 (12:15) | 0.688 (15:30) | 31 (15:30) | null (0.0) | 11:00 (66.703) | null (0.0) | null (0.0) | 14:18 (20.97) | null (0.0) | 0.233 | 34 | 121 (0) |
| 45% | 1.117 | 1.821 | 0.288 | 3.373 | 3.846 | 2.817 | 60.003 (11:00) | 38.375 (12:00) | 1.601 (15:45) | 32 (15:45) | null (0.0) | 11:00 (65.291) | null (0.0) | null (0.0) | 14:03 (18.773) | null (0.0) | 0.182 | 35 | 124 (0) |
| 50% | 1.074 | 2.076 | 0.294 | 3.383 | 4.102 | 2.823 | 61.473 (11:00) | 42.044 (12:00) | 1.439 (14:45) | 31 (14:45) | null (0.0) | 11:00 (68.781) | null (0.0) | null (0.0) | 14:18 (20.106) | null (0.0) | 0.265 | 43 | 124 (0) |
| Day 2 | |||||||||||||||||||
| 0% | 1.096 | 1.096 | null | 3.121 | 3.121 | null | 58.518 (11:45) | 51.829 (12:30) | 26.606 (15:00) | 39 (17:15) | null (0.0) | 11:33 (63.199) | null (0.0) | null (0.0) | 12:48 (39.28) | null (0.0) | 0.259 | 0 | 138 (0) |
| 5% | 1.176 | 1.281 | 0.113 | 3.247 | 3.306 | 2.643 | 59.415 (11:45) | 52.191 (12:30) | 30.51 (15:00) | 40 (18:15) | null (0.0) | 11:33 (63.194) | null (0.0) | null (0.0) | 12:48 (41.272) | null (0.0) | 0.304 | 10 | 135 (0) |
| 10% | 1.085 | 1.193 | 0.389 | 3.178 | 3.218 | 2.919 | 60.196 (11:45) | 52.337 (12:30) | 30.127 (15:00) | 41 (17:00) | null (0.0) | 11:33 (64.909) | null (0.0) | null (0.0) | 13:03 (40.822) | null (0.0) | 0.256 | 14 | 134 (0) |
| 15% | 1.151 | 1.391 | 0.2 | 3.278 | 3.417 | 2.729 | 61.225 (11:45) | 52.543 (12:15) | 32.795 (15:00) | 41 (16:45) | null (0.0) | 11:33 (63.284) | null (0.0) | null (0.0) | 12:48 (40.797) | null (0.0) | 0.221 | 17 | 135 (0) |
| 20% | 1.124 | 1.372 | 0.255 | 3.261 | 3.397 | 2.785 | 60.883 (11:45) | 52.229 (12:30) | 32.268 (15:00) | 43 (19:30) | null (0.0) | 11:33 (64.901) | null (0.0) | null (0.0) | 13:18 (40.597) | null (0.0) | 0.261 | 18 | 132 (0) |
| 25% | 1.016 | 1.279 | 0.341 | 3.182 | 3.304 | 2.87 | 61.045 (11:45) | 52.692 (12:30) | 36.474 (15:00) | 44 (17:30) | null (0.0) | 11:33 (64.99) | null (0.0) | null (0.0) | 13:18 (38.655) | null (0.0) | 0.297 | 28 | 127 (0) |
| 30% | 1.27 | 1.914 | 0.246 | 3.49 | 3.94 | 2.775 | 64.286 (11:45) | 53.156 (12:15) | 37.457 (15:00) | 45 (17:30) | null (0.0) | 11:33 (65.432) | null (0.0) | null (0.0) | 12:48 (45.128) | null (0.0) | 0.334 | 32 | 127 (0) |
| 35% | 1.001 | 1.663 | 0.225 | 3.258 | 3.689 | 2.754 | 63.777 (11:45) | 53.146 (12:15) | 37.457 (15:00) | 45 (17:30) | null (0.0) | 11:33 (66.343) | null (0.0) | null (0.0) | 12:48 (45.749) | null (0.0) | 0.391 | 39 | 126 (0) |
| 40% | 1.099 | 2.169 | 0.313 | 3.414 | 4.193 | 2.842 | 66.159 (11:30) | 54.667 (12:15) | 37.933 (14:45) | 47 (17:30) | null (0.0) | 11:33 (66.982) | null (0.0) | null (0.0) | 13:33 (42.714) | null (0.0) | 0.544 | 46 | 124 (0) |
| 45% | 1.161 | 1.977 | 0.33 | 3.436 | 4.002 | 2.859 | 65.816 (11:45) | 55.169 (12:15) | 37.457 (15:00) | 46 (17:30) | null (0.0) | 11:33 (67.203) | null (0.0) | null (0.0) | 13:48 (41.513) | null (0.0) | 0.372 | 37 | 127 (0) |
| 50% | 0.915 | 2.179 | 0.509 | 3.322 | 4.204 | 3.038 | 66.037 (11:45) | 56.143 (12:15) | 38.057 (14:45) | 50 (20:45) | null (0.0) | 11:33 (68.342) | null (0.0) | null (0.0) | 13:33 (43.078) | null (0.0) | 0.039 | 59 | 124 (0) |
| Day 1 | Time to Treatment [h/pt] | ALOS [h/pt] | Crowding [%] | Peak Crowding ED [#] (Time of Peak [When]) | Time Start Use [When] (Time in Use [%]) | Time Full [When] (Time Full [%]) | Time in WR (h/pt) | Times TR Blocked for cont. (#) | Times TR Seized [#] (Times WZ Seized [#]) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PCR | Tot. | Ord. | Con. | Tot. | Ord. | Con. | >15 | >25 | >30 | ED | E. TR | Tri. | WZ | E. TR | Tri. | WZ | |||
| 0% | 0.674 | 0.674 | null | 2.699 | 2.699 | null | 42.379 (11:00) | 20.182 (13:30) | 0.0 (null) | 26 (14:45) | null (0.0) | 11:00 (52.876) | null (0.0) | null (0.0) | 14:19 (4.697) | null (0.0) | 0.034 | 0 | 124 (0) |
| 5% | 0.704 | 0.734 | 0.028 | 2.75 | 2.759 | 2.559 | 44.838 (11:00) | 21.362 (12:15) | 0.0 (null) | 26 (14:45) | null (0.0) | 11:00 (53.752) | 17:30 (0.0) | null (0.0) | 14:18 (5.162) | null (0.0) | 0.035 | 0 | 125 (1) |
| 10% | 0.766 | 0.842 | 0.028 | 2.839 | 2.868 | 2.558 | 45.567 (11:00) | 24.282 (13:30) | 0.0 (null) | 27 (15:30) | null (0.0) | 11:00 (56.789) | 13:45 (0.0) | null (0.0) | 14:18 (5.766) | null (0.0) | 0.04 | 0 | 132 (8) |
| 15% | 0.771 | 0.847 | 0.028 | 2.844 | 2.873 | 2.557 | 45.306 (11:00) | 24.141 (13:30) | 0.0 (null) | 27 (15:30) | null (0.0) | 11:00 (56.803) | 13:45 (0.0) | null (0.0) | 14:18 (5.766) | null (0.0) | 0.04 | 0 | 131 (7) |
| 20% | 0.979 | 1.181 | 0.03 | 3.094 | 3.208 | 2.56 | 53.5 (11:00) | 32.911 (11:30) | 0.0 (null) | 30 (15:45) | null (0.0) | 11:00 (60.379) | 11:00 (0.0) | null (0.0) | 14:18 (12.496) | null (0.0) | 0.084 | 2 | 140 (16) |
| 25% | 0.97 | 1.2 | 0.031 | 3.095 | 3.226 | 2.561 | 50.377 (11:00) | 30.928 (12:15) | 0.0 (null) | 30 (14:45) | null (0.0) | 11:00 (61.067) | 11:00 (0.0) | null (0.0) | 14:18 (11.23) | null (0.0) | 0.089 | 3 | 140 (16) |
| 30% | 1.057 | 1.487 | 0.045 | 3.234 | 3.515 | 2.574 | 57.362 (11:00) | 35.921 (12:00) | 3.04 (14:30) | 34 (14:45) | null (0.0) | 11:00 (61.926) | 11:00 (2.341) | null (0.0) | 14:35 (15.781) | 14:15 (0.0) | 0.115 | 8 | 145 (21) |
| 35% | 1.16 | 1.803 | 0.057 | 3.373 | 3.832 | 2.586 | 58.932 (11:00) | 39.084 (12:15) | 0.949 (14:45) | 32 (14:45) | null (0.0) | 11:00 (62.505) | 11:00 (0.0) | null (0.0) | 14:20 (18.893) | null (0.0) | 0.159 | 12 | 147 (23) |
| 40% | 1.232 | 2.166 | 0.079 | 3.486 | 4.196 | 2.609 | 64.307 (11:00) | 42.508 (11:30) | 1.669 (14:45) | 32 (14:45) | null (0.0) | 11:00 (64.334) | 11:00 (0.0) | null (0.0) | 14:05 (20.459) | null (0.0) | 0.226 | 15 | 154 (30) |
| 45% | 1.179 | 2.199 | 0.082 | 3.451 | 4.231 | 2.611 | 63.18 (11:00) | 45.553 (12:00) | 2.433 (14:30) | 32 (14:45) | null (0.0) | 11:00 (66.505) | 11:00 (0.0) | null (0.0) | 13:48 (25.838) | null (0.0) | 0.27 | 17 | 154 (33) |
| 50% | 0.989 | 2.049 | 0.168 | 3.301 | 4.082 | 2.697 | 64.686 (11:00) | 45.038 (11:30) | 1.668 (14:30) | 33 (14:45) | null (0.0) | 11:00 (69.976) | 11:00 (0.0) | null (0.0) | 13:50 (27.207) | null (0.0) | 0.343 | 28 | 156 (33) |
| Day 2 | |||||||||||||||||||
| 0% | 1.096 | 1.096 | null | 3.121 | 3.121 | null | 58.518 (11:45) | 51.829 (12:30) | 26.606 (15:00) | 39 (17:15) | null (0.0) | 11:33 (63.199) | null (0.0) | null (0.0) | 12:48 (39.28) | null (0.0) | 0.259 | 0 | 138 (0) |
| 5% | 1.238 | 1.356 | 0.028 | 3.308 | 3.382 | 2.558 | 59.819 (11:45) | 52.629 (12:30) | 29.551 (15:00) | 41 (17:30) | null (0.0) | 11:33 (63.194) | 11:45 (0.0) | null (0.0) | 12:48 (40.724) | null (0.0) | 0.352 | 0 | 144 (8) |
| 10% | 1.076 | 1.201 | 0.03 | 3.156 | 3.227 | 2.558 | 59.276 (11:45) | 52.726 (12:30) | 29.776 (15:00) | 41 (17:00) | null (0.0) | 11:33 (64.964) | 12:45 (0.0) | null (0.0) | 13:03 (42.865) | null (0.0) | 0.21 | 1 | 146 (10) |
| 15% | 1.216 | 1.437 | 0.028 | 3.322 | 3.464 | 2.556 | 59.612 (11:45) | 53.622 (12:30) | 31.446 (15:00) | 42 (17:30) | null (0.0) | 11:33 (65.97) | 13:00 (0.0) | null (0.0) | 12:48 (42.227) | null (0.0) | 0.327 | 0 | 148 (13) |
| 20% | 1.212 | 1.604 | 0.039 | 3.367 | 3.633 | 2.568 | 61.392 (11:45) | 53.888 (12:30) | 35.687 (15:00) | 46 (17:30) | null (0.0) | 11:33 (64.683) | 11:45 (0.0) | null (0.0) | 13:03 (39.153) | null (0.0) | 0.259 | 8 | 158 (25) |
| 25% | 1.205 | 1.59 | 0.063 | 3.36 | 3.618 | 2.593 | 60.794 (11:45) | 54.584 (12:15) | 35.962 (15:00) | 45 (17:30) | null (0.0) | 11:33 (66.281) | 11:45 (0.0) | null (0.0) | 13:04 (41.051) | null (0.0) | 0.279 | 7 | 152 (23) |
| 30% | 1.3 | 1.959 | 0.064 | 3.504 | 3.99 | 2.592 | 64.576 (11:45) | 54.954 (12:30) | 36.761 (15:00) | 44 (17:00) | null (0.0) | 11:33 (66.798) | 11:45 (0.0) | null (0.0) | 13:33 (41.286) | null (0.0) | 0.441 | 12 | 160 (32) |
| 35% | 1.338 | 2.262 | 0.074 | 3.578 | 4.291 | 2.604 | 63.709 (11:45) | 54.547 (12:30) | 38.723 (13:45) | 45 (16:45) | null (0.0) | 11:33 (68.524) | 11:45 (2.444) | null (0.0) | 13:50 (40.626) | 13:15 (0.0) | 0.45 | 16 | 158 (28) |
| 40% | 1.338 | 2.599 | 0.122 | 3.623 | 4.631 | 2.651 | 65.434 (11:45) | 55.725 (12:15) | 37.457 (15:00) | 50 (17:30) | null (0.0) | 11:33 (68.349) | 12:00 (2.482) | null (0.0) | 13:03 (43.676) | 15:31 (0.0) | 0.424 | 16 | 162 (37) |
| 45% | 1.27 | 2.657 | 0.192 | 3.582 | 4.689 | 2.721 | 67.118 (11:45) | 55.725 (12:15) | 37.457 (15:00) | 50 (19:30) | null (0.0) | 11:33 (70.772) | 12:00 (12.209) | null (0.0) | 13:03 (41.651) | 15:30 (0.0) | 0.32 | 27 | 155 (30) |
| 50% | 0.929 | 2.529 | 0.106 | 3.291 | 4.565 | 2.636 | 66.839 (11:30) | 56.3 (12:15) | 39.062 (13:45) | 48 (18:15) | null (0.0) | 11:33 (67.85) | 12:00 (0.372) | null (0.0) | 13:33 (43.083) | 13:45 (0.0) | 0.164 | 25 | 162 (40) |
| Day 1 | Time to Treatment [h/pt] | ALOS [h/pt] | Crowding [%] | Peak Crowding ED [#] (Time of Peak [When]) | Time Start Use [When] (Time in Use [%]) | Time Full [When] (Time Full [%]) | Time in WR (h/pt) | Times TR Blocked for cont. (#) | Times TR Seized [#] (Times WZ Seized [#]) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PCR | Tot. | Ord. | Con. | Tot. | Ord. | Con. | >15 | >25 | >30 | ED | E. TR | Tri. | WZ | E. TR | Tri. | WZ | |||
| 0% | 0.165 | 0.165 | null | 2.193 | 2.193 | null | 26.212 (11:00) | 6.598 (14:45) | 0.0 (null) | 23 (15:15) | 11:00 (44.386) | 12:03 (15.609) | null (0.0) | 11:00 (28.482) | null (0.0) | null (44.386) | 0.028 | 0 | 124 (0) |
| 5% | 0.175 | 0.181 | 0.028 | 2.223 | 2.209 | 2.56 | 26.755 (11:00) | 7.811 (12:15) | 0.0 (null) | 23 (15:15) | 11:00 (44.406) | 12:03 (18.12) | null (0.0) | 11:00 (28.507) | null (0.0) | null (44.406) | 0.028 | 0 | 124 (0) |
| 10% | 0.181 | 0.186 | 0.139 | 2.26 | 2.213 | 2.669 | 27.939 (11:00) | 8.427 (12:15) | 0.0 (null) | 23 (14:45) | 11:00 (44.392) | 11:03 (18.87) | null (0.0) | 11:00 (28.462) | null (0.0) | null (44.392) | 0.028 | 6 | 124 (0) |
| 15% | 0.196 | 0.219 | 0.086 | 2.309 | 2.246 | 2.617 | 29.535 (11:00) | 9.68 (14:30) | 0.0 (null) | 25 (15:30) | 11:00 (47.51) | 12:03 (18.66) | null (0.0) | 11:00 (29.757) | null (0.0) | null (47.51) | 0.028 | 8 | 124 (0) |
| 20% | 0.203 | 0.231 | 0.054 | 2.311 | 2.258 | 2.585 | 29.42 (11:00) | 10.91 (12:15) | 0.0 (null) | 24 (14:45) | 11:00 (46.275) | 12:03 (21.252) | null (0.0) | 11:00 (28.675) | null (0.0) | null (46.275) | 0.028 | 3 | 124 (0) |
| 25% | 0.202 | 0.232 | 0.067 | 2.323 | 2.259 | 2.598 | 28.564 (11:00) | 10.584 (12:15) | 0.0 (null) | 23 (14:15) | 11:00 (49.405) | 12:03 (21.731) | null (0.0) | 11:00 (28.661) | null (0.0) | null (49.405) | 0.028 | 6 | 124 (0) |
| 30% | 0.211 | 0.243 | 0.128 | 2.377 | 2.27 | 2.658 | 30.745 (11:00) | 12.229 (13:45) | 0.0 (null) | 23 (14:45) | 11:00 (46.351) | 11:00 (21.089) | null (0.0) | 11:00 (28.668) | null (0.0) | null (46.351) | 0.028 | 8 | 124 (0) |
| 35% | 0.244 | 0.324 | 0.106 | 2.455 | 2.35 | 2.637 | 35.734 (11:00) | 14.392 (12:00) | 0.0 (null) | 25 (14:45) | 11:00 (47.67) | 11:33 (24.815) | null (0.0) | 11:00 (32.844) | null (0.0) | null (47.67) | 0.028 | 13 | 124 (0) |
| 40% | 0.243 | 0.34 | 0.097 | 2.47 | 2.367 | 2.627 | 34.264 (11:00) | 15.922 (12:00) | 0.0 (null) | 25 (15:45) | 11:00 (50.027) | 12:03 (23.69) | null (0.0) | 11:00 (34.072) | null (0.0) | null (50.027) | 0.028 | 14 | 124 (0) |
| 45% | 0.274 | 0.414 | 0.119 | 2.54 | 2.442 | 2.649 | 35.515 (11:00) | 17.864 (12:15) | 0.0 (null) | 26 (14:45) | 11:00 (50.657) | 12:03 (24.881) | null (0.0) | 11:00 (33.71) | null (0.0) | null (50.657) | 0.028 | 18 | 124 (0) |
| 50% | 0.265 | 0.381 | 0.151 | 2.546 | 2.409 | 2.68 | 34.927 (11:00) | 17.119 (13:45) | 0.0 (null) | 26 (15:45) | 11:00 (50.989) | 11:03 (24.835) | null (0.0) | 11:00 (33.548) | null (0.0) | null (50.989) | 0.028 | 22 | 124 (0) |
| Day 2 | |||||||||||||||||||
| 0% | 0.514 | 0.514 | null | 2.541 | 2.541 | null | 46.305 (11:45) | 31.866 (12:30) | 1.355 (17:15) | 31 (17:15) | 11:33 (50.352) | 12:18 (41.288) | null (0.0) | 11:48 (46.744) | 15:18 (3.031) | null (50.352) | 0.038 | 0 | 146 (0) |
| 5% | 0.589 | 0.626 | 0.114 | 2.652 | 2.652 | 2.644 | 47.581 (11:45) | 35.333 (12:30) | 3.535 (15:30) | 34 (17:30) | 11:33 (57.59) | 12:18 (43.765) | null (0.0) | 11:48 (47.055) | 15:18 (6.392) | null (57.59) | 0.053 | 7 | 146 (0) |
| 10% | 0.647 | 0.729 | 0.186 | 2.75 | 2.756 | 2.718 | 47.755 (11:45) | 38.239 (12:30) | 5.356 (15:30) | 34 (17:30) | 11:33 (57.577) | 12:18 (45.799) | null (0.0) | 11:48 (48.158) | 15:18 (8.703) | null (57.577) | 0.06 | 11 | 146 (0) |
| 15% | 0.655 | 0.755 | 0.168 | 2.768 | 2.782 | 2.697 | 47.844 (11:45) | 41.006 (12:15) | 4.133 (15:15) | 35 (17:00) | 11:33 (52.309) | 12:18 (43.965) | null (0.0) | 11:48 (46.861) | 15:03 (9.531) | null (52.309) | 0.07 | 14 | 146 (0) |
| 20% | 0.692 | 0.824 | 0.201 | 2.826 | 2.851 | 2.731 | 47.813 (11:45) | 40.281 (12:30) | 5.037 (15:15) | 35 (17:15) | 11:33 (59.423) | 12:18 (45.878) | null (0.0) | 11:48 (48.204) | 15:18 (9.743) | null (59.423) | 0.067 | 17 | 146 (0) |
| 25% | 0.692 | 0.824 | 0.201 | 2.826 | 2.851 | 2.731 | 47.813 (11:45) | 40.281 (12:30) | 5.037 (15:15) | 35 (17:15) | 11:33 (59.423) | 12:18 (45.878) | null (0.0) | 11:48 (48.204) | 15:18 (9.743) | null (59.423) | 0.067 | 17 | 146 (0) |
| 30% | 0.738 | 0.991 | 0.185 | 2.923 | 3.019 | 2.715 | 49.347 (11:30) | 43.576 (12:15) | 6.922 (15:15) | 34 (17:00) | 11:33 (73.999) | 12:18 (45.724) | null (0.0) | 11:48 (48.263) | 15:18 (14.42) | null (73.999) | 0.088 | 24 | 146 (0) |
| 35% | 0.727 | 1.091 | 0.221 | 2.965 | 3.118 | 2.751 | 53.815 (11:30) | 45.377 (12:15) | 13.294 (15:15) | 36 (17:00) | 11:33 (74.99) | 12:18 (47.386) | null (0.0) | 11:48 (56.18) | 15:18 (18.36) | null (74.99) | 0.121 | 35 | 146 (0) |
| 40% | 0.857 | 1.331 | 0.179 | 3.091 | 3.359 | 2.71 | 54.879 (11:30) | 46.072 (12:15) | 14.001 (15:15) | 36 (17:30) | 11:33 (73.043) | 12:18 (48.971) | null (0.0) | 11:48 (55.009) | 15:04 (18.712) | null (73.043) | 0.194 | 33 | 146 (0) |
| 45% | 0.84 | 1.393 | 0.234 | 3.107 | 3.42 | 2.764 | 53.371 (11:30) | 47.933 (12:15) | 19.29 (15:15) | 39 (18:15) | 11:33 (73.035) | 12:18 (49.939) | null (0.0) | 11:46 (51.908) | 15:48 (19.507) | null (73.035) | 0.227 | 38 | 146 (0) |
| 50% | 0.827 | 1.488 | 0.318 | 3.139 | 3.517 | 2.847 | 54.228 (11:30) | 48.574 (12:15) | 15.906 (15:15) | 38 (17:30) | 11:33 (76.708) | 12:18 (50.2) | null (0.0) | 11:48 (53.962) | 15:48 (18.469) | null (76.708) | 0.172 | 47 | 146 (0) |
| Day 1 | Time to Treatment [h/pt] | ALOS [h/pt] | Crowding [%] | Peak Crowding ED [#] (Time of Peak [When]) | Time Start Use [When] (Time in Use [%]) | Time Full [When] (Time Full [%]) | Time in WR (h/pt) | Times TR Blocked for cont. (#) | Times TR Seized [#] (Times WZ Seized [#]) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PCR | Tot. | Ord. | Con. | Tot. | Ord. | Con. | >15 | >25 | >30 | ED | E. TR | Tri. | WZ | E. TR | Tri. | WZ | |||
| 0% | 0.165 | 0.165 | null | 2.193 | 2.193 | null | 26.212 (11:00) | 6.598 (14:45) | 0.0 (null) | 23 (15:15) | 11:00 (44.386) | 12:03 (15.609) | null (0.0) | 11:00 (28.482) | null (0.0) | null (44.386) | 0.028 | 0 | 124 (0) |
| 5% | 0.175 | 0.181 | 0.028 | 2.223 | 2.209 | 2.56 | 26.755 (11:00) | 7.811 (12:15) | 0.0 (null) | 23 (15:15) | 11:00 (44.406) | 12:03 (18.12) | null (0.0) | 11:00 (28.507) | null (0.0) | null (44.406) | 0.028 | 0 | 124 (0) |
| 10% | 0.187 | 0.203 | 0.028 | 2.262 | 2.231 | 2.559 | 27.457 (11:00) | 9.66 (14:00) | 0.0 (null) | 23 (15:15) | 11:00 (45.451) | 12:03 (17.86) | 13:45 (0.0) | 11:00 (28.217) | null (0.0) | null (45.451) | 0.028 | 0 | 128 (4) |
| 15% | 0.185 | 0.201 | 0.028 | 2.26 | 2.229 | 2.557 | 27.472 (11:00) | 9.518 (14:00) | 0.0 (null) | 23 (15:15) | 11:00 (45.449) | 12:03 (17.852) | 13:45 (0.0) | 11:00 (28.226) | null (0.0) | null (45.449) | 0.028 | 0 | 127 (3) |
| 20% | 0.21 | 0.245 | 0.029 | 2.32 | 2.273 | 2.561 | 29.543 (11:00) | 12.354 (12:15) | 0.0 (null) | 25 (14:45) | 11:00 (46.277) | 12:03 (21.085) | 12:15 (0.0) | 11:00 (31.066) | null (0.0) | null (46.277) | 0.029 | 1 | 130 (6) |
| 25% | 0.221 | 0.273 | 0.029 | 2.356 | 2.301 | 2.559 | 29.784 (11:00) | 12.654 (12:15) | 0.0 (null) | 25 (14:15) | 11:00 (43.523) | 12:03 (21.427) | 12:15 (0.0) | 11:00 (31.639) | null (0.0) | null (43.523) | 0.03 | 1 | 131 (7) |
| 30% | 0.242 | 0.33 | 0.032 | 2.42 | 2.359 | 2.562 | 32.25 (11:00) | 15.652 (12:00) | 0.0 (null) | 25 (14:30) | 11:00 (49.199) | 12:05 (21.03) | 12:01 (0.0) | 11:00 (31.712) | null (0.0) | null (49.199) | 0.03 | 3 | 135 (11) |
| 35% | 0.312 | 0.492 | 0.034 | 2.539 | 2.522 | 2.565 | 33.365 (11:00) | 18.449 (12:15) | 0.0 (null) | 28 (14:45) | 11:00 (49.472) | 12:03 (26.089) | 11:00 (0.0) | 11:00 (34.341) | null (0.0) | null (49.472) | 0.029 | 5 | 137 (13) |
| 40% | 0.354 | 0.622 | 0.034 | 2.613 | 2.653 | 2.564 | 37.235 (11:00) | 19.639 (12:15) | 0.0 (null) | 29 (14:45) | 11:00 (50.572) | 12:03 (25.51) | 11:30 (0.269) | 11:00 (34.004) | null (0.0) | 14:30 (50.572) | 0.032 | 9 | 144 (20) |
| 45% | 0.361 | 0.646 | 0.034 | 2.624 | 2.676 | 2.565 | 36.845 (11:00) | 19.818 (12:15) | 0.0 (null) | 29 (14:45) | 11:00 (50.504) | 11:00 (26.152) | 11:15 (0.0) | 11:00 (35.205) | null (0.0) | null (50.504) | 0.03 | 10 | 143 (19) |
| 50% | 0.364 | 0.744 | 0.065 | 2.675 | 2.776 | 2.595 | 40.444 (11:00) | 22.111 (12:00) | 0.0 (null) | 28 (15:45) | 11:00 (55.496) | 11:00 (30.774) | 11:30 (0.0) | 11:00 (41.512) | null (0.0) | null (55.496) | 0.03 | 18 | 148 (24) |
| Day 2 | |||||||||||||||||||
| 0% | 0.514 | 0.514 | null | 2.541 | 2.541 | null | 46.305 (11:45) | 31.866 (12:30) | 1.355 (17:15) | 31 (17:15) | 11:33 (50.352) | 12:18 (41.288) | null (0.0) | 11:48 (46.744) | 15:18 (3.031) | null (50.352) | 0.038 | 0 | 146 (0) |
| 5% | 0.597 | 0.646 | 0.028 | 2.664 | 2.673 | 2.559 | 46.668 (11:45) | 36.775 (12:30) | 2.845 (15:30) | 33 (17:30) | 11:33 (54.857) | 12:18 (44.25) | 13:00 (0.0) | 11:48 (46.844) | 15:18 (6.221) | null (54.857) | 0.046 | 0 | 153 (7) |
| 10% | 0.588 | 0.645 | 0.03 | 2.662 | 2.673 | 2.56 | 46.788 (11:45) | 36.702 (12:30) | 3.846 (15:15) | 33 (17:00) | 11:33 (51.952) | 12:18 (44.251) | 12:45 (0.0) | 11:48 (46.792) | 15:20 (3.713) | null (51.952) | 0.048 | 1 | 155 (9) |
| 15% | 0.65 | 0.747 | 0.028 | 2.746 | 2.776 | 2.556 | 47.568 (11:45) | 39.45 (12:30) | 4.96 (15:30) | 34 (17:30) | 11:33 (65.171) | 12:18 (45.113) | 13:00 (0.0) | 11:48 (49.022) | 15:18 (7.109) | null (65.171) | 0.059 | 0 | 157 (11) |
| 20% | 0.845 | 1.134 | 0.033 | 3.006 | 3.164 | 2.563 | 49.997 (11:45) | 46.002 (12:15) | 11.996 (15:15) | 39 (17:30) | 11:33 (66.143) | 12:18 (46.723) | 12:15 (0.0) | 11:48 (51.553) | 15:03 (17.136) | null (66.143) | 0.147 | 5 | 168 (22) |
| 25% | 0.824 | 1.109 | 0.033 | 2.987 | 3.139 | 2.563 | 49.995 (11:45) | 46.332 (12:15) | 12.182 (15:15) | 39 (17:30) | 11:33 (66.143) | 12:18 (46.903) | 12:15 (0.0) | 11:48 (50.973) | 15:03 (17.266) | null (66.143) | 0.144 | 5 | 169 (23) |
| 30% | 0.819 | 1.204 | 0.049 | 3.015 | 3.234 | 2.578 | 50.729 (11:45) | 45.112 (12:30) | 9.723 (15:15) | 38 (17:30) | 11:33 (66.914) | 12:18 (46.96) | 12:30 (0.0) | 11:48 (50.767) | 15:05 (17.34) | null (66.914) | 0.14 | 7 | 167 (21) |
| 35% | 0.878 | 1.356 | 0.054 | 3.092 | 3.387 | 2.585 | 53.225 (11:45) | 46.156 (12:30) | 15.138 (13:45) | 36 (17:30) | 11:33 (66.915) | 12:20 (50.162) | 12:16 (0.0) | 11:48 (52.112) | 15:18 (16.982) | null (66.915) | 0.171 | 12 | 174 (28) |
| 40% | 0.87 | 1.59 | 0.047 | 3.136 | 3.625 | 2.577 | 55.925 (11:30) | 46.716 (12:30) | 16.544 (15:30) | 37 (16:30) | 11:33 (66.993) | 12:20 (51.765) | 12:16 (0.0) | 11:48 (56.692) | 15:33 (17.146) | null (66.993) | 0.18 | 17 | 183 (37) |
| 45% | 0.987 | 1.83 | 0.066 | 3.257 | 3.863 | 2.596 | 56.174 (11:30) | 49.787 (12:15) | 18.998 (14:45) | 38 (16:45) | 11:33 (66.988) | 12:33 (51.641) | 12:16 (4.456) | 11:48 (54.967) | 15:18 (23.72) | 12:46 (66.988) | 0.274 | 22 | 176 (30) |
| 50% | 0.961 | 1.99 | 0.087 | 3.264 | 4.026 | 2.617 | 57.365 (11:45) | 50.398 (12:30) | 20.445 (15:15) | 38 (17:30) | 11:33 (75.424) | 12:18 (53.59) | 12:30 (0.0) | 11:48 (56.004) | 15:03 (25.072) | null (75.424) | 0.34 | 23 | 186 (40) |
References
- Cucinotta, D.; Vanelli, M. WHO Declares COVID-19 a Pandemic. Acta Biomed. 2020, 91, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Ciotti, M.; Ciccozzi, M.; Terrinoni, A.; Jiang, W.-C.; Wang, C.-B.; Bernardini, S. The COVID-19 Pandemic. Crit. Rev. Clin. Lab. Sci. 2020, 57, 365–388. [Google Scholar] [CrossRef] [PubMed]
- Acuti Martellucci, C.; Flacco, M.E.; Cappadona, R.; Bravi, F.; Mantovani, L.; Manzoli, L. SARS-CoV-2 Pandemic: An Overview. Adv. Biol. Regul. 2020, 77, 100736. [Google Scholar] [CrossRef]
- Helse Stavanger, S. Koronavirus—Rutiner for Medarbeidere. Available online: https://helse-stavanger.no/om-oss/for-ansatte/koronavirus-rutiner-for-ansatte (accessed on 7 October 2022).
- Fredheim, G. Helse- og Omsorgsdepartementet: Regjeringens Strategi og Beredskapsplan for Håndteringen av COVID-19-Pandemien. Available online: https://www.regjeringen.no/no/dokumenter/regjeringens-strategi-og-beredskapsplan-for-handteringen-av-covid-19-pandemien/id2907427/ (accessed on 26 April 2023).
- Liu, Y.; Gayle, A.A.; Wilder-Smith, A.; Rocklöv, J. The Reproductive Number of COVID-19 Is Higher Compared to SARS Coronavirus. J. Travel Med. 2020, 27, taaa021. [Google Scholar] [CrossRef]
- Ribaric, N.L.; Vincent, C.; Jonitz, G.; Hellinger, A.; Ribaric, G. Hidden Hazards of SARS-CoV-2 Transmission in Hospitals: A Systematic Review. Indoor Air 2022, 32, e12968. [Google Scholar] [CrossRef]
- People with Certain Medical Conditions. Available online: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html (accessed on 19 May 2023).
- Capalbo, C.; Aceti, A.; Simmaco, M.; Bonfini, R.; Rocco, M.; Ricci, A.; Napoli, C.; Rocco, M.; Alfonsi, V.; Teggi, A.; et al. The Exponential Phase of the COVID-19 Pandemic in Central Italy: An Integrated Care Pathway. Int. J. Environ. Res. Public Health 2020, 17, 3792. [Google Scholar] [CrossRef] [PubMed]
- Korber, B.; Fischer, W.M.; Gnanakaran, S.; Yoon, H.; Theiler, J.; Abfalterer, W.; Hengartner, N.; Giorgi, E.E.; Bhattacharya, T.; Foley, B.; et al. Tracking Changes in SARS-CoV-2 Spike: Evidence That D614G Increases Infectivity of the COVID-19 Virus. Cell 2020, 182, 812–827.e19. [Google Scholar] [CrossRef]
- Rutherford, P.A.; Provost, L.P.; Kotagal, U.R.; Luther, K.; Anderson, A. Institute for Healthcare Improvement: Achieving Hospital-Wide Patient Flow. 2017. Available online: https://www.ihi.org/resources/Pages/IHIWhitePapers/Achieving-Hospital-wide-Patient-Flow.aspx (accessed on 19 February 2021).
- McHugh, M.; VanDyke, K.; McClelland, M.; Moss, D. Improving Patient Flow and Reducing Emergency Department Crowding: A Guide for Hospitals; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2012. [Google Scholar]
- Mason, S. Keynote Address: United Kingdom Experiences of Evaluating Performance and Quality in Emergency Medicine. Acad. Emerg. Med. 2011, 18, 1234–1238. [Google Scholar] [CrossRef]
- Mohiuddin, S.; Busby, J.; Savović, J.; Richards, A.; Northstone, K.; Hollingworth, W.; Donovan, J.L.; Vasilakis, C. Patient Flow within UK Emergency Departments: A Systematic Review of the Use of Computer Simulation Modelling Methods. BMJ Open 2017, 7, e015007. [Google Scholar] [CrossRef]
- Vanbrabant, L.; Braekers, K.; Ramaekers, K.; Van Nieuwenhuyse, I. Simulation of Emergency Department Operations: A Comprehensive Review of KPIs and Operational Improvements. Comput. Ind. Eng. 2019, 131, 356–381. [Google Scholar] [CrossRef]
- Bansal, K.; Kumar, S. Mutational Cascade of SARS-CoV-2 Leading to Evolution and Emergence of Omicron Variant. Virus Res. 2022, 315, 198765. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, P.; Ray, P.K. Patient Flow Modelling and Performance Analysis of Healthcare Delivery Processes in Hospitals: A Review and Reflections. Comput. Ind. Eng. 2014, 78, 299–312. [Google Scholar] [CrossRef]
- Nataraja, R.M.; Oo, Y.M.; Kyaw, K.K.; Webb, N.R.; Ljuhar, D.; Pacilli, M.; Win, N.N.; Kimber, C.; Aye, A. Clinical Impact of the Introduction of Pediatric Intussusception Air Enema Reduction Technology in a Low- to Middle-Income Country Using Low-Cost Simulation-Based Medical Education. Simul. Healthc. 2020, 15, 7. [Google Scholar] [CrossRef]
- Aljahany, M.; Alassaf, W.; Alibrahim, A.A.; Kentab, O.; Alotaibi, A.; Alresseeni, A.; Algarni, A.; Algaeed, H.A.; Aljaber, M.I.; Alruwaili, B.; et al. Use of In Situ Simulation to Improve Emergency Department Readiness for the COVID-19 Pandemic. Prehosp. Disaster Med. 2021, 36, 6–13. [Google Scholar] [CrossRef]
- Salmon, A.; Rachuba, S.; Briscoe, S.; Pitt, M. A Structured Literature Review of Simulation Modelling Applied to Emergency Departments: Current Patterns and Emerging Trends. Oper. Res. Health Care 2018, 19, 1–13. [Google Scholar] [CrossRef]
- Castanheira-Pinto, A.; Gonçalves, B.S.; Lima, R.M.; Dinis-Carvalho, J. Modeling, Assessment and Design of an Emergency Department of a Public Hospital through Discrete-Event Simulation. Appl. Sci. 2021, 11, 805. [Google Scholar] [CrossRef]
- Hamza, N.; Majid, M.A.; Hujainah, F. SIM-PFED: A Simulation-Based Decision Making Model of Patient Flow for Improving Patient Throughput Time in Emergency Department. IEEE Access 2021, 9, 103419–103439. [Google Scholar] [CrossRef]
- Hamza, N.; Abdul Majid, M.; Adam, K.; Akma Abu Bakar, N. A Review on Simulation and Modelling for Patient Flow in Emergency Department. IOP Conf. Ser. Mater. Sci. Eng. 2019, 551, 012037. [Google Scholar] [CrossRef]
- Friesen, M.R.; McLeod, R.D. A Survey of Agent-Based Modeling of Hospital Environments. IEEE Access 2014, 2, 227–233. [Google Scholar] [CrossRef]
- Terning, G.; Brun, E. Systemic Conceptual Modeling of Patient Flow in a Hospital Emergency Department: A Case Example. In Proceedings of the System Dynamics Society Record of the 38th International Conference of the System Dynamics Society, Bergen, Norway, 19–24 July 2020. [Google Scholar]
- Randers, J. Elements of the System Dynamics Method. J. Oper. Res. Soc. 1997, 48, 1144–1145. [Google Scholar] [CrossRef]
- Albin, S.; Forrester, J.W.; Breierova, L. Building a System Dynamics Model: Part 1: Conceptualization; MIT: Cambridge, MA, USA, 2001. [Google Scholar]
- Luna, L.F.; Andersen, D.L. Using Qualitative Methods in the Conceptualization and Assessment of System Dynamics Models. In Proceedings of the 20th International System Dynamics Conference, Palermo, Italy, 28 July–1 August 2002. [Google Scholar]
- Terning, G.; Brun, E.C.; El-Thalji, I. The Patient Flow Effect of Pandemic Policies: A Hybrid Simulation Study in a Norwegian Emergency Department. Healthcare 2023, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Borshchev, A. The Big Book of Simulation Modeling: Multimethod Modeling with AnyLogic 6; AnyLogic North America: Chicago, IL, USA, 2013; ISBN 978-0-9895731-7-7. [Google Scholar]
- Ören, T.; Zeigler, B.P.; Tolk, A. (Eds.) Body of Knowledge for Modeling and Simulation: A Handbook by the Society for Modeling and Simulation International; Simulation Foundations, Methods and Applications; Springer International Publishing: Cham, Switzerland, 2023; ISBN 978-3-031-11084-9. [Google Scholar]
- Terning, G.; Brun, E.C.; El-Thalji, I. Modeling Patient Flow in an Emergency Department under COVID-19 Pandemic Conditions: A Hybrid Modeling Approach. Healthcare 2022, 10, 840. [Google Scholar] [CrossRef] [PubMed]
- Suh, H. Om oss—Stavanger Universitetssjukehus—Helse Stavanger HF. Available online: https://helse-stavanger.no/om-oss (accessed on 21 June 2019).
- Minge, A. Hun Skal Hele Tiden Være Orakelet og ta Raske og Rette Avgjørelse. Denne Dagen Varte Pausen i 30 Sekunder. Available online: https://www.aftenbladet.no/magasin/i/EWgGxa/hun-skal-hele-tiden-vaere-orakelet-og-ta-raske-og-rette-avgjoerelse-den (accessed on 3 November 2020).
- Cho, Y.-J.; Yeo, I.-H.; Lee, D.-E.; Kim, J.-K.; Kim, Y.-J.; Kim, C.-H.; Choe, J.-Y.; Park, J.-B.; Seo, K.-S.; Yu, B.-H.; et al. Collateral Effect of the Coronavirus Disease 2019 Pandemic on Emergency Department Visits in Korea. Medicina 2022, 59, 90. [Google Scholar] [CrossRef] [PubMed]
- Cummins, N.M.; Garavan, C.; Barry, L.A.; Devlin, C.; Corey, G.; Cummins, F.; Ryan, D.; McCarthy, G.; Galvin, R. The Impact of COVID-19 on an Irish Emergency Department (ED): A Cross-Sectional Study Exploring the Factors Influencing ED Utilisation Prior to and during the Pandemic from the Patient Perspective. BMC Emerg. Med. 2022, 22, 176. [Google Scholar] [CrossRef]
- Krogstad, U.; Lindahl, A.; Saastad, E.; Hafstad, E. Akuttmottak-En Risikosone for Pasientsikkerhet. Læringsnotat Fra Meldeordningen i Kunnskapssenteret 2015; Nasjonalt kunnskapssenter for helsetjenesten: Oslo, Sweden, 2015. [Google Scholar]
- Skinner, J.; Higbea, R.; Buer, D.; Horvath, C. Using predictive analytics to align ED staffing resources with patient demand: A hospital in Grand Rapids, Mich., used management theory and data analysis to design and implement a much more precise model for setting staffing levels in its emergency department. Healthc. Financ. Manag. 2018, 72, 56–62. [Google Scholar]
- Phan, T.; Brozak, S.; Pell, B.; Gitter, A.; Xiao, A.; Mena, K.D.; Kuang, Y.; Wu, F. A Simple SEIR-V Model to Estimate COVID-19 Prevalence and Predict SARS-CoV-2 Transmission Using Wastewater-Based Surveillance Data. Sci. Total Environ. 2023, 857, 159326. [Google Scholar] [CrossRef]




| Abbreviation | Resource Description |
|---|---|
| PT | Pre-Triage: The pre-triage is an introduced physical location outside of the emergency department. Admitted patients enter here for the healthcare workers to assess whether the incoming patient should be regarded as infected by COVID-19 (COVID+) or not infected by COVID-19 (COVID−). Depending on the status, the patient will, in the case of COVID−, be directed the regular route via the emergency department reception or, in the case of COVID+, be fast-tracked directly to an available treatment room for proper isolation and treatment of the emergent condition the patient suffers. |
| E.TR. | Extra Treatment Rooms: Because of the COVID-19 pandemic situation, extra treatment rooms were introduced to be available for the emergency department. These additional treatment rooms were introduced to compensate for the higher occupancy of patients in the emergency department at the onset of the pandemic. In the case of the emergency department, they introduced four extra-treatment rooms. |
| WZ | A designated area was allocated for the precise purpose of enabling the fast-tracking of the patients classified as COVID+. In the instance where a patient who is COVID− has completed their treatment and requires either restitution or non-intensive follow-up, the treatment room may be deemed unnecessary for their further stay in the emergency department. Suppose no further treatment rooms are available for incoming COVID+ patients entering the emergency department. In that case, the COVID− patient may vacate the treatment room to allow for an incoming COVID+ patient to expeditiously receive proper isolation, thus minimizing cross-infection among other patients and healthcare workers within the emergency department. |
| Abbreviation | Patient Flow Performance Indicators (PFPI) and Description | Unit |
|---|---|---|
| TTT | Time to Treatment: This performance indicator calculates the patient population’s average time within the emergency department before the treatment starts in their assigned treatment room. The value is in this simulation study given on a per-patient basis, i.e., the number of hours of waiting per patient. A lower value is better, all else equal, compared to a higher value, as less waiting means that each patient faster receives health care for the condition they got admitted for. This patient performance indicator is reported for the aggregate total group of patient agents (Tot.), the patients classified with the COVID+ status, and the patients classified with the COVID− status. | Hours per patient [h/pt] |
| ALOS | The average length of stay: The performance indicator is calculated, showing the total amount of time spent in the emergency department on a per-patient basis. It tracks the time taken from entering the emergency department, the point of admission, until the patient has been discharged from the emergency department. The performance indicator is measured as an average for all the patient agents, meaning the value gives the average hours of time each patient spent in the emergency department. Similar to the previous patient flow performance indicator, TTT, this performance indicator is also reported for the aggregate total group of patient agents (Tot.), the patients classified with the COVID+ status, and the patients classified with the COVID− status. | Hours per patient [h/pt] |
| Crowding | The patient flow performance indicator called ‘crowding’ calculates for each and every moment how many patients are present in the emergency department. This patient flow performance indicator is, including its naming, from the case emergency department’s patient flow improvements project. In the implementation of this metric, we show how much of the total simulated time the crowding has been beyond three different thresholds of interest as defined by the case emergency department in their internal working emphasis on the improvement of patient flow conditions and response for over-crowding problematics. The three threshold levels are set at 15, 25, and 30 patients in the emergency department. | % |
| Peak Crowding | Peak crowding, as its name suggests, is concerned about the peak of crowding, the previous performance indicator. The given number (#) tells how many patients were residing at the emergency department at the point in time when the number was at its maximum that day. In addition, the metric also tracks the time during the day the peak occurs. Thus, the first portion states the number of patients, while the second portion states how much the clock was at that given point. | # & Time |
| Time Start Use/Time in Use | This patient flow performance indicator states the time different resources of particular interest start being used during the day. In this performance measure implementation, it has been chosen to track when the extra treatment rooms (E.TR.), triage (Tri.), and waiting zone (WZ) are used. Secondly, the performance indicator calculates how much the given resource is used in proportion to the total elapsed time of that time, e.g., a reading in the Tri.-column at ‘11:00′ means that the triage that day was not used until 11:00. The second value shows the percentage amount of that day this resource has been used at least by one patient. | Time |
| Time Full | This performance measure, as the name suggests, keeps track of when particular resources of interest, the extra treatment rooms (E.TR.), triage (Tri.), and waiting zone (WZ), are full, meaning they cannot further take any more patients. Similar to the previous performance indicator, this also has dual tracking. Firstly, the time when the given resource is full is given next, a percentage of how long the elapsed time the resource has been full. | Time & % |
| Time in WR | This performance measure calculates how long patients on average, spent in the emergency department waiting room. The indicator is calculated as an average across all the patients. | h |
| Times TR blocked for COVID+. | This performance indicator tracks the total number of times (#) a patient agent classified as COVID+ is blocked from entering the treatment room after pre-triage. This is a critical point for the emergency department as it is heavily loaded with the patients so much that they cannot immediately fast-track a patient to an isolated room. | # |
| Times TR Assigned | This performance measure tracks the number of times (#) a treatment room (TR) has been assigned to a patient. This refer to the term ‘seize’ used in simulation modeling discipline; how many times a certain resource is utilized for the purpose treatment of a patient. Each time a patient is assigned to a treatment room is resource-consuming, especially considering the sanitization of the rooms. | # |
| Abbreviations | Model Inputs Description |
|---|---|
| Tot. | Total patient population: This denotes both the patient groups who are classified as COVID+ and COVID−. This category was made to show the combined effects of the total patient groups. |
| COVID− | This is the population of patient agents that in the pre-triage is deemed to not be infected by COVID-19. These patients will, after the pre-triage control, progress to the emergency department in the usual manner. |
| COVID+ | This points to the portion of the patient agents that in the pre-triage is found to be infected by COVID-19. As these patient agents pose an increased risk associated with the infection status, the patient agents will be fast-tracked to a treatment room. |
| PIR | Patient infection rate: The variable determining the proportion of how many COVID+ patients will be inserted in the different simulation runs. In the present study, the PIR is varied between 0 and 50%, with a 5 percentage points increment between each run. |
| Sc. No. | Explanation | Model and Resource Configuration | ||
|---|---|---|---|---|
| PIR [%] | WZ | E.TR. | ||
| Sc. 1 | Base case—no added resources: Scenario simulating situation during pandemic operation. However, none of the extra resources are introduced. What are introduced in this series of scenarios are only the new policies, i.e., channeling and expediting the COVID+ patients according to the infection control policies. Thus, the E.TR. and WZ are not in use for this scenario. | 0 → 50 | ||
| Sc. 2 | Adding the waiting zone (WZ): A simulation series runs with a single parameter variation. Peri-pandemic operation with differing proportions of patient infection rates: 0–50% with 5 percentage point increments between each simulation run. This series of scenario runs has the waiting zone (WZ) enabled for utilization when the circumstances are as described in Section 2.4—‘Case Emergency Department, Interventions, and Resources’ and listed in Table 1. | 0 → 50 | ✓ | |
| Sc. 3 | Adding extra treatment rooms: A series of simulation runs with single parameter variation. Peri-pandemic operation with differing proportions of patient infection rates: 0–50% with 5 percentage point increments between each simulation run. This series of scenario runs has the extra treatment rooms (E.TR.) enabled for utilization. | 0 → 50 | ✓ | |
| Sc. 3.2 Sc. 3.4 Sc. 3.6 | A sub-group of three simulation series of simulation runs: measure the differential impact of the increased number of E.TR.; In Sc. 3.2, the number of E.TR is two; in Sc. 3.4, the number of E.TR. is four; lastly, in Sc. 3.6 the number of E.TR. is six. | 0 → 50 | ✓ | |
| Sc. 4 | Adding waiting zone and extra treatment rooms: series of simulation runs with single parameter variation. Peri-pandemic operation with differing proportions of patient infection rates: 0–50% with 5 percentage point increments between each simulation run. This series of scenario runs includes both the WZ and the E.TR. enabled for utilization. | 0 → 50 | ✓ | ✓ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terning, G.; El-Thalji, I.; Brun, E.C. The Impact of Patient Infection Rate on Emergency Department Patient Flow: Hybrid Simulation Study in a Norwegian Case. Healthcare 2023, 11, 1904. https://doi.org/10.3390/healthcare11131904
Terning G, El-Thalji I, Brun EC. The Impact of Patient Infection Rate on Emergency Department Patient Flow: Hybrid Simulation Study in a Norwegian Case. Healthcare. 2023; 11(13):1904. https://doi.org/10.3390/healthcare11131904
Chicago/Turabian StyleTerning, Gaute, Idriss El-Thalji, and Eric Christian Brun. 2023. "The Impact of Patient Infection Rate on Emergency Department Patient Flow: Hybrid Simulation Study in a Norwegian Case" Healthcare 11, no. 13: 1904. https://doi.org/10.3390/healthcare11131904
APA StyleTerning, G., El-Thalji, I., & Brun, E. C. (2023). The Impact of Patient Infection Rate on Emergency Department Patient Flow: Hybrid Simulation Study in a Norwegian Case. Healthcare, 11(13), 1904. https://doi.org/10.3390/healthcare11131904










