A Comparative Assessment of Approvals and Discontinuations of Systemic Antibiotics and Other Therapeutic Areas
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Food and Drug Administration Safety and Innovation Act (FDASIA) 2013 § 3187 (2013) 112th Congress (2011–2012). Available online: https://www.congress.gov/bill/112th-congress/senate-bill/3187 (accessed on 20 June 2022).
- H.R.6—21st Century Cures Act, 114th Congress (2015–2016). 2015. Available online: https://www.congress.gov/bill/114th-congress/house-bill/6 (accessed on 20 June 2022).
- Title 21—Food and Drugs Chapter I. Food and Drug Administration Department of Health and Human Services Subchapter D—Drugs for Human Use. CFR-Code of Federal Regulations. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?CFRPart=314&showFR=1 (accessed on 9 July 2022).
- Code of Federal Regulations. 21 CFR 314.150. Available online: https://www.ecfr.gov/current/title-21/chapter-I/subchapter-D/part-314/subpart-D/section-314.150 (accessed on 20 June 2022).
- Outterson, K.; Powers, J.H.; Seoane-Vazquez, E.; Rodriguez-Monguio, R.; Kesselheim, A.S. Approval and withdrawal of new antibiotics and other antiinfectives in the U.S., 1980–2009. J. Law Med. Ethics 2013, 41, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Deak, D.; Outterson, K.; Powers, J.H.; Kesselheim, A.S. Progress in the Fight Against Multidrug-Resistant Bacteria? A Review of U.S. Food and Drug Administration-Approved Antibiotics, 2010–2015. Ann. Intern. Med. 2016, 165, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Darrow, J.J.; Najafzadeh, M.; Stefanini, K.; Kesselheim, A.S. Regulatory approval characteristics of antimicrobial versus non-antimicrobial products, 1984–2018: An evaluation of Food and Drug Administration flexibilities. Lancet Infect. Dis. 2020, 20, e159–e164. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration (FDA). New Drugs at FDA: CDER’s New Molecular Entities and New Therapeutic Biological Products. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/ (accessed on 20 June 2022).
- Approved Drug Products with Therapeutic Equivalence Evaluations—Orange Book. Available online: https://www.fda.gov/drugs/drug-approvals-and-databases/approved-drug-products-therapeutic-equivalence-evaluations-orange-book (accessed on 1 May 2023).
- Approved Cellular and Gene Therapy Products. Available online: https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/approved-cellular-and-gene-therapy-products (accessed on 1 May 2023).
- Purple Book. Database of Licensed Biological Products. Available online: https://www.purplebooksearch.fda.gov (accessed on 20 June 2022).
- Federal Register. Available online: https://www.federalregister.gov/ (accessed on 20 June 2022).
- World Health Organization Collaborating Center for Drug Statistics Methodology Anatomical Therapeutic Chemical (ATC) Classification System. Available online: https://www.whocc.no/atc_ddd_index/ (accessed on 20 June 2022).
- Qureshi, Z.P.; Seoane-Vazquez, E.; Rodriguez-Monguio, R.; Stevenson, K.B.; Szeinbach, S.L. Market withdrawal of new molecular entities approved in the United States from 1980 to 2009. Pharmacoepidemiol. Drug Saf. 2011, 20, 772–777. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. MedWatch: The FDA Safety Information and Adverse Event Reporting Program. Available online: https://www.fda.gov/safety/medwatch-fda-safety-information-and-adverse-event-reporting-program (accessed on 19 November 2022).
- US Food and Drug Administration. Drug Safety Communications. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/drug-safety-communications (accessed on 1 May 2023).
- Doshi, P.; Hur, P.; Jones, M.; Albarmawi, H.; Jefferson, T.; Morgan, D.J.; Spears, P.A.; Powers, J.H., III. Informed Consent to Study Purpose in Randomized Clinical Trials of Antibiotics, 1991 Through 2011. JAMA Intern. Med. 2017, 177, 1452–1459. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Laxminarayan, R. Are physicians’ prescribing decisions sensitive to drug prices? Evidence from a free-antibiotics program. Health Econ. 2015, 24, 158–174. [Google Scholar] [CrossRef] [PubMed]
- Clancy, C.J.; Potoski, B.A.; Buehrle, D.; Nguyen, M.H. Estimating the Treatment of Carbapenem-Resistant Enterobacteriaceae Infections in the United States Using Antibiotic Prescription Data. Open Forum Infect. Dis. 2019, 6, ofz344. [Google Scholar] [CrossRef] [PubMed]
- Yahav, D.; Shepshelovich, D.; Tau, N. Cost Analysis of New Antibiotics to Treat Multidrug-Resistant Bacterial Infections: Mind the Gap. Infect. Dis. Ther. 2021, 10, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Mitra-Majumdar, M.; Powers, J.H.; Brown, B.L.; Kesselheim, A.S. Evidence at time of regulatory approval and cost of new antibiotics in 2016–19: Cohort study of FDA approved drugs. BMJ Med. 2022, 1, e000227. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration Center for Drug Evaluation and Research. Application Number: 209445Orig1s000. Multi-Discipline Review. 16 December 2019. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/209445Orig1s000MultidisciplineR.pdf (accessed on 9 November 2022).
- Bassetti, M.; Echols, R.; Matsunaga, Y.; Ariyasu, M.; Doi, Y.; Ferrer, R.; Lodise, T.P.; Naas, T.; Niki, Y.; Paterson, D.L.; et al. Efficacy and safety of cefiderocol or best available therapy for the treatment of serious infections caused by carbapenem-resistant Gram-negative bacteria (CREDIBLE-CR): A randomised, open-label, multicentre, pathogen-focused, descriptive, phase 3 trial. Lancet Infect. Dis. 2021, 21, 226–240. [Google Scholar] [CrossRef] [PubMed]
- Gotham, D.; Moja, L.; van der Heijden, M.; Paulin, S.; Smith, I.; Beyer, P. Reimbursement models to tackle market failures for antimicrobials: Approaches taken in France, Germany, Sweden, the United Kingdom, and the United States. Health Policy 2021, 125, 296–306. [Google Scholar] [CrossRef] [PubMed]
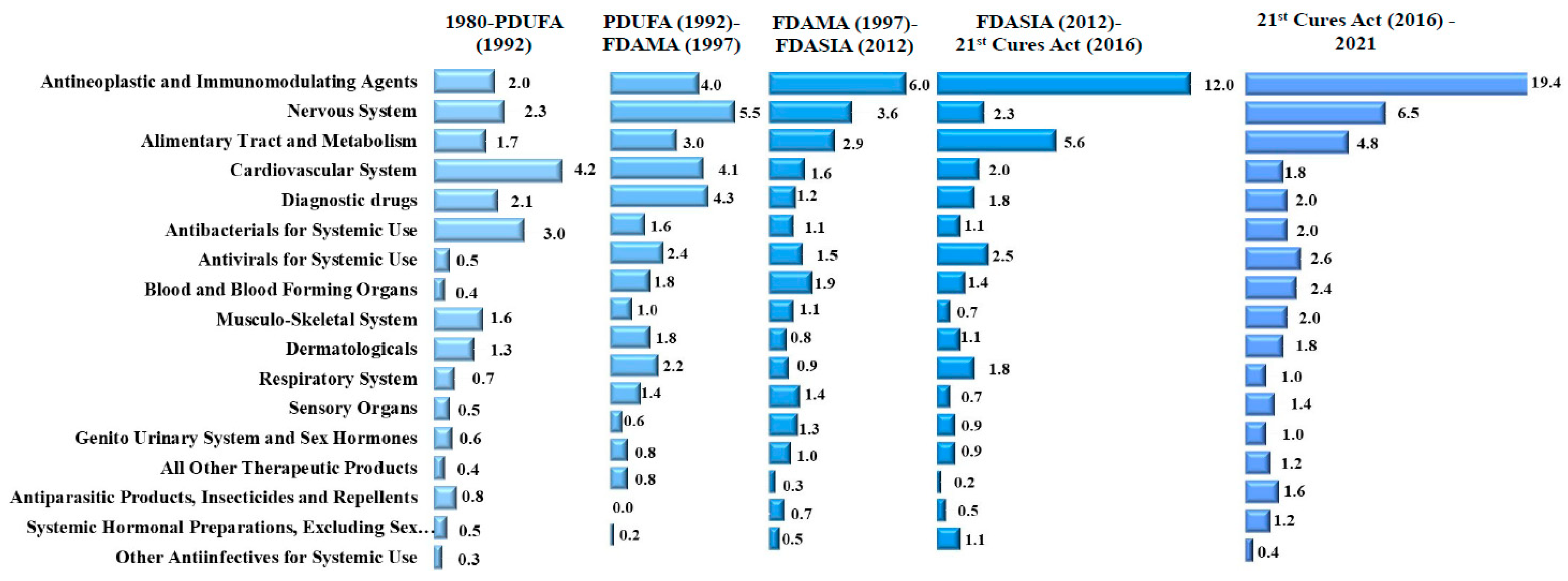

| Therapeutic Class | 1980–PDUFA (1992) | PDUFA (1992)–FDAMA (1997) | FDAMA (1997)–FDASIA (2012) | FDASIA (2012)–21st Century Cures Act (2016) | 21st Century Cures Act (2016)–2021 | Total |
|---|---|---|---|---|---|---|
| Antineoplastic and Immunomodulating Agents | 26 (2.0, 8.8%) | 20 (4.0, 11.2%) | 88 (6.0, 21.7%) | 53 (12.0, 32.7%) | 98 (19.5, 36.7%) | 285 (6.8, 21.8%) |
| Nervous System | 30 (2.3, 10.1%) | 28 (5.5, 15.6%) | 52 (3.6, 12.8%) | 10 (2.3, 6.2%) | 34 (6.8, 12.7%) | 154 (3.7, 11.8%) |
| Alimentary Tract and Metabolism | 22 (1.7, 7.4%) | 15 (3.0, 8.4%) | 43 (2.9, 10.6%) | 25 (5.6, 15.4%) | 24 (4.8, 9.0%) | 129 (3.1, 9.8%) |
| Cardiovascular System | 54 (4.2, 18.2%) | 21 (4.1, 11.7%) | 24 (1.6, 5.9%) | 9 (2.0, 5.6%) | 8 (1.6, 3.0%) | 116 (2.8, 8.9%) |
| Diagnostic drugs | 27 (2.1, 9.1%) | 22 (4.3, 12.3%) | 17 (1.2, 4.2%) | 8 (1.8, 4.9%) | 10 (2.0, 3.7%) | 84 (2.0, 6.4%) |
| Antibacterials for Systemic Use | 38 (3.0, 12.8%) | 8 (1.6, 4.5%) | 16 (1.1, 3.9%) | 5 (1.1, 3.1%) | 10 (2.0, 3.7%) | 77 (1.8, 5.9%) |
| Antivirals for Systemic Use | 7 (0.5, 2.4%) | 12 (2.4, 6.7%) | 22 (1.5, 5.4%) | 11 (2.5, 6.8%) | 13 (2.6, 4.9%) | 65 (1.5, 5.0%) |
| Blood and Blood Forming Organs | 5 (0.4, 1.7%) | 9 (1.8, 5.0%) | 28 (1.9, 6.9%) | 6 (1.4, 3.7%) | 12 (2.4, 4.5%) | 60 (1.4, 4.6%) |
| Musculo-Skeletal System | 21 (1.6, 7.1%) | 5 (1.0, 2.8%) | 16 (1.1, 3.9%) | 3 (0.7, 1.9%) | 10 (2.0, 3.7%) | 55 (1.3, 4.2%) |
| Dermatologicals | 17 (1.3, 5.7%) | 9 (1.8, 5.0%) | 11 (0.8, 2.7%) | 5 (1.1, 3.1%) | 9 (1.8, 3.4%) | 51 (1.2, 3.9%) |
| Respiratory System | 9 (0.7, 3.0%) | 11 (2.2, 6.1%) | 13 (0.9, 3.2%) | 8 (1.8, 4.9%) | 5 (1.0, 1.9%) | 46 (1.1, 3.5%) |
| Sensory Organs | 7 (0.5, 2.4%) | 7 (1.4, 3.9%) | 21 (1.4, 5.2%) | 3 (0.7, 1.9%) | 7 (1.4, 2.6%) | 45 (1.1, 3.4%) |
| Genito Urinary System and Sex Hormones | 8 (0.6, 2.7%) | 3 (0.6, 1.7%) | 19 (1.3, 4.7%) | 4 (0.9, 2.5%) | 5 (1.0, 1.9%) | 39 (0.9, 3.0%) |
| Antiparasitic Products, Insecticides and Repellents | 10 (0.8, 3.4%) | 4 (0.8, 2.2%) | 5 (0.3, 1.2%) | 1 (0.2, 0.6%) | 8 (1.6, 3.0%) | 28 (0.7, 2.1%) |
| Systemic Hormonal Preparations, Excluding Sex Hormones and Insulins | 6 (0.5, 2.0%) | (0.0, 0.0%) | 10 (0.7, 2.5%) | 2 (0.5, 1.2%) | 7 (1.4, 2.6%) | 25 (0.6, 1.9%) |
| Other Antiinfectives for Systemic Use | 4 (0.3, 1.4%) | 1 (0.2, 0.6%) | 7 (0.5, 1.7%) | 5 (1.1, 3.1%) | 2 (0.4, 0.7%) | 19 (0.5, 1.5%) |
| All Other Therapeutic Products | 5 (0.4, 1.7%) | 4 (0.8, 2.2%) | 14 (1.0, 3.4%) | 4 (0.9, 2.5%) | 5 (1.0, 1.9%) | 32 (0.8, 2.4%) |
| Total | 296 (23.1, 100%) | 179 (35.4, 100%) | 406 (27.7, 100%) | 162 (36.6, 100%) | 267 (53.2, 100%) | 1310 (31.2, 100%) |
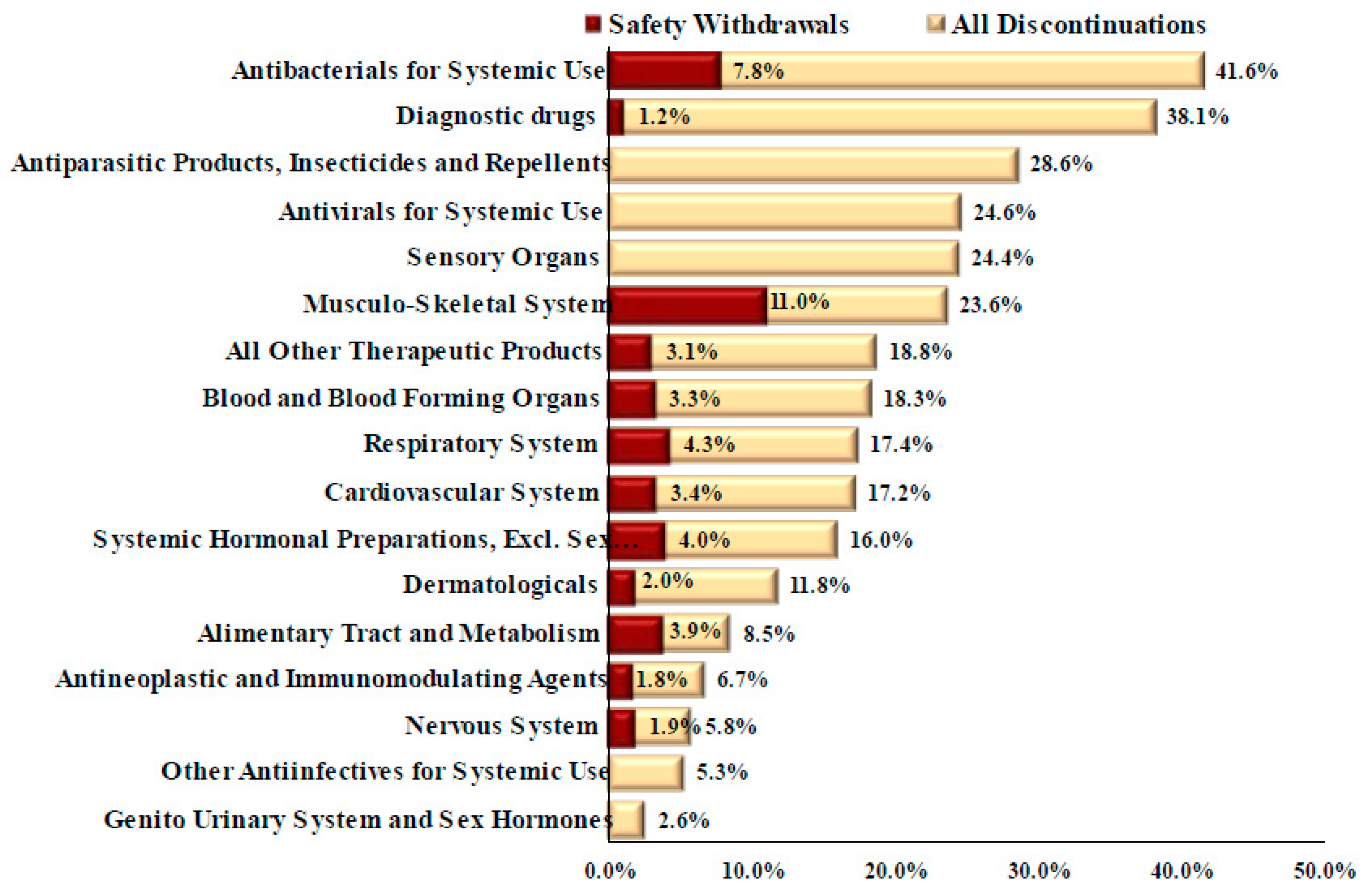
| Therapeutic Class | 1980–PDUFA (1992) | PDUFA (1992)–FDAMA (1997) | FDAMA (1997)–FDASIA (2012) | FDASIA (2012)–21st Century Cures Act (2016) | 21st Century Cures Act (2016)–2021 | Total (1981–2021) |
|---|---|---|---|---|---|---|
| Antineoplastic and Immunomodulating Agents | 7 (0.5, 2.4%) | - | 8 (0.5, 2.0%) | 2 (0.5, 1.2%) | 3 (0.6, 1.1%) | 20 (0.5, 1.5%) |
| Nervous System | 5 (0.4, 1.7%) | 2 (0.4, 1.1%) | 2 (0.1, 0.5%) | - | - | 9 (0.2, 0.7%) |
| Alimentary Tract and Metabolism | 2 (0.2, 0.7%) | 3 (0.6, 1.7%) | 5 (0.3, 1.2%) | 2 (0.5, 1.2%) | - | 12 (0.3, 0.9%) |
| Cardiovascular System | 12 (0.9, 4.1%) | 5 (1.0, 2.8%) | 2 (0.1, 0.5%) | 1 (0.2, 0.6%) | - | 20 (0.5, 1.5%) |
| Diagnostic drugs | 14 (1.1, 4.7%) | 11 (2.2, 6.1%) | 7 (0.5, 1.7%) | - | - | 32 (0.8, 2.4%) |
| Antibacterials for Systemic Use | 21 (1.6, 7.1%) | 4 (0.8, 2.2%) | 7 (0.5, 1.7%) | - | - | 32 (0.8, 2.4%) |
| Antivirals for Systemic Use | 3 (0.2, 1.0%) | 5 (1.0, 2.8%) | 5 (0.3, 1.2%) | 3 (0.7, 1.9%) | - | 16 (0.4, 1.2%) |
| Blood and Blood Forming Organs | 1 (0.1, 0.3%) | 4 (0.8, 2.2%) | 5 (0.3, 1.2%) | - | 1 (0.2, 0.4%) | 11 (0.3, 0.8%) |
| Musculo-Skeletal System | 7 (0.5, 2.4%) | 2 (0.4, 1.1%) | 3 (0.2, 0.7%) | 1 (0.2, 0.6%) | - | 13 (0.3, 1.0%) |
| Dermatologicals | 3 (0.2, 1.0%) | - | 3 (0.2, 0.7%) | - | - | 6 (0.1, 0.5%) |
| Respiratory System | 5 (0.4, 1.7%) | 1 (0.2, 0.6%) | 2 (0.1, 0.5%) | - | - | 8 (0.2, 0.6%) |
| Sensory Organs | 2 (0.2, 0.7%) | 2 (0.4, 1.1%) | 5 (0.3, 1.2%) | 2 (0.5, 1.2%) | - | 11 (0.3, 0.8%) |
| Genito Urinary System and Sex Hormones | 1 (0.1, 0.3%) | - | - | - | - | 1 (0.0, 0.1%) |
| Antiparasitic Products, Insecticides and Repellents | 5 (0.4, 1.7%) | 1 (0.2, 0.6%) | 1 (0.1, 0.2%) | - | 1 (0.2, 0.4%) | 8 (0.2, 0.6%) |
| Systemic Hormonal Preparations, Excluding Sex Hormones and Insulins | 3 (0.2, 1.0%) | - | 1 (0.1, 0.2%) | - | - | 4 (0.1, 0.3%) |
| Other Antiinfectives for Systemic Use | 1 (0.1, 0.3%) | - | 0 (0.0, 0.0%) | - | - | 1 (0.0, 0.1%) |
| All Other Therapeutic Products | 2 (0.2, 0.7%) | 1 (0.2, 0.6%) | 3 (0.2, 0.7%) | - | - | 6 (0.1, 0.5%) |
| Total | 94 (7.3, 31.9%) | 41 (8.1, 22.8%) | 59 (4.0, 14.5%) | 11 (2.5, 6.8%) | 5 (1.0, 1.9%) | 210 (5.0, 16.0%) |
| Pharmacological and Chemical Classes | 1980–PDUFA (1992) | PDUFA (1992)–FDAMA (1997) | FDAMA (1997)–FDASIA (2012) | FDASIA (2012)–21st Century Cures Act (2016) | 21st Century Cures Act (2016)–2021 | Total (1981–2021) |
|---|---|---|---|---|---|---|
| Aminoglycoside Antibacterials | 2 (2, 100.0%) | - | - | - | 1 (0, 0.0%) | 3 (2, 66.7%) |
| Other aminoglycosides | 2 (2, 100.0%) | - | - | - | 1 (0, 0.0%) | 3 (2, 66.7%) |
| Beta-Lactam Antibacterials, Penicillins | 7 (4, 57.1%) | 1 (0, 0.0%) | - | - | - | 8 (4, 50.0%) |
| Combinations of penicillins, incl. beta-lactamase inhibitors | 2 (0, 0.0%) | 1 (0, 0.0%) | - | - | - | 3 (0, 0.0%) |
| Penicillins with extended spectrum | 5 (4, 80.0%) | - | - | - | - | 5 (4, 80.0%) |
| Macrolides, Lincosamides and Streptogramins | 2 (0, 0.0%) | 1 (1, 100.0%) | 2 (1, 50.0%) | - | - | 5 (2, 40.0%) |
| Macrolides | 2 (0, 0.0%) | 1 (1, 100.0%) | 1 (1, 100.0%) | - | - | 4 (2, 50.0%) |
| Streptogramins | - | - | 1 (0, 0.0%) | - | - | 1 (0, 0.0%) |
| Other Antibacterials | - | 1 (0, 0.0%) | 3 (0, 0.0%) | 3 (0, 0.0%) | 2 (0, 0.0%) | 9 (0, 0.0%) |
| Glycopeptide antibacterials | - | - | 1 (0, 0.0%) | 2 (0, 0.0%) | - | 3 (0, 0.0%) |
| Imidazole derivatives | - | - | - | - | 1 (0, 0.0%) | 1 (0, 0.0%) |
| Other antibacterials | - | 1 (0, 0.0%) | 2 (0, 0.0%) | 1 (0, 0.0%) | 1 (0, 0.0%) | 5 (0, 0.0%) |
| Other Beta-Lactam Antibacterials | 20 (10, 50.0%) | 3 (1, 33.3%) | 5 (2, 40.0%) | 2 (0, 0.0%) | 3 (0, 0.0%) | 33 (13, 39.4%) |
| Carbapenems | 1 (0, 0.0%) | 1 (0, 0.0%) | 2 (1, 50.0%) | - | 2 (0, 0.0%) | 6 (1, 16.7%) |
| Fourth-generation cephalosporins | - | 1 (0, 0.0%) | - | - | - | 1 (0, 0.0%) |
| Monobactams | 1 (0, 0.0%) | - | - | - | - | 1 (0, 0.0%) |
| Other cephalosporins and penems | - | - | 1 (0, 0.0%) | 1 (0, 0.0%) | 1 (0, 0.0%) | 3 (0, 0.0%) |
| Second-generation cephalosporins | 8 (5, 62.5%) | - | - | - | - | 8 (5, 62.5%) |
| Third-generation cephalosporins | 10 (5, 50.0%) | 1 (1, 100.0%) | 2 (1, 50.0%) | 1 (0, 0.0%) | - | 14 (7, 50.0%) |
| Quinolone Antibacterials | 7 (5, 71.4%) | 2 (2, 100.0%) | 5 (4, 80.0%) | - | 1 (0, 0.0%) | 15 (11, 73.3%) |
| Fluoroquinolones | 6 (4, 66.7%) | 2 (2, 100.0%) | 5 (4, 80.0%) | - | 1 (0, 0.0%) | 14 (10, 71.4%) |
| Other quinolones | 1 (1, 100.0%) | - | - | - | - | 1 (1, 100.0%) |
| Tetracyclines | - | - | 1 (0, 0.0%) | - | 3 (0, 0.0%) | 4 (0, 0.0%) |
| Tetracyclines | - | - | 1 (0, 0.0%) | - | 3 (0, 0.0%) | 4 (0, 0.0%) |
| Total | 38 (21, 55.3%) | 8 (4, 50.0%) | 16 (7, 43.8%) | 5 (0, 0.0%) | 10 (0, 0.0%) | 77 (32, 41.6%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodriguez-Monguio, R.; Seoane-Vazquez, E.; Powers, J.H., III. A Comparative Assessment of Approvals and Discontinuations of Systemic Antibiotics and Other Therapeutic Areas. Healthcare 2023, 11, 1759. https://doi.org/10.3390/healthcare11121759
Rodriguez-Monguio R, Seoane-Vazquez E, Powers JH III. A Comparative Assessment of Approvals and Discontinuations of Systemic Antibiotics and Other Therapeutic Areas. Healthcare. 2023; 11(12):1759. https://doi.org/10.3390/healthcare11121759
Chicago/Turabian StyleRodriguez-Monguio, Rosa, Enrique Seoane-Vazquez, and John H. Powers, III. 2023. "A Comparative Assessment of Approvals and Discontinuations of Systemic Antibiotics and Other Therapeutic Areas" Healthcare 11, no. 12: 1759. https://doi.org/10.3390/healthcare11121759
APA StyleRodriguez-Monguio, R., Seoane-Vazquez, E., & Powers, J. H., III. (2023). A Comparative Assessment of Approvals and Discontinuations of Systemic Antibiotics and Other Therapeutic Areas. Healthcare, 11(12), 1759. https://doi.org/10.3390/healthcare11121759







