History of Mammography: Analysis of Breast Imaging Diagnostic Achievements over the Last Century
Abstract
1. Introduction
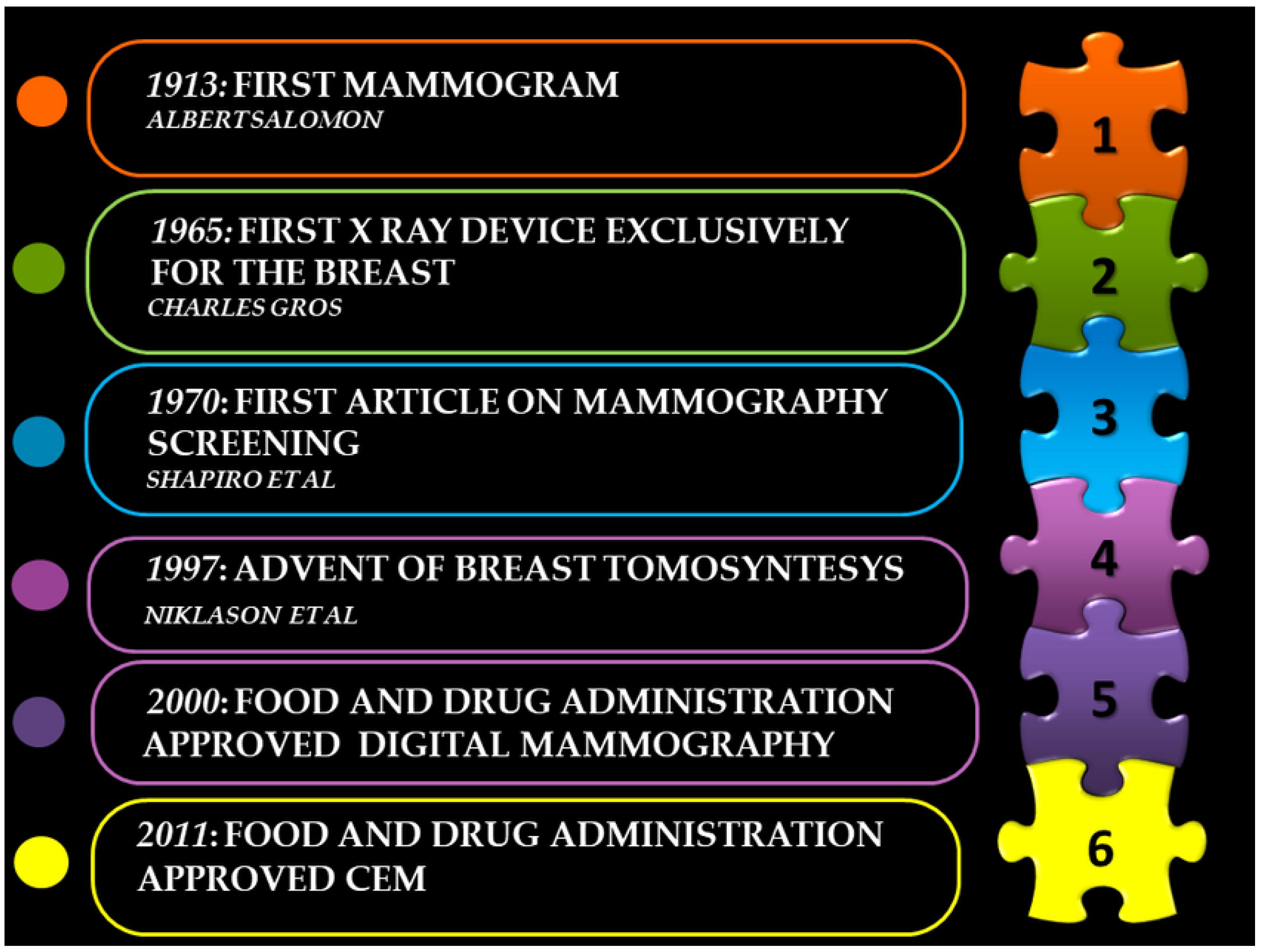
2. Origin of Mammography
3. Technical Progress
4. Mammography Screening and Reduction in Breast Cancer Mortality
5. Digital Breast Tomosynthesis (DBT)
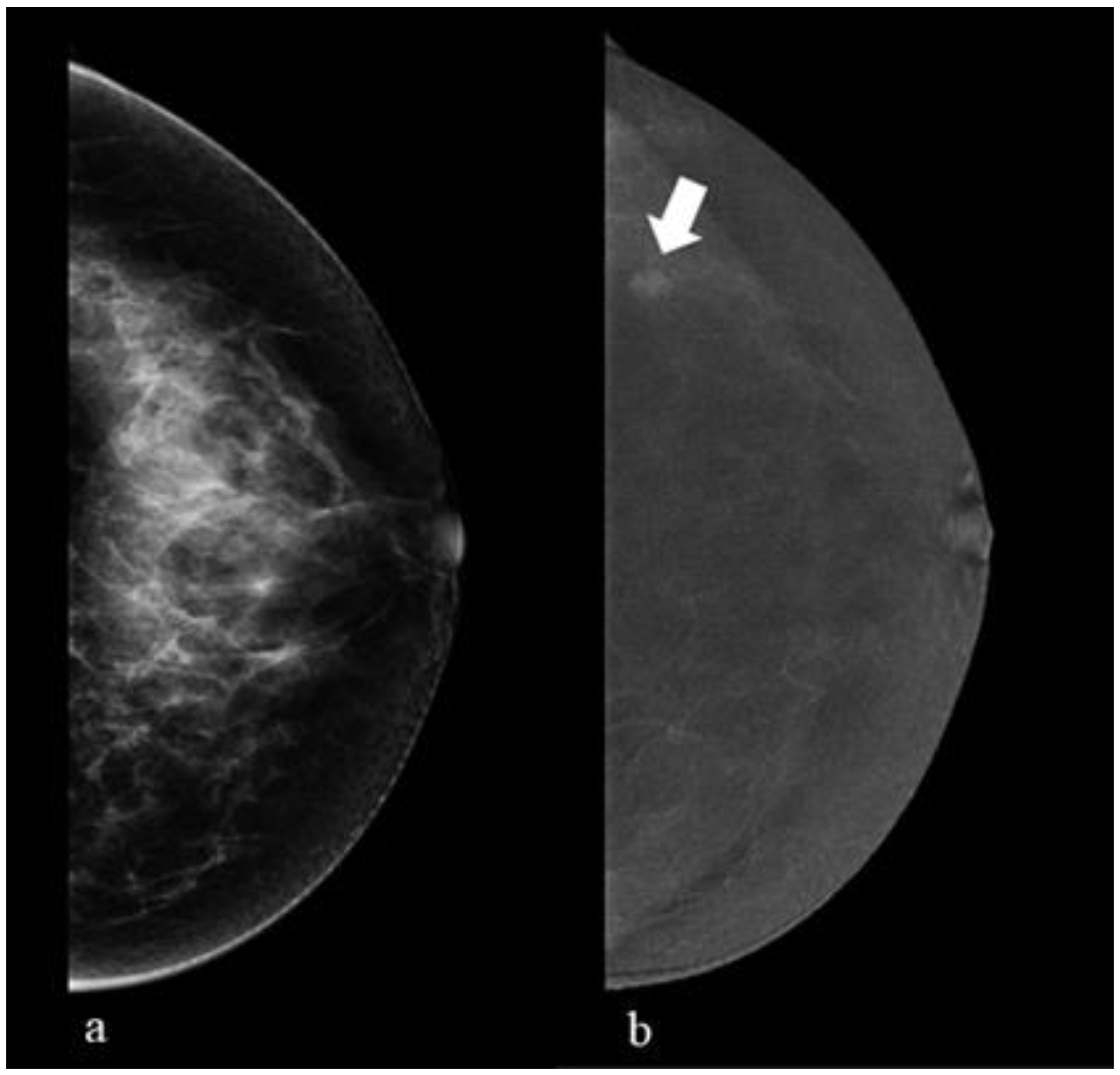
6. Contrast-Enhanced Mammography (CEM)
7. Breast MRI
8. Breast Ultrasound
9. Future Directions
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2020, 71, 209–249. [Google Scholar] [CrossRef]
- Nyström, L.; Rutqvist, L.E.; Wall, S.; Lindgren, A.; Lindqvist, M.; Rydén, S.; Andersson, I.; Bjurstam, N.; Fagerberg, G.; Frisell, J.; et al. Breast cancer screening with mammography: Overview of Swedish randomised trials. Lancet 1993, 341, 973–978, Erratum in Lancet 1993, 342, 1372. [Google Scholar] [CrossRef]
- Tabár, L.; Fagerberg, C.J.G.; Gad, A.; Baldetorp, L.; Holmberg, L.H.; Gröntoft, O.; Ljungquist, U.; Lundström, B.; Månson, J.C.; Eklund, G. Reduction in mortality from breast cancer after mass screening with mammography: Randomized trial from the breast cancer screening working group of the Swedish national board of health and welfare. Lancet 1985, 1, 829–832. [Google Scholar] [CrossRef]
- Tubiana, M. Wilhelm Conrad Röntgen et la découverte des rayons X [Wilhelm Conrad Röntgen and the discovery of X-rays]. Bull. Acad. Natl. Med. 1996, 180, 97–108. (In French) [Google Scholar]
- Salomon, A. Betrage zur pathologie und clinic der mammkarzinome. Arch. Kiln Chir. 1913, 101, 573–668. [Google Scholar]
- Kleinschmidt, O. Brustdruse. In Die Klinik der Bosartigen Geschwulste; Zweife, P., Payr, E., Hirzel, S., Eds.; Von Hirzel: Leipzig, Germany, 1927; pp. 5–90. [Google Scholar]
- Vogel, W. Die roentgendarstellung der mammatumoren. Arch. Klin. Chir. 1932, 171, 618–626. [Google Scholar]
- Warren, S.L. A roentgenologic study of the breast. Am. J. Roentgenel. Radiat. Ther. 1930, 24, 113–124. [Google Scholar]
- Gershon-Cohen, J.; Colcher, A.E. Evaluation of roentgen diagnosis of early carcinoma of breast. J. Am. Med. Assoc. 1937, 108, 867–871. [Google Scholar] [CrossRef]
- Gershon-Cohen, J.; Strickler, A. Roentgenologic Examination of the Normal Breast: Its Evaluation in Demonstrating Early Neoplastic Changes. Am. J. Roentgenol. Radium Ther. 1938, 40, 189–201. [Google Scholar]
- Ingleby, H.; Gershon-Cohen, J. Comparative Anatomy, Pathology, and Roentgenology of the Breast; University of Pennsylvania Press: Philadelphia, PA, USA, 1960. [Google Scholar]
- Leborgne, R. Diagnosis of tumours of the breast by simple roentgenography; calcifications in carcinomas. Am. J. Roentgenol. Radium Ther. 1951, 65, 1–11. [Google Scholar]
- Leborgne, R. The Breast in Roentgen Diagnosis; Impresora: Montevideo, Uruguay, 1953. [Google Scholar]
- Haus, A.G. Historical technical developments in mammography. Technol. Cancer Res. Treat. 2002, 1, 119–126. [Google Scholar] [CrossRef]
- Roach, J.F.; Hiileboe, H.E. Xeroradiography. Am. J. Roontgenol. Radium Thor. Nucl. Med. 1955, 73, 5–9. [Google Scholar] [CrossRef]
- Ruzicka, F.F., Jr.; Kaufman, L.; Shapiro, G.; Perez, J.V.; Grossi, C.E. Xeromammography and film mammography, a comparative study. Radiology 1965, 85, 260–269. [Google Scholar] [CrossRef]
- Gould, H.R.; Ruzicka, F.F.; Sanchez-Ubeda, R.; Perez, J. Xeroradiography of the breast. Am. J. Roontgonol. Radium Thor. Nucl. Med. 1960, 84, 220–223. [Google Scholar]
- Wolfe, J.N. Xerography of the breast. Radiology 1968, 91, 231–240. [Google Scholar] [CrossRef]
- Martin, J.E. Xerornammography—An improved diagnostic method: Review of 250 biopsied cases. Am. J. Roontgonol. Radium Thor. NucI Med. 1973, 117, 90–96. [Google Scholar] [CrossRef]
- Gold, R.; Bassett, L.; Widoff, B. Radiologic history exhibit: Highlights from the history of mammography. Radiographics 1990, 10, 111–1131. [Google Scholar] [CrossRef]
- Kimme-Smith, C. New and future developments in screen-film mammography equipment and techniques. Radiol. Clin. N. Am. 1992, 30, 55–66. [Google Scholar] [CrossRef]
- Egan, R. Roles of Mammography in the Early Detection of Breast Cancer. Cancer 1969, 24, 1197–1200. [Google Scholar] [CrossRef]
- Egan, R. Experience with mammography in a tumor institution: Evaluation of 1000 cases. AJR 1960, 75, 894–900. [Google Scholar]
- Barnes, G.T.; Brezovich, I.A. The intensify of scattered radiation in mammography. Radiology 1978, 126, 243–247. [Google Scholar] [CrossRef]
- Chan, H.P.; Frank, P.H.; Doi, K.; Iida, N.; Higashida, Y. Development of ultra-high strip density (UHSD) grids: A new anti-scatter technique for mammography. Radiology 1985, 154, 807–815. [Google Scholar] [CrossRef]
- Chen, H.; Danielsson, M.; Xu, C.; Cederström, B. On image quality metrics and the usefulness of grids in digital mammography. J. Med. Imaging 2015, 2, 013501. [Google Scholar] [CrossRef]
- Feig, S.A. Mammographic equipment: Principles, features, selection. Radiol. Clin. N. Am. 1987, 25, 897–911. [Google Scholar] [CrossRef]
- Judith White, F.D.A. Approves System for Digital Mammography. JNCI J. Natl. Cancer Inst. 2000, 92, 442. [Google Scholar] [CrossRef]
- Pisano, E.D.; Gatsonis, C.; Hendrick, E.; Yaffe, M.; Baum, J.K.; Acharyya, S.; Conant, E.F.; Fajardo, L.L.; Bassett, L.; D’Orsi, C.; et al. Diagnostic performance of digital versus film mammography for breast-cancer screening. N. Engl. J. Med. 2005, 353, 1773–1783. [Google Scholar] [CrossRef]
- Habbema, J.; Oortmarssen, G.J.V.; van Putten, D.J.; Lubbe, J.T.; Maas, P.J.V.D. Age-specific reduction in breast cancer mortality by screening: An analysis of the results of the Health Insurance Plan of Greater New York study. J. Natl. Cancer Inst. 1986, 77, 317–320. [Google Scholar] [PubMed]
- Strax, P. Detection of breast cancer. Cancer 1990, 66, 1336–1340. [Google Scholar] [CrossRef]
- Shapiro, S.; Strax, P.; Venet, L. Periodic Breast Cancer Screening in Reducing Mortality from Breast Cancer. J. Am. Med. Assoc. 1971, 215, 1777–1785. [Google Scholar] [CrossRef]
- Andersson, I.; Aspegren, K.; Janzon, L.; Landberg, T.; Lindholm, K.; Linell, F.; Ljungberg, O.; Ranstam, J.; Sigfússon, B. Mammographic screening and mortality from breast cancer: The Malmö mammographic screening trial. BMJ 1988, 297, 943–948. [Google Scholar] [CrossRef]
- Tabár, L.; Vitak, B.; Chen, T.H.; Yen, A.M.; Cohen, A.; Tot, T.; Chiu, S.Y.; Chen, S.L.; Fann, J.C.; Rosell, J.; et al. Swedish two-county trial: Impact of mammographic screening on breast cancer mortality during 3 decades. Radiology 2011, 260, 658–663. [Google Scholar] [CrossRef]
- Frisell, J.; Eklund, G.; Hellström, L.; Lidbrink, E.; Rutqvist, L.E.; Somell, A. Randomized study of mammography screening--preliminary report on mortality in the Stockholm trial. Breast Cancer Res. Treat. 1991, 18, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Bjurstam, N.; Björneld, L.; Duffy, S.W.; Smith, T.C.; Cahlin, E.; Eriksson, O.; Hafström, L.O.; Lingaas, H.; Mattsson, J.; Persson, S.; et al. The Gothenburg breast screening trial: First results on mortality, incidence, and mode of detection for women ages 39-49 years at randomization. Cancer 1997, 80, 2091–2099. [Google Scholar] [CrossRef]
- Moss, S.M.; Wale, C.; Smith, R.; Evans, A.; Cuckle, H.; Duffy, S.W. Effect of mammographic screening from age 40 years on breast cancer mortality in the UK Age trial at 17 years’ follow-up: A randomised controlled trial. Lancet Oncol. 2015, 16, 1123–1132, Erratum in Lancet Oncol. 2015, 16, e427. [Google Scholar] [CrossRef]
- Tabar, L.; Fagerberg, G.; Chen, H.H.; Duffy, S.W.; Smart, C.R.; Gad, A.; Smith, R.A. Efficacy of breast cancer screening by age. New results from the Swedish Two-County Trial. Cancer 1995, 75, 2507–2517. [Google Scholar] [CrossRef]
- Bhide, A.; Datar, S.; Stebbins, K. Mammography: Case Histories of Significant Medical Advances; Harvard Business School Accounting & Management Unit Working Paper, No. 20-002; Harvard Business School: Boston, MA, USA, 2019. [Google Scholar]
- Burnside, E.S.; Sickles, E.A.; Bassett, L.W.; Rubin, D.L.; Lee, C.H.; Ikeda, D.M.; Mendelson, E.B.; Wilcox, P.A.; Butler, P.F.; D’Orsi, C.J. The ACR BI-RADS experience: Learning from history. J. Am. Coll. Radiol. 2009, 6, 851–860. [Google Scholar] [CrossRef]
- D’Orsi, C.; Sickles, E.; Mendelson, E.B.; Morris, E.A. ACR BI-RADS Atlas, Breast Imaging Reporting and Data System; American College of Radiology: Reston, VA, USA, 2013. [Google Scholar]
- Mann, R.M.; Athanasiou, A.; Baltzer, P.A.T.; Camps-Herrero, J.; Clauser, P.; Fallenberg, E.M.; Forrai, G.; Fuchsjäger, M.H.; Helbich, T.H.; Killburn-Toppin, F.; et al. European Society of Breast Imaging (EUSOBI). Breast cancer screening in women with extremely dense breasts recommendations of the European Society of Breast Imaging (EUSOBI). Eur. Radiol. 2022, 32, 4036–4045. [Google Scholar] [CrossRef]
- Honig, E.L.; Mullen, L.A.; Amir, T.; Alvin, M.D.; Jones, M.K.; Ambinder, E.B.; Falomo, E.T.; Harvey, S.C. Factors Impacting False Positive Recall in Screening Mammography. Acad. Radiol. 2019, 26, 1505–1512. [Google Scholar] [CrossRef]
- Morris, E.; Feig, S.A.; Drexler, M.; Lehman, C. Implications of Overdiagnosis: Impact on Screening Mammography Practices. Popul. Health Manag. 2015, 18 (Suppl. S1), S3–S11. [Google Scholar] [CrossRef]
- Burgess, A.E.; Jacobson, F.L.; Judy, P.F. Human observer detection experiments with mammograms and power-law noise. Med. Phys. 2001, 28, 419–437. [Google Scholar] [CrossRef]
- Ziedses des Plantes, B.G. Eine Neue Methode Zur Differenzierung in der Rontgenographie (Planigraphie). Acta Radiol. 1932, 13, 182–192. [Google Scholar] [CrossRef]
- Niklason, L.T.; Christian, B.T.; Niklason, L.E.; Kopans, D.B.; Castleberry, D.E.; Opsahl-Ong, B.H.; Landberg, C.E.; Slanetz, P.J.; Giardino, A.A.; Moore, R.; et al. Digital tomosynthesis in breast imaging. Radiology 1997, 205, 399–406. [Google Scholar] [CrossRef]
- Lynge, E.; Vejborg, I.; Andersen, Z.; von Euler-Chelpin, M.; Napolitano, G. Mammographic Density and Screening Sensitivity, Breast Cancer Incidence and Associated Risk Factors in Danish Breast Cancer Screening. J. Clin. Med. 2019, 8, 2021. [Google Scholar] [CrossRef] [PubMed]
- Tirada, N.; Li, G.; Dreizin, D.; Robinson, L.; Khorjekar, G.; Dromi, S.; Ernst, T. Digital Breast Tomosynthesis: Physics, Artifacts, and Quality Control Considerations. Radiographics 2019, 39, 413–426. [Google Scholar] [CrossRef]
- Skaane, P.; Bandos, A.I.; Gullien, R.; Eben, E.B.; Ekseth, U.; Haakenaasen, U.; Izadi, M.; Jebsen, I.N.; Jahr, G.; Krager, M.; et al. Prospective trial comparing full-field digital mammography (FFDM) versus combined FFDM and tomosynthesis in a population-based screening program using independent double reading with arbitration. Eur. Radiol. 2013, 23, 2061–2071. [Google Scholar] [CrossRef]
- Skaane, P.; Bandos, A.I.; Gullien, R.; Eben, E.B.; Ekseth, U.; Haakenaasen, U.; Izadi, M.; Jebsen, I.N.; Jahr, G.; Krager, M.; et al. Comparison of digital mammography alone and digital mammography plus tomosynthesis in a population-based screening program. Radiology 2013, 267, 47–56. [Google Scholar] [CrossRef]
- Ciatto, S.; Houssami, N.; Bernardi, D.; Caumo, F.; Pellegrini, M.; Brunelli, S.; Tuttobene, P.; Bricolo, P.; Fantò, C.; Valentini, M.; et al. Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (STORM): A prospective comparison study. Lancet Oncol. 2013, 14, 583–589. [Google Scholar] [CrossRef]
- McDonald, E.S.; Oustimov, A.; Weinstein, S.P.; Synnestvedt, M.B.; Schnall, M.; Conant, E.F. Effectiveness of Digital Breast Tomosynthesis Compared With Digital Mammography: Outcomes Analysis From 3 Years of Breast Cancer Screening. JAMA Oncol. 2016, 2, 737–743. [Google Scholar] [CrossRef]
- McDonald, E.S.; McCarthy, A.M.; Akhtar, A.L.; Synnestvedt, M.B.; Schnall, M.; Conant, E.F. Baseline Screening Mammography: Performance of Full-Field Digital Mammography Versus Digital Breast Tomosynthesis. AJR Am. J. Roentgenol. 2015, 205, 1143–1148. [Google Scholar] [CrossRef]
- Lourenco, A.P.; Barry-Brooks, M.; Baird, G.L.; Tuttle, A.; Mainiero, M.B. Changes in recall type and patient treatment following implementation of screening digital breast tomosynthesis. Radiology 2015, 274, 337–342. [Google Scholar] [CrossRef]
- Friedewald, S.M.; Rafferty, E.A.; Rose, S.L.; Durand, M.A.; Plecha, D.M.; Greenberg, J.S.; Hayes, M.K.; Copit, D.S.; Carlson, K.L.; Cink, T.M.; et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA 2014, 311, 2499–2507. [Google Scholar] [CrossRef]
- Giess, C.S.; Pourjabbar, S.; Ip, I.K.; Lacson, R.; Alper, E.; Khorasani, R. Comparing Diagnostic Performance of Digital Breast Tomosynthesis and Full-Field Digital Mammography in a Hybrid Screening Environment. AJR Am. J. Roentgenol. 2017, 209, 929–934. [Google Scholar] [CrossRef]
- Sharpe, R.E., Jr.; Venkataraman, S.; Phillips, J.; Dialani, V.; FeinZachary, V.J.; Prakash, S.; Slanetz, P.J.; Mehta, T.S. Increased Cancer Detection Rate and Variations in the Recall Rate Resulting from Implementation of 3D Digital Breast Tomosynthesis into a Population-based Screening Program. Radiology 2016, 278, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Skaane, P. Breast cancer screening with digital breast tomosynthesis. Breast Cancer 2017, 24, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Kopans, D.; Gavenonis, S.; Halpern, E.; Moore, R. Calcifications in the breast and digital breast tomosynthesis. Breast J. 2011, 17, 638–644. [Google Scholar] [CrossRef]
- Lee, W.K.; Chung, J.; Cha, E.S.; Lee, J.E.; Kim, J.H. Digital breast tomosynthesis and breast ultrasound: Additional roles in dense breasts with category 0 at conventional digital mammography. Eur. J. Radiol. 2016, 85, 291–296. [Google Scholar] [CrossRef]
- Hicken, N.F. Mammography: The Roentgenographic Diagnosis of Breast Tumors by Means of Contrast Media. Surg. Gynecol. Obstet. 1937, 64, 593–603. [Google Scholar]
- Chang, C.H.; Sibala, J.L.; Fritz, S.L.; Dwyer, S.J., III; Templeton, A.W.; Lin, F.; Jewell, W.R. Computed tomography in detection and diagnosis of breast cancer. Cancer 1980, 46 (Suppl. S4), 939–946. [Google Scholar] [CrossRef]
- Akashi-Tanaka, S.; Fukutomi, T.; Miyakawa, K.; Uchiyama, N.; Nanasawa, T.; Tsuda, H. Clinical use of contrast-enhanced computed tomography for decision making in breast-conserving surgery. Breast Cancer 1997, 4, 280–284. [Google Scholar] [CrossRef]
- Brody, W.R.; Macovski, A.; Lehmann, L.; DiBianca, F.A.; Volz, D.; Edelheit, L.S. Intravenous angiography using scanned projection radiography: Preliminary investigation of a new method. Investig. Radiol. 1980, 15, 220–223. [Google Scholar] [CrossRef]
- Jong, R.A.; Yaffe, M.J.; Skarpathiotakis, M.; Shumak, R.S.; Danjoux, N.M.; Gunesekara, A.; Plewes, D.B. Contrast-enhanced digital mammography: Initial clinical experience. Radiology 2003, 228, 842–850. [Google Scholar] [CrossRef]
- Diekmann, F.; Diekmann, S.; Jeunehomme, F.; Muller, S.; Hamm, B.; Bick, U. Digital mammography using iodine-based contrast media: Initial clinical experience with dynamic contrast medium enhancement. Investig. Radiol. 2005, 40, 397–404. [Google Scholar] [CrossRef]
- Dromain, C.; Balleyguier, C.; Muller, S.; Mathieu, M.C.; Rochard, F.; Opolon, P.; Sigal, R. Evaluation of tumor angiogenesis of breast carcinoma using contrast-enhanced digital mammography. AJR Am. J. Roentgenol. 2006, 187, W528–W537. [Google Scholar] [CrossRef]
- Dromain, C.; Thibault, F.; Muller, S.; Rimareix, F.; Delaloge, S.; Tardivon, A.; Balleyguier, C. Dual-energy contrast-enhanced digital mammography: Initial clinical results. Eur. Radiol. 2011, 21, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Zanardo, M.; Cozzi, A.; Trimboli, R.M.; Labaj, O.; Monti, C.B.; Schiaffino, S.; Carbonaro, L.A.; Sardanelli, F. Technique, protocols and adverse reactions for contrast-enhanced spectral mammography (CESM): A systematic review. Insights Imaging 2019, 10, 76. [Google Scholar] [CrossRef]
- Cozzi, A.; Magni, V.; Zanardo, M.; Schiaffino, S.; Sardanelli, F. Contrast-enhanced Mammography: A Systematic Review and Meta-Analysis of Diagnostic Performance. Radiology 2022, 302, 568–581. [Google Scholar] [CrossRef]
- Łuczyńska, E.; Heinze-Paluchowska, S.; Hendrick, E.; Dyczek, S.; Ryś, J.; Herman, K.; Blecharz, P.; Jakubowicz, J. Comparison between breast MRI and contrast-enhanced spectral mammography. Med. Sci. Monit. 2015, 21, 1358–1367. [Google Scholar] [CrossRef] [PubMed]
- Nicosia, L.; Bozzini, A.C.; Palma, S.; Montesano, M.; Signorelli, G.; Pesapane, F.; Latronico, A.; Bagnardi, V.; Frassoni, S.; Sangalli, C.; et al. Contrast-Enhanced Spectral Mammography and tumor size assessment: A valuable tool for appropriate surgical management of breast lesions. Radiol. Med. 2022, 127, 1228–1234. [Google Scholar] [CrossRef] [PubMed]
- Nicosia, L.; Bozzini, A.C.; Signorelli, G.; Palma, S.; Pesapane, F.; Frassoni, S.; Bagnardi, V.; Pizzamiglio, M.; Farina, M.; Trentin, C.; et al. Contrast-Enhanced Spectral Mammography in the Evaluation of Breast Microcalcifications: Controversies and Diagnostic Management. Healthcare 2023, 11, 511. [Google Scholar] [CrossRef]
- Nicosia, L.; Bozzini, A.C.; Palma, S.; Montesano, M.; Pesapane, F.; Ferrari, F.; Dominelli, V.; Rotili, A.; Meneghetti, L.; Frassoni, S.; et al. A Score to Predict the Malignancy of a Breast Lesion Based on Different Contrast Enhancement Patterns in Contrast-Enhanced Spectral Mammography. Cancers 2022, 14, 4337. [Google Scholar] [CrossRef]
- Cozzi, A.; Schiaffino, S.; Fanizza, M.; Magni, V.; Menicagli, L.; Monaco, C.G.; Benedek, A.; Spinelli, D.; Di Leo, G.; Di Giulio, G.; et al. Contrast-enhanced mammography for the assessment of screening recalls: A two-centre study. Eur. Radiol. 2022, 32, 7388–7399. [Google Scholar] [CrossRef]
- Nicosia, L.; Bozzini, A.C.; Pesapane, F.; Rotili, A.; Marinucci, I.; Signorelli, G.; Frassoni, S.; Bagnardi, V.; Origgi, D.; De Marco, P.; et al. Breast Digital Tomosynthesis versus Contrast-Enhanced Mammography: Comparison of Diagnostic Application and Radiation Dose in a Screening Setting. Cancers 2023, 15, 2413. [Google Scholar] [CrossRef] [PubMed]
- Iotti, V.; Ravaioli, S.; Vacondio, R.; Coriani, C.; Caffarri, S.; Sghedoni, R.; Nitrosi, A.; Ragazzi, M.; Gasparini, E.; Masini, C.; et al. Contrast-enhanced spectral mammography in neoadjuvant chemotherapy monitoring: A comparison with breast magnetic resonance imaging. Breast Cancer Res. 2017, 19, 106. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Phillips, J.; Sung, J.S.; Lewin, J.M.; Newell, M.S. ACR BI-RADS®. In ACR BI-RADS® Contrast Enhanced Mammography (CEM) (A Supplement to ACR BI-RADS® Mammography 2013) Atlas, Breast Imaging Reporting and Data System; American College of Radiology: Reston, VA, USA, 2022. [Google Scholar]
- Heywang, S.; Fenzl, G.; Hahn, D.; Krischke, I.; Edmaier, M.; Eiermann, W.; Bassermann, R. MR imaging of the breast: Comparison with mammography and ultrasound. J. Comput. Assist. Tomogr. 1986, 10, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, W.; Zeitler, E. MR imaging of the breast: Fast imaging sequences with and without Gd-DTPA. Preliminary observations. Radiology 1989, 170, 681–686. [Google Scholar]
- Kuhl, C.K.; Schild, H.H.; Morakkabati, N. Dynamic bilateral contrast-enhanced MR imaging of the breast: Trade-off between spatial and temporal resolution. Radiology 2005, 236, 789–800. [Google Scholar] [CrossRef]
- Rahmat, K.; Mumin, N.A.; Hamid, M.T.R.; Hamid, S.A.; Ng, W.L. MRI Breast: Current Imaging Trends, Clinical Applications, and Future Research Directions. Curr. Med. Imaging 2022, 18, 1347–1361. [Google Scholar] [CrossRef]
- Kuhl, C.K.; Schmutzler, R.K.; Leutner, C.C.; Kempe, A.; Wardelmann, E.; Hocke, A.; Maringa, M.; Pfeifer, U.; Krebs, D.; Schild, H.H. Breast MR imaging screening in 192 women proved or suspected to be carriers of a breast cancer susceptibility gene: Preliminary results. Radiology 2000, 215, 267–279. [Google Scholar] [CrossRef]
- Kriege, M.; Brekelmans, C.T.; Boetes, C.; Besnard, P.E.; Zonderland, H.M.; Obdeijn, I.M.; Manoliu, R.A.; Kok, T.; Peterse, H.; Tilanus-Linthorst, M.M.; et al. Efficacy of MRI and mammography for breast cancer screening in women with a familial or genetic predisposition. N. Engl. J. Med. 2004, 351, 427–437. [Google Scholar] [CrossRef]
- Hussein, H.; Abbas, E.; Keshavarzi, S.; Fazelzad, R.; Bukhanov, K.; Kulkarni, S.; Au, F.; Ghai, S.; Alabousi, A.; Freitas, V. Supplemental Breast Cancer Screening in Women with Dense Breasts and Negative Mammography: A Systematic Review and Meta-Analysis. Radiology 2023, 306, e221785. [Google Scholar] [CrossRef]
- Millet, I.; Pages, E.; Hoa, D.; Merigeaud, S.; Curros Doyon, F.; Prat, X.; Taourel, P. Pearls and pitfalls in breast MRI. Br. J. Radiol. 2012, 85, 197–207. [Google Scholar] [CrossRef]
- Wild, J.J.; Neal, D. Use of high-frequency ultrasonic waves for detecting changes of texture in living tissue. Lancet 1951, 1, 655–657. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, P.J. The history of breast ultrasound. J. Ultrasound Med. 2004, 23, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Kossoff, G.; Jellins, J. The physics of breast echography. Semin. Ultrasound 1982, 3, 5–12. [Google Scholar]
- Jellins, J.; Kossoff, G.; Boyd, J. The complementary role of Doppler to the B-mode examination of the breast. J. Ultrasound Med. 1983, 2, 10–29. [Google Scholar]
- Fornage, B.D.; Sneige, N.; Faroux, M.J.; Andry, E. Sonographic appearance and ultrasound-guided fine-needle aspiration biopsy of breast carcinomas smaller than 1 cm3. J. Ultrasound Med. 1990, 9, 559–568. [Google Scholar] [CrossRef]
- Fornage, B.D.; Toubas, O.; Morel, M. Clinical, mammographic, and sonographic determination of preoperative breast cancer size. Cancer 1987, 60, 765–771. [Google Scholar] [CrossRef]
- Amy, D.; Durante, E.; Tot, T. The lobar approach to breast ultrasound imaging and surgery. J. Med. Ultrason. 2015, 42, 331–339. [Google Scholar] [CrossRef]
- Geisel, J.; Raghu, M.; Hooley, R. The Role of Ultrasound in Breast Cancer Screening: The Case for and Against Ultrasound. Semin. Ultrasound CT MR 2018, 39, 25–34. [Google Scholar] [CrossRef]
- Nicosia, L.; Ferrari, F.; Bozzini, A.C.; Latronico, A.; Trentin, C.; Meneghetti, L.; Pesapane, F.; Pizzamiglio, M.; Balesetreri, N.; Cassano, E. Automatic breast ultrasound: State of the art and future perspectives. Ecancermedicalscience 2020, 14, 1062. [Google Scholar] [CrossRef]
- Trimboli, R.M.; Giorgi Rossi, P.; Battisti, N.M.L.; Cozzi, A.; Magni, V.; Zanardo, M.; Sardanelli, F. Do we still need breast cancer screening in the era of targeted therapies and precision medicine? Insights Imaging 2020, 11, 105, PMC7519022. [Google Scholar] [CrossRef] [PubMed]
- Applications of artificial intelligence in breast imaging. Radiol. Clin. N. Am. 2021, 59, 139–148. [CrossRef] [PubMed]
- Becker, A.S.; Marcon, M.; Ghafoor, S.; Wurnig, M.C.; Frauenfelder, T.; Boss, A. Deep Learning in Mammography: Diagnostic Accuracy of a Multipurpose Image Analysis Software in the Detection of Breast Cancer. Investig. Radiol. 2017, 52, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Chitalia, R.; Miliotis, M.; Jahani, N.; Tastsoglou, S.; McDonald, E.S.; Belenky, V.; Cohen, E.A.; Newitt, D.; Van’t Veer, L.J.; Esserman, L.; et al. Radiomic tumor phenotypes augment molecular profiling in predicting recurrence free survival after breast neoadjuvant chemotherapy. Commun. Med. 2023, 3, 46. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, C.K. What the Future Holds for the Screening, Diagnosis, and Treatment of Breast Cancer. Radiology 2023, 306, e223338. [Google Scholar] [CrossRef]
- Wang, G.; Shi, D.; Guo, Q.; Zhang, H.; Wang, S.; Ren, K. Radiomics Based on Digital Mammography Helps to Identify Mammographic Masses Suspicious for Cancer. Front. Oncol. 2022, 12, 843436. [Google Scholar] [CrossRef]
- Nicosia, L.; Bozzini, A.C.; Ballerini, D.; Palma, S.; Pesapane, F.; Raimondi, S.; Gaeta, A.; Bellerba, F.; Origgi, D.; De Marco, P.; et al. Radiomic Features Applied to Contrast Enhancement Spectral Mammography: Possibility to Predict Breast Cancer Molecular Subtypes in a Non-Invasive Manner. Int. J. Mol. Sci. 2022, 23, 15322. [Google Scholar] [CrossRef]
- Li, J.; Song, Y.; Xu, S.; Wang, J.; Huang, H.; Ma, W.; Jiang, X.; Wu, Y.; Cai, H.; Li, L. Predicting underestimation of ductal carcinoma in situ: A comparison between radiomics and conventional approaches. Int. J. Comput. Assist. Radiol. Surg. 2019, 14, 709–721. [Google Scholar] [CrossRef]
- Carter, S.M.; Rogers, W.; Win, K.T.; Frazer, H.; Richards, B.; Houssami, N. The ethical, legal and social implications of using artificial intelligence systems in breast cancer care. Breast 2020, 49, 25–32. [Google Scholar] [CrossRef]
- Mendat, C.C.; Mislan, D.; Hession-Kunz, L. Patient comfort from the technologist perspective: Factors to consider in mammographic imaging. Int. J. Womens Health 2017, 9, 359–364. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicosia, L.; Gnocchi, G.; Gorini, I.; Venturini, M.; Fontana, F.; Pesapane, F.; Abiuso, I.; Bozzini, A.C.; Pizzamiglio, M.; Latronico, A.; et al. History of Mammography: Analysis of Breast Imaging Diagnostic Achievements over the Last Century. Healthcare 2023, 11, 1596. https://doi.org/10.3390/healthcare11111596
Nicosia L, Gnocchi G, Gorini I, Venturini M, Fontana F, Pesapane F, Abiuso I, Bozzini AC, Pizzamiglio M, Latronico A, et al. History of Mammography: Analysis of Breast Imaging Diagnostic Achievements over the Last Century. Healthcare. 2023; 11(11):1596. https://doi.org/10.3390/healthcare11111596
Chicago/Turabian StyleNicosia, Luca, Giulia Gnocchi, Ilaria Gorini, Massimo Venturini, Federico Fontana, Filippo Pesapane, Ida Abiuso, Anna Carla Bozzini, Maria Pizzamiglio, Antuono Latronico, and et al. 2023. "History of Mammography: Analysis of Breast Imaging Diagnostic Achievements over the Last Century" Healthcare 11, no. 11: 1596. https://doi.org/10.3390/healthcare11111596
APA StyleNicosia, L., Gnocchi, G., Gorini, I., Venturini, M., Fontana, F., Pesapane, F., Abiuso, I., Bozzini, A. C., Pizzamiglio, M., Latronico, A., Abbate, F., Meneghetti, L., Battaglia, O., Pellegrino, G., & Cassano, E. (2023). History of Mammography: Analysis of Breast Imaging Diagnostic Achievements over the Last Century. Healthcare, 11(11), 1596. https://doi.org/10.3390/healthcare11111596







