A Multiaxial Rehabilitation Programme for Workers with COVID-19 Sequelae Using a Conventional and Technological-Robotic Approach: The Proposal of INAIL and Fondazione Don Carlo Gnocchi
Abstract
:1. Introduction
2. Long COVID or Post-COVID Syndrome
2.1. Respiratory Sequelae
2.2. Cardiological Sequelae
2.3. Neurological Sequelae
2.4. Psychological Sequelae
2.5. Metabolic Sequelae
3. Long COVID, Rehabilitation, and Technologies: Scientific Evidence
4. The INAIL Proposal for Long COVID Rehabilitation
- Respiratory rehabilitation: based on personalised evaluation and treatment, which includes exercise training, education, techniques of bronchial clearance, and behavioural modification designed to improve the physical and psychological condition of people with respiratory diseases.
- Cardiac rehabilitation: based on healthy behaviour and education, lifestyle risk factor management, medical risk management, long-term strategies, and exercise training with ergometers at different frequencies and intensities aimed to improve cardiac function.
- Musculoskeletal rehabilitation: based on traditional and aquatic exercise training and/or technological and robotic solutions aimed at improving muscle strength, endurance, overall motor performance, and the gradual recovery/adaptation of daily life activities.
- Neuropsychological rehabilitation: based on cognitive-behavioural techniques aimed at improving higher functions and psychological aspects.
5. Fondazione Don Carlo Gnocchi for Long COVID Rehabilitation
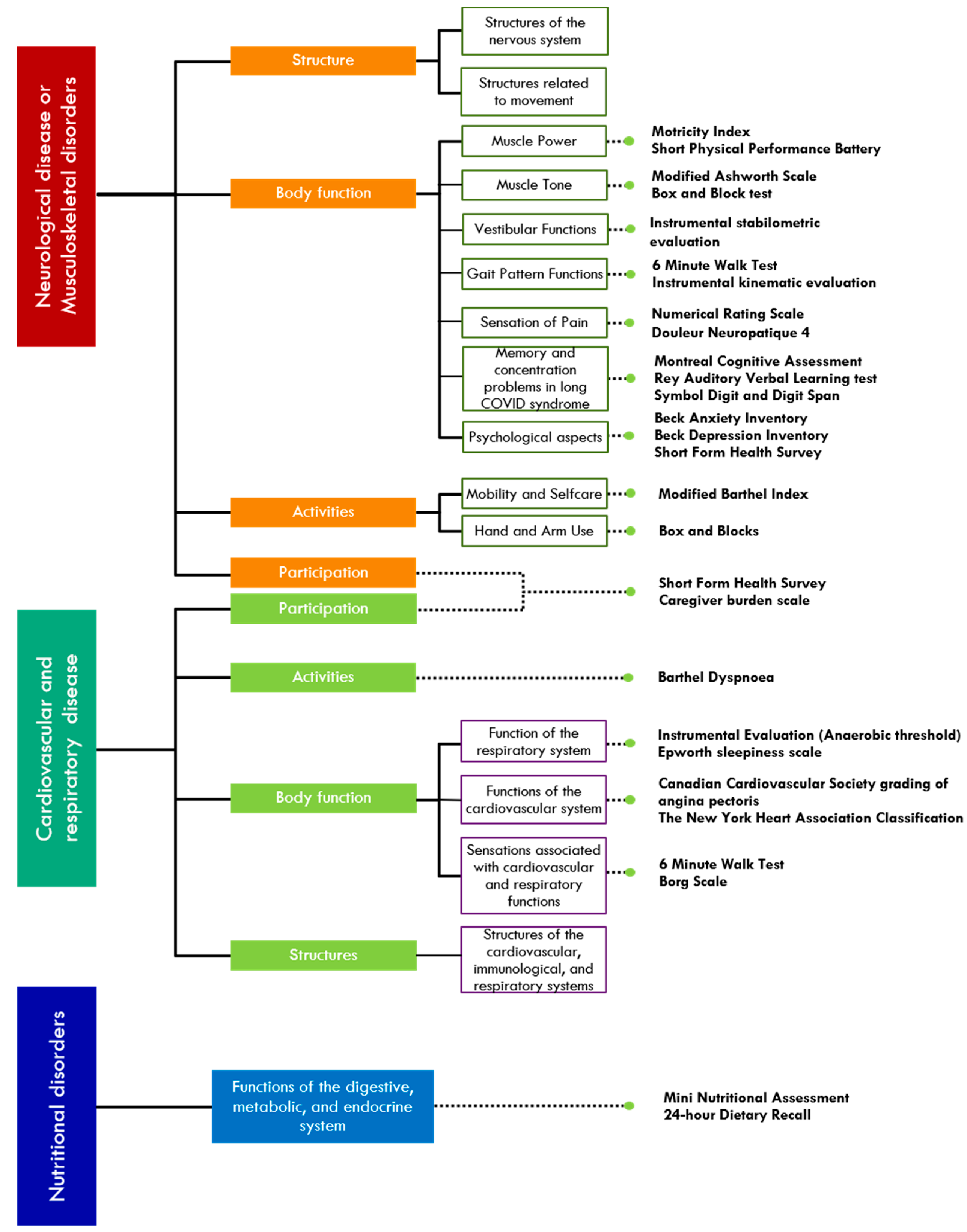
6. Integrated Multi-Axial Rehabilitation Protocol INAIL-FDG
6.1. Respiratory and Cardiological Rehabilitation
6.2. Neuromotor Rehabilitation
- sensorimotor training to restore normal motor programmes, maintain joint mobility of the upper and lower limbs through passive and active assisted mobilisations, and control pain when present;
- programmes to increase muscle strength and endurance with incremental exercises appropriate to the patient’s performance, and programmes to recover segmental movement and then more complex motor functions;
- training from autonomies in safe postural transitions;
- postural control exercises;
- balance and gait recovery exercise;
- activities aimed at family and social reintegration.
- intensified treatment;
- objective measurement of patient’s achieved goals;
- personalised treatment;
- stimulation of neurocognitive as well as motor aspects [77].
- The robotic device can be:
- End-effector, where movements are generated from the most distal segment of the extremity and no alignment between patient-robot joints is required [78];
- Exoskeletons, which have a one-to-one correspondence between robots and human joints, and every single joint is guided along a pre-programmed trajectory [78]. They can support and assist complex motor functions of the upper and lower limbs.
- There are also advanced technological systems such as:
- sensor-based systems that allow for the control of a wide range of limb movements, but also of the whole body in space (trunk and gait control);
- electromechanical systems with body weight or a district relief system (e.g., upper limb).
6.3. Cognitive Rehabilitation
6.3.1. Memory and Concentration Problems in Long COVID Syndrome
- targeted reinforcement of deficit skills through the administration of exercises of increasing difficulty (e.g., short-term memory training or attention training);
- adaptation of the patient’s behaviours and setting of new compensation strategies (e.g., for memory difficulties the use of tools such as calendars and alarm clocks; for concentration difficulties the avoidance of distracting situations and contexts, to do only one thing at a time);
- evaluation of the results obtained is mandatory at the end of the treatment.
6.3.2. Psychological Aspects
6.3.3. Nutritional Aspects
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carvalho Aguiar Melo, M.; de Sousa Soares, D. Impact of social distancing on mental health during the COVID-19 pandemic: An urgent discussion. Int. J. Soc. Psychiatry 2020, 66, 625–626. [Google Scholar] [CrossRef] [PubMed]
- d’Ettorre, G.; Gentilini Cacciola, E.; Santinelli, L.; De Girolamo, G.; Spagnolello, O.; Russo, A.; Tarsitani, L.; Ciccozzi, M.; Mastroianni, C.M.; d’Ettorre, G.; et al. COVID-19 sequelae in working age patients: A systematic review. J. Med. Virol. 2022, 94, 858–868. [Google Scholar] [CrossRef] [PubMed]
- Mahase, E. COVID-19: What do we know about “long covid”? BMJ 2020, 370, m2815. [Google Scholar] [CrossRef] [PubMed]
- Yelin, D.; Margalit, I.; Yahav, D.; Runold, M.; Bruchfeld, J. Long COVID-19-it’s not over until? Clin. Microbiol. Infect 2021, 27, 506–508. [Google Scholar] [CrossRef]
- Malkova, A.; Kudryavtsev, I.; Starshinova, A.; Kudlay, D.; Zinchenko, Y.; Glushkova, A.; Yablonskiy, P.; Shoenfeld, Y. Post COVID-19 Syndrome in Patients with Asymptomatic/Mild Form. Pathogens 2021, 10, 1408. [Google Scholar] [CrossRef]
- Qin, E.S.; Hough, C.L.; Andrews, J.; Bunnell, A.E. Intensive care unit-acquired weakness and the COVID-19 pandemic: A clinical review. PM&R 2022, 14, 227–238. [Google Scholar] [CrossRef]
- AL-Mhanna, S.B.; Mohamed, M.; Noor, N.M.; Afolabi, H.A.; Irekeola, A.A.; Bello, K.E.; Aldhahi, M.I.; Wan Ghazali, W.S. Effectiveness of Pulmonary Rehabilitation among COVID-19 Patients: A Systematic Review and Meta-Analysis. Healthcare 2022, 10, 2130. [Google Scholar] [CrossRef]
- Bailly, M.; Pélissier, L.; Coudeyre, E.; Evrard, B.; Bingula, R.; Rochette, C.; Mériade, L.; Blavignac, C.; Fournier, A.-C.; Bignon, Y.-J.; et al. Systematic Review of COVID-19-Related Physical Activity-Based Rehabilitations: Benefits to Be Confirmed by More Robust Methodological Approaches. Int. J. Environ. Res. Public Health 2022, 19, 9025. [Google Scholar] [CrossRef]
- Rolin, S.; Chakales, A.; Verduzco-Gutierrez, M. Rehabilitation Strategies for Cognitive and Neuropsychiatric Manifestations of COVID-19. Curr. Phys. Med. Rehabil. Rep. 2022, 10, 182–187. [Google Scholar] [CrossRef]
- Nopp, S.; Moik, F.; Klok, F.A.; Gattinger, D.; Petrovic, M.; Vonbank, K.; Koczulla, A.R.; Ay, C.; Zwick, R.H. Outpatient Pulmonary Rehabilitation in Patients with Long COVID Improves Exercise Capacity, Functional Status, Dyspnea, Fatigue, and Quality of Life. Respiration 2022, 101, 593–601. [Google Scholar] [CrossRef]
- Calabrò, R.S.; Cassio, A.; Mazzoli, D.; Andrenelli, E.; Bizzarini, E.; Campanini, I.; Carmignano, S.M.; Cerulli, S.; Chisari, C.; Colombo, V.; et al. What does evidence tell us about the use of gait robotic devices in patients with multiple sclerosis? A comprehensive systematic review on functional outcomes and clinical recommendations. Eur. J. Phys. Rehabil. Med. 2021, 57, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Morone, G.; Palomba, A.; Martino Cinnera, A.; Agostini, M.; Aprile, I.; Arienti, C.; Paci, M.; Casanova, E.; Marino, D.; LA Rosa, G.; et al. Systematic review of guidelines to identify recommendations for upper limb robotic rehabilitation after stroke. Eur. J. Phys. Rehabil. Med. 2021, 57, 238–245. [Google Scholar] [CrossRef]
- Straudi, S.; Baluardo, L.; Arienti, C.; Bozzolan, M.; Lazzarini, S.G.; Agostini, M.; Aprile, I.; Paci, M.; Casanova, E.; Marino, D.; et al. Effectiveness of robot-assisted arm therapy in stroke rehabilitation: An overview of systematic reviews. NeuroRehabilitation 2022, 51, 559–576. [Google Scholar] [CrossRef]
- Bressi, F.; Cricenti, L.; Campagnola, B.; Bravi, M.; Miccinilli, S.; Santacaterina, F.; Sterzi, S.; Straudi, S.; Agostini, M.; Paci, M.; et al. Effects of robotic upper limb treatment after stroke on cognitive patterns: A systematic review. NeuroRehabilitation 2022, 51, 541–558. [Google Scholar] [CrossRef] [PubMed]
- Sykes, D.L.; Holdsworth, L.; Jawad, N.; Gunasekera, P.; Morice, A.H.; Crooks, M.G. Post-COVID-19 Symptom Burden: What is Long-COVID and How Should We Manage It? Lung 2021, 199, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, A.; Iqbal, K.; Arshad Ali, S.; Azim, D.; Farid, E.; Baig, M.D.; Bin Arif, T.; Raza, M. The COVID-19 Sequelae: A Cross-Sectional Evaluation of Post-recovery Symptoms and the Need for Rehabilitation of COVID-19 Survivors. Cureus 2021, 13, e13080. [Google Scholar] [CrossRef]
- Sudre, C.H.; Murray, B.; Varsavsky, T.; Graham, M.S.; Penfold, R.S.; Bowyer, R.C.; Pujol, J.C.; Klaser, K.; Antonelli, M.; Canas, L.S.; et al. Attributes and predictors of long COVID. Nat. Med. 2021, 27, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Pincherle, A.; Jöhr, J.; Pancini, L.; Leocani, L.; Dalla Vecchia, L.; Ryvlin, P.; Schiff, N.D.; Diserens, K. Intensive Care Admission and Early Neuro-Rehabilitation. Lessons for COVID-19? Front. Neurol. 2020, 11, 880. [Google Scholar] [CrossRef] [PubMed]
- Polastri, M.; Nava, S.; Clini, E.; Vitacca, M.; Gosselink, R. COVID-19 and pulmonary rehabilitation: Preparing for phase three. Eur. Respir. J. 2020, 55, 2001822. [Google Scholar] [CrossRef]
- Kiekens, C.; Boldrini, P.; Andreoli, A.; Avesani, R.; Gamna, F.; Grandi, M.; Lombardi, F.; Lusuardi, M.; Molteni, F.; Perboni, A.; et al. Rehabilitation and respiratory management in the acute and early post-acute phase. “Instant paper from the field” on rehabilitation answers to the COVID-19 emergency. Eur. J. Phys. Rehabil. Med. 2020, 56, 323–326. [Google Scholar] [CrossRef]
- Picone, P.; Sanfilippo, T.; Guggino, R.; Scalisi, L.; Monastero, R.; Baschi, R.; Mandalà, V.; San Biagio, L.; Rizzo, M.; Giacomazza, D.; et al. Neurological Consequences, Mental Health, Physical Care, and Appropriate Nutrition in Long-COVID-19. Cell Mol. Neurobiol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Torres-Castro, R.; Vasconcello-Castillo, L.; Alsina-Restoy, X.; Solis-Navarro, L.; Burgos, F.; Puppo, H.; Vilaró, J. Respiratory function in patients post-infection by COVID-19: A systematic review and meta-analysis. Pulmonology 2021, 27, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Peramo-Álvarez, F.P.; López-Zúñiga, M.Á.; López-Ruz, M.Á. Medical sequels of COVID-19. Med. Clin. (Engl. Ed.) 2021, 157, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Daher, A.; Balfanz, P.; Cornelissen, C.; Müller, A.; Bergs, I.; Marx, N.; Müller-Wieland, D.; Hartmann, B.; Dreher, M.; Müller, T. Follow up of patients with severe coronavirus disease 2019 (COVID-19): Pulmonary and extrapulmonary disease sequelae. Respir. Med. 2020, 174, 106197. [Google Scholar] [CrossRef] [PubMed]
- van den Borst, B.; Peters, J.B.; Brink, M.; Schoon, Y.; Bleeker-Rovers, C.P.; Schers, H.; van Hees, H.W.H.; van Helvoort, H.; van den Boogaard, M.; van der Hoeven, H.; et al. Comprehensive Health Assessment 3 Months After Recovery From Acute Coronavirus Disease 2019 (COVID-19). Clin. Infect. Dis. 2021, 73, e1089–e1098. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef]
- Centorbi, C.; Garau, E.; Borsi, L.; Brambilla, V.; Brambilla, L.; Lazzeroni, D. Cardiovascular Post-Acute COVID-19 Syndrome: Definition, Clinical Scenarios, Diagnosis, and Management. In New Insights on Cardiomyopathy; IntechOpen: London, UK, 2022. [Google Scholar]
- Huang, L.; Zhao, P.; Tang, D.; Zhu, T.; Han, R.; Zhan, C.; Liu, W.; Zeng, H.; Tao, Q.; Xia, L. Cardiac Involvement in Patients Recovered From COVID-2019 Identified Using Magnetic Resonance Imaging. JACC Cardiovasc Imaging 2020, 13, 2330–2339. [Google Scholar] [CrossRef]
- Puntmann, V.O.; Carerj, M.L.; Wieters, I.; Fahim, M.; Arendt, C.; Hoffmann, J.; Shchendrygina, A.; Escher, F.; Vasa-Nicotera, M.; Zeiher, A.M.; et al. Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered From Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 1265–1273. [Google Scholar] [CrossRef]
- Rajpal, S.; Tong, M.S.; Borchers, J.; Zareba, K.M.; Obarski, T.P.; Simonetti, O.P.; Daniels, C.J. Cardiovascular Magnetic Resonance Findings in Competitive Athletes Recovering From COVID-19 Infection. JAMA Cardiol. 2021, 6, 116–118. [Google Scholar] [CrossRef]
- Keyhanian, K.; Umeton, R.P.; Mohit, B.; Davoudi, V.; Hajighasemi, F.; Ghasemi, M. SARS-CoV-2 and nervous system: From pathogenesis to clinical manifestation. J. Neuroimmunol. 2020, 350, 577436. [Google Scholar] [CrossRef]
- Halpin, S.; O’Connor, R.; Sivan, M. Long COVID and chronic COVID syndromes. J. Med. Virol. 2020, 93, 1242. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Torre, F.; Mínguez-Olaondo, A.; López-Bravo, A.; Tijero, B.; Grozeva, V.; Walcker, M.; Azkune-Galparsoro, H.; López de Munain, A.; Alcaide, A.B.; Quiroga, J.; et al. Dysautonomia in COVID-19 Patients: A Narrative Review on Clinical Course, Diagnostic and Therapeutic Strategies. Front. Neurol. 2022, 13, 886609. [Google Scholar] [CrossRef] [PubMed]
- Mäkinen, O.J.; Bäcklund, M.E.; Liisanantti, J.; Peltomaa, M.; Karlsson, S.; Kalliomäki, M.-L. Persistent pain in intensive care survivors: A systematic review. Br. J. Anaesth 2020, 125, 149–158. [Google Scholar] [CrossRef]
- Hosey, M.M.; Needham, D.M. Survivorship after COVID-19 ICU stay. Nat. Rev. Dis. Primers 2020, 6, 60. [Google Scholar] [CrossRef]
- Malik, G.R.; Wolfe, A.R.; Soriano, R.; Rydberg, L.; Wolfe, L.F.; Deshmukh, S.; Ko, J.H.; Nussbaum, R.P.; Dreyer, S.D.; Jayabalan, P.; et al. Injury-prone: Peripheral nerve injuries associated with prone positioning for COVID-19-related acute respiratory distress syndrome. Br. J. Anaesth 2020, 125, e478–e480. [Google Scholar] [CrossRef] [PubMed]
- Zubair, A.S.; McAlpine, L.S.; Gardin, T.; Farhadian, S.; Kuruvilla, D.E.; Spudich, S. Neuropathogenesis and Neurologic Manifestations of the Coronaviruses in the Age of Coronavirus Disease 2019: A Review. JAMA Neurol. 2020, 77, 1018–1027. [Google Scholar] [CrossRef]
- Carvalho, P.M.d.M.; Moreira, M.M.; de Oliveira, M.N.A.; Landim, J.M.M.; Neto, M.L.R. The psychiatric impact of the novel coronavirus outbreak. Psychiatry Res. 2020, 286, 112902. [Google Scholar] [CrossRef]
- Taquet, M.; Luciano, S.; Geddes, J.R.; Harrison, P.J. Bidirectional associations between COVID-19 and psychiatric disorder: Retrospective cohort studies of 62 354 COVID-19 cases in the USA. Lancet Psychiatry 2021, 8, 130–140. [Google Scholar] [CrossRef]
- Singh, A.K.; Singh, R. Hyperglycemia without diabetes and new-onset diabetes are both associated with poorer outcomes in COVID-19. Diabetes Res. Clin. Pract. 2020, 167, 108382. [Google Scholar] [CrossRef]
- Yong, S.J. Long COVID or post-COVID-19 syndrome: Putative pathophysiology, risk factors, and treatments. Infect. Dis. 2021, 53, 737–754. [Google Scholar] [CrossRef]
- Demeco, A.; Marotta, N.; Barletta, M.; Pino, I.; Marinaro, C.; Petraroli, A.; Moggio, L.; Ammendolia, A. Rehabilitation of patients post-COVID-19 infection: A literature review. J. Int. Med. Res. 2020, 48, 300060520948382. [Google Scholar] [CrossRef]
- Twisk, F.N.M.; Maes, M. A review on cognitive behavorial therapy (CBT) and graded exercise therapy (GET) in myalgic encephalomyelitis (ME)/chronic fatigue syndrome (CFS): CBT/GET is not only ineffective and not evidence-based, but also potentially harmful for many patients with ME/CFS. Neuro Endocrinol. Lett. 2009, 30, 284–299. [Google Scholar] [PubMed]
- Fugazzaro, S.; Contri, A.; Esseroukh, O.; Kaleci, S.; Croci, S.; Massari, M.; Facciolongo, N.C.; Besutti, G.; Iori, M.; Salvarani, C.; et al. Rehabilitation Interventions for Post-Acute COVID-19 Syndrome: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 5185. [Google Scholar] [CrossRef]
- Rivera, R.; Carballea, D.; Benitez, D. Implementation of Cognitive Telerehabilitation during COVID-19 Pandemic. Arch. Phys. Med. Rehabil. 2022, 103, e6. [Google Scholar] [CrossRef]
- Vieira, A.G.d.S.; Pinto, A.C.P.N.; Garcia, B.M.S.P.; Eid, R.A.C.; Mól, C.G.; Nawa, R.K. Telerehabilitation improves physical function and reduces dyspnoea in people with COVID-19 and post-COVID-19 conditions: A systematic review. J. Physiother. 2022, 68, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Murray, E.; Goodfellow, H.; Bindman, J.; Blandford, A.; Bradbury, K.; Chaudhry, T.; Fernandez-Reyes, D.; Gomes, M.; Hamilton, F.L.; Heightman, M.; et al. Development, deployment and evaluation of digitally enabled, remote, supported rehabilitation for people with long COVID-19 (Living With COVID-19 Recovery): Protocol for a mixed-methods study. BMJ Open 2022, 12, e057408. [Google Scholar] [CrossRef]
- Inail Consulenza Statistico Attuariale. Scheda Nazionale Infortuni Sul Lavoro Da Covid-19. Scheda Nazionale Infortuni Sul Lavoro da COVID-19. I Dati Delle Denunce al 31 Dicembre 2022. N.R 31. 2023. Available online: https://www.inail.it/cs/internet/docs/alg-scheda-tecnica-contagi-covid-31-dicembre-2022.pdf (accessed on 1 March 2023).
- Rossi, P.; La Russa, C.; Bramante, L.; Mele, A.; Savino, E. Il modello multi-assiale nella riabilitazione da COVID-19. In Rivista Degli Infortuni e Delle Malattie Professionali; pp. 39–44. Available online: https://www.inail.it/cs/internet/comunicazione/pubblicazioni/rivista-infortuni.html (accessed on 1 March 2023).
- Aprile, I.; Germanotta, M.; Cruciani, A.; Loreti, S.; Pecchioli, C.; Cecchi, F.; Montesano, A.; Galeri, S.; Diverio, M.; Falsini, C.; et al. Upper Limb Robotic Rehabilitation After Stroke: A Multicenter, Randomized Clinical Trial. J. Neurol. Phys. Ther. 2020, 44, 3–14. [Google Scholar] [CrossRef]
- Aprile, I.; Guardati, G.; Cipollini, V.; Papadopoulou, D.; Mastrorosa, A.; Castelli, L.; Monteleone, S.; Redolfi, A.; Galeri, S.; Germanotta, M. Robotic Rehabilitation: An Opportunity to Improve Cognitive Functions in Subjects With Stroke. An Explorative Study. Front. Neurol. 2020, 11, 588285. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. International Classification of Functioning, Disability and Health: ICF. Available online: https://apps.who.int/iris/handle/10665/42407 (accessed on 1 March 2023).
- Wijkstra, P.J.; TenVergert, E.M.; van der Mark, T.W.; Postma, D.S.; Van Altena, R.; Kraan, J.; Koëter, G.H. Relation of lung function, maximal inspiratory pressure, dyspnoea, and quality of life with exercise capacity in patients with chronic obstructive pulmonary disease. Thorax 1994, 49, 468–472. [Google Scholar] [CrossRef]
- Gosselink, R.; De Vos, J.; van den Heuvel, S.P.; Segers, J.; Decramer, M.; Kwakkel, G. Impact of inspiratory muscle training in patients with COPD: What is the evidence? Eur. Respir. J. 2011, 37, 416–425. [Google Scholar] [CrossRef]
- Gimeno-Santos, E.; Frei, A.; Steurer-Stey, C.; de Batlle, J.; Rabinovich, R.A.; Raste, Y.; Hopkinson, N.S.; Polkey, M.I.; van Remoortel, H.; Troosters, T.; et al. Determinants and outcomes of physical activity in patients with COPD: A systematic review. Thorax 2014, 69, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, B. ACSM’s Guidelines for Exercise Testing and Prescription 9th Ed. 2014. J. Can. Chiropr. Assoc. 2014, 58, 328. [Google Scholar]
- O’Shea, S.D.; Taylor, N.F.; Paratz, J. Peripheral muscle strength training in COPD: A systematic review. Chest 2004, 126, 903–914. [Google Scholar] [CrossRef] [PubMed]
- Probst, V.S.; Troosters, T.; Pitta, F.; Decramer, M.; Gosselink, R. Cardiopulmonary stress during exercise training in patients with COPD. Eur. Respir. J 2006, 27, 1110–1118. [Google Scholar] [CrossRef]
- Janaudis-Ferreira, T.; Hill, K.; Goldstein, R.; Wadell, K.; Brooks, D. Arm exercise training in patients with chronic obstructive pulmonary disease: A systematic review. J. Cardiopulm Rehabil. Prev. 2009, 29, 277–283. [Google Scholar] [CrossRef]
- Costi, S.; Crisafulli, E.; Degli Antoni, F.; Beneventi, C.; Fabbri, L.M.; Clini, E.M. Effects of unsupported upper extremity exercise training in patients with COPD: A randomized clinical trial. Chest 2009, 136, 387–395. [Google Scholar] [CrossRef]
- Vogiatzis, I.; Nanas, S.; Roussos, C. Interval training as an alternative modality to continuous exercise in patients with COPD. Eur. Respir. J. 2002, 20, 12–19. [Google Scholar] [CrossRef]
- Vogiatzis, I.; Simoes, D.C.M.; Stratakos, G.; Kourepini, E.; Terzis, G.; Manta, P.; Athanasopoulos, D.; Roussos, C.; Wagner, P.D.; Zakynthinos, S. Effect of pulmonary rehabilitation on muscle remodelling in cachectic patients with COPD. Eur. Respir. J. 2010, 36, 301–310. [Google Scholar] [CrossRef]
- Poulain, M.; Durand, F.; Palomba, B.; Ceugniet, F.; Desplan, J.; Varray, A.; Préfaut, C. 6-minute walk testing is more sensitive than maximal incremental cycle testing for detecting oxygen desaturation in patients with COPD. Chest 2003, 123, 1401–1407. [Google Scholar] [CrossRef] [PubMed]
- Leung, R.W.M.; Alison, J.A.; McKeough, Z.J.; Peters, M.J. Ground walk training improves functional exercise capacity more than cycle training in people with chronic obstructive pulmonary disease (COPD): A randomised trial. J. Physiother. 2010, 56, 105–112. [Google Scholar] [CrossRef]
- Kodric, M.; Garuti, G.; Colomban, M.; Russi, B.; Porta, R.D.; Lusuardi, M.; Confalonieri, M. The effectiveness of a bronchial drainage technique (ELTGOL) in COPD exacerbations. Respirology 2009, 14, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Pfleger, A.; Theissl, B.; Oberwaldner, B.; Zach, M.S. Self-administered chest physiotherapy in cystic fibrosis: A comparative study of high-pressure PEP and autogenic drainage. Lung 1992, 170, 323–330. [Google Scholar] [CrossRef]
- Volsko, T.A.; DiFiore, J.; Chatburn, R.L. Performance comparison of two oscillating positive expiratory pressure devices: Acapella versus Flutter. Respir. Care 2003, 48, 124–130. [Google Scholar] [PubMed]
- Hansen, L.G.; Warwick, W.J. High-frequency chest compression system to aid in clearance of mucus from the lung. Biomed. Instrum Technol. 1990, 24, 289–294. [Google Scholar] [PubMed]
- Bach, J.R. Update and perspective on noninvasive respiratory muscle aids. Part 2: The expiratory aids. Chest 1994, 105, 1538–1544. [Google Scholar] [CrossRef]
- Huppmann, P.; Sczepanski, B.; Boensch, M.; Winterkamp, S.; Schönheit-Kenn, U.; Neurohr, C.; Behr, J.; Kenn, K. Effects of inpatient pulmonary rehabilitation in patients with interstitial lung disease. Eur. Respir. J. 2013, 42, 444–453. [Google Scholar] [CrossRef]
- Holland, A.E.; Hill, C.J.; Glaspole, I.; Goh, N.; McDonald, C.F. Predictors of benefit following pulmonary rehabilitation for interstitial lung disease. Respir. Med. 2012, 106, 429–435. [Google Scholar] [CrossRef]
- Vitacca, M.; Paneroni, M.; Baiardi, P.; De Carolis, V.; Zampogna, E.; Belli, S.; Carone, M.; Spanevello, A.; Balbi, B.; Bertolotti, G. Development of a Barthel Index based on dyspnea for patients with respiratory diseases. Int. J. Chron Obstruct Pulmon Dis. 2016, 11, 1199–1206. [Google Scholar] [CrossRef]
- Johns, M.W. A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef]
- Smith, E.R. The angina grading system of the Canadian Cardiovascular Society. Can. J. Cardiol. 2002, 18, 439–442. [Google Scholar]
- Bredy, C.; Ministeri, M.; Kempny, A.; Alonso-Gonzalez, R.; Swan, L.; Uebing, A.; Diller, G.-P.; Gatzoulis, M.A.; Dimopoulos, K. New York Heart Association (NYHA) classification in adults with congenital heart disease: Relation to objective measures of exercise and outcome. Eur. Heart J. Qual Care Clin. Outcomes 2018, 4, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Borg, G.A.V. Psychophysical bases of perceived exertion. Med. Sci. Sport. Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef]
- Aprile, I.; Guardati, G.; Cipollini, V.; Papadopoulou, D.; Monteleone, S.; Redolfi, A.; Garattini, R.; Sacella, G.; Noro, F.; Galeri, S.; et al. Influence of cognitive impairment on the recovery of subjects with subacute stroke undergoing upper limb robotic rehabilitation. Brain Sci. 2021, 11, 587. [Google Scholar] [CrossRef] [PubMed]
- Molteni, F.; Gasperini, G.; Cannaviello, G.; Guanziroli, E. Exoskeleton and End-Effector Robots for Upper and Lower Limbs Rehabilitation: Narrative Review. PM R 2018, 10, S174–S188. [Google Scholar] [CrossRef] [PubMed]
- Bohannon, R.W. Motricity index scores are valid indicators of paretic upper extremity strength following stroke. J. Phys. Ther. Sci. 1999, 11, 59–61. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A Short Physical Performance Battery Assessing Lower Extremity Function: Association With Self-Reported Disability and Prediction of Mortality and Nursing Home Admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef]
- Enright, P.L. The six-minute walk test. Respir. Care 2003, 48, 783–785. [Google Scholar]
- Desrosiers, J.; Bravo, G.; Hébert, R.; Dutil, É.; Mercier, L. Validation of the Box and Block Test as a measure of dexterity of elderly people: Reliability, validity, and norms studies. Arch. Phys. Med. Rehabil. 1994, 75, 751–755. [Google Scholar] [CrossRef]
- Bohannon, R.W.; Smith, M.B. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys. Ther. 1987, 67, 206–207. [Google Scholar] [CrossRef]
- Shah, S.; Vanclay, F.; Cooper, B. Improving the sensitivity of the Barthel Index for stroke rehabilitation. J. Clin. Epidemiol. 1989, 42, 703–709. [Google Scholar] [CrossRef]
- Downie, W.W.; Leatham, P.A.; Rhind, V.M.; Wright, V.; Branco, J.A.; Anderson, J.A. Studies with pain rating scales. Ann. Rheum Dis. 1978, 37, 378–381. [Google Scholar] [CrossRef] [PubMed]
- Bouhassira, D.; Attal, N.; Alchaar, H.; Boureau, F.; Brochet, B.; Bruxelle, J.; Cunin, G.; Fermanian, J.; Ginies, P.; Grun-Overdyking, A.; et al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain 2005, 114, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Giardini, M.; Arcolin, I.; Guglielmetti, S.; Godi, M.; Capelli, A.; Corna, S. Balance performance in patients with post-acute COVID-19 compared to patients with an acute exacerbation of chronic obstructive pulmonary disease and healthy subjects. Int. J. Rehabil. Res. 2022, 45, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Martín, A.J.; De Villa, S.G.; García, F.J.R.; Domínguez, J.J.G.; Diciolla, N.; Sánchez, M.J.Y.; Marques, A.; Jiménez, A.R. IMU-based monitoring of discharged patients with COVID-19 for the assessment of in-home recovering. In Proceedings of the 2022 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Messina, Italy, 22–24 June 2022; pp. 1–2. [Google Scholar]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Monaco, M.; Costa, A.; Caltagirone, C.; Carlesimo, G.A. Forward and backward span for verbal and visuo-spatial data: Standardization and normative data from an Italian adult population. Neurol. Sci. 2013, 34, 749–754. [Google Scholar] [CrossRef]
- Smith, A. Symbol Digit Modalities Test; Western Psychological Services Los Angeles: Los Angeles, CA, USA, 1973. [Google Scholar]
- Rey, A. L’examen Clinique en Psychologie. [The Clinical Examination in Psychology]; L’examen clinique en psychologie; Presses Universitaries De France: Oxford, UK, 1958; p. 222. [Google Scholar]
- Aprile, I.; Falchini, F.; Mili, E.; Mastrorosa, A.; Langone, E.; Mosca, R.; Larocca, S.; Lategana, M.; Aiello, L.; Lorusso, A.; et al. Effects of Social Distancing on Quality of Life and Emotional-Affective Sphere of Caregivers and Older Patients Hospitalized in Rehabilitation Departments during COVID-19 Quarantine: An Observational Study. Diagnostics 2022, 12, 1299. [Google Scholar] [CrossRef]
- Beck, A.T.; Epstein, N.; Brown, G.; Steer, R. Beck anxiety inventory. J. Consult. Clin. Psychol. 1993. [Google Scholar] [CrossRef]
- Beck, A.T.; Steer, R.A.; Brown, G. Beck, A.T.; Steer, R.A.; Brown, G. Beck depression inventory–II. In Psychological assessment; American Psychological Association: Washington, DC, USA, 1996. [Google Scholar]
- Apolone, G.; Mosconi, P. The Italian SF-36 Health Survey: Translation, validation and norming. J. Clin. Epidemiol. 1998, 51, 1025–1036. [Google Scholar] [CrossRef]
- Key, S. Caregiver Burden Scale. Gerontologist 1980, 20, 649–655. [Google Scholar]
- Vellas, B.; Guigoz, Y.; Garry, P.J.; Nourhashemi, F.; Bennahum, D.; Lauque, S.; Albarede, J.-L. The mini nutritional assessment (MNA) and its use in grading the nutritional state of elderly patients. Nutrition 1999, 15, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Karvetti, R.-L. Validity of the 24-h dietary recall. J. Am. Diet. Assoc. 1985, 85, 1437–1442. [Google Scholar] [CrossRef] [PubMed]
- Peretti, A.; Amenta, F.; Tayebati, S.K.; Nittari, G.; Mahdi, S.S. Telerehabilitation: Review of the State-of-the-Art and Areas of Application. JMIR Rehabil. Assist Technol. 2017, 4, e7. [Google Scholar] [CrossRef] [PubMed]
- Haleem, A.; Javaid, M.; Singh, R.P.; Suman, R. Telemedicine for healthcare: Capabilities, features, barriers, and applications. Sens. Int. 2021, 2, 100117. [Google Scholar] [CrossRef]
- Bressi, F.; Campagnola, B.; Cricenti, L.; Santacaterina, F.; Miccinilli, S.; Di Pino, G.; Fiori, F.; D’Alonzo, M.; Di Lazzaro, V.; Ricci, L.; et al. Upper limb home-based robotic rehabilitation in chronic stroke patients: A pilot study. Front. Neurorobot. 2023, 17, 1130770. [Google Scholar] [CrossRef] [PubMed]



| Pubmed | (Rehab*[tiab]) AND (COVID-19[tiab] OR “COVID 19”[tiab] OR “COVID”[tiab] OR long-covid[tiab] OR COVID-2019[tiab] OR SARS-CoV-2[tiab] OR 2019-nCoV[tiab] OR 2019-SARS-CoV-2[tiab]) AND (robot*[tiab] OR “robot assisted”[tiab] OR “robot-assisted”[tiab] OR exoskelet*[tiab] OR “end effector”[tiab] OR end-effector[tiab] OR electromechani*[tiab] OR electro-mechani*[tiab] OR “Virtual Reality” [tiab] OR VR[tiab] OR Kinect[tiab] OR “wii” [tiab] OR technology[tiab] OR “augmented reality” [tiab]) |
| Scopus | TITLE-ABS-KEY (rehab*) AND TITLE-ABS-KEY (COVID-19 OR “COVID 19” OR long-covid OR covid OR COVID-2019 OR SARS-CoV-2 OR 2019-ncov OR 2019-SARS-CoV-2) AND TITLE-ABS-KEY (robot* OR “robot assisted” OR “robot-assisted” OR exoskelet* OR “end effector” OR end-effector OR electromechani* OR electro-mechani* OR “Virtual Reality” OR vr OR kinect OR “wii” OR technology OR “augmented reality”) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aprile, I.; Bramante, L.; La Russa, C.; Germanotta, M.; Barletta, V.T.; Falchini, F.; Brambilla, L.; Guglielmelli, E.; Rossi, P. A Multiaxial Rehabilitation Programme for Workers with COVID-19 Sequelae Using a Conventional and Technological-Robotic Approach: The Proposal of INAIL and Fondazione Don Carlo Gnocchi. Healthcare 2023, 11, 1593. https://doi.org/10.3390/healthcare11111593
Aprile I, Bramante L, La Russa C, Germanotta M, Barletta VT, Falchini F, Brambilla L, Guglielmelli E, Rossi P. A Multiaxial Rehabilitation Programme for Workers with COVID-19 Sequelae Using a Conventional and Technological-Robotic Approach: The Proposal of INAIL and Fondazione Don Carlo Gnocchi. Healthcare. 2023; 11(11):1593. https://doi.org/10.3390/healthcare11111593
Chicago/Turabian StyleAprile, Irene, Lucia Bramante, Chiara La Russa, Marco Germanotta, Valeria Teresa Barletta, Francesca Falchini, Lorenzo Brambilla, Eugenio Guglielmelli, and Patrizio Rossi. 2023. "A Multiaxial Rehabilitation Programme for Workers with COVID-19 Sequelae Using a Conventional and Technological-Robotic Approach: The Proposal of INAIL and Fondazione Don Carlo Gnocchi" Healthcare 11, no. 11: 1593. https://doi.org/10.3390/healthcare11111593
APA StyleAprile, I., Bramante, L., La Russa, C., Germanotta, M., Barletta, V. T., Falchini, F., Brambilla, L., Guglielmelli, E., & Rossi, P. (2023). A Multiaxial Rehabilitation Programme for Workers with COVID-19 Sequelae Using a Conventional and Technological-Robotic Approach: The Proposal of INAIL and Fondazione Don Carlo Gnocchi. Healthcare, 11(11), 1593. https://doi.org/10.3390/healthcare11111593








