Survival and Its Correlates in Multiple Sclerosis Patients under a Universal Health Insurance Program in Taiwan: An 18-Year Nationwide Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources
2.2. Subjects
2.3. Variable Description and Definition
2.4. Analytical Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wallin, M.T.; Culpepper, W.J.; Nichols, E.; Bhutta, Z.A.; Gebrehiwot, T.T.; Hay, S.I.; Khalil, I.A.; Krohn, K.J.; Liang, X.; Naghavi, M. Global, regional, and national burden of multiple sclerosis 1990–2016: A systematic analysis for the global burden of disease study 2016. Lancet Neurol. 2019, 18, 269–285. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Neurological Disorders and Stroke. Multiple Sclerosis Information Page. National Institute of Neurological Disorders and Stroke; 2019. Available online: https://www.ninds.nih.gov/Disorders/All-Disorders/Multiple-Sclerosis-Information-Page#disorders-r1.2022/1/142022 (accessed on 9 April 2023).
- Dyment, D.A.; Ebers, G.C.; Sadovnick, A.D. Genetics of multiple sclerosis. Lancet Neurol. 1981, 3, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Pugliatti, M.; Harbo, H.F.; Holmøy, T.; Kampman, M.T.; Myhr, K.M.; Riise, T.; Wolfson, C. Environmental risk factors in multiple sclerosis. Acta Neurol. Scand. 2008, 117, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Feige, J.; Moser, T.; Bieler, L.; Schwenker, K.; Hauer, L.; Sellner, J. Vitamin d supplementation in multiple sclerosis: A critical analysis of potentials and threats. Nutrients 2020, 12, 783. [Google Scholar] [CrossRef]
- El-Etr, M.; Ghoumari, A.; Sitruk-Ware, R.; Schumacher, M. Hormonal influences in multiple sclerosis: New therapeutic benefits for steroids. Maturitas 2011, 68, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Voskuhl, R.R.; Gold, S.M. Sex-related factors in multiple sclerosis: Genetic, hormonal and environmental contributions. Nat. Rev. Neurol. 2012, 8, 255. [Google Scholar] [CrossRef]
- McAlpine, D.; Compston, A. Mcalpine’s Multiple Sclerosis; Elsevier Health Sciences: Philadelphia, PA, USA, 2005. [Google Scholar]
- Ghezzi, A.; Banwell, B.; Boyko, A.; Amato, M.P.; Anlar, B.; Blinkenberg, M.; Boon, M.; Filippi, M.; Jozwiak, S.; Ketelslegers, I. Meeting review: The management of multiple sclerosis in children: A european view. Mult. Scler. J. 2010, 16, 1258–1267. [Google Scholar] [CrossRef]
- Miller, D.H.; Leary, S.M. Primary-progressive multiple sclerosis. Lancet Neurol. 2007, 6, 903–912. [Google Scholar] [CrossRef]
- Lunde, H.M.B.; Assmus, J.; Myhr, K.-M.; Bø, L.; Grytten, N. Survival and cause of death in multiple sclerosis: A 60-year longitudinal population study. J. Neurol. Neurosurg. Psychiatry 2017, 88, 621–625. [Google Scholar] [CrossRef]
- Kingwell, E.; van der Kop, M.; Zhao, Y.; Shirani, A.; Zhu, F.; Oger, J.; Tremlett, H. Relative mortality and survival in multiple sclerosis: Findings from british columbia, canada. J. Neurol. Neurosurg. Psychiatry 2011, 83, 61–66. [Google Scholar] [CrossRef]
- Brønnum-Hansen, H.; Koch-Henriksen, N.; Stenager, E. Trends in survival and cause of death in danish patients with multiple sclerosis. Brain 2004, 127, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Phadke, J.G. Survival pattern and cause of death in patients with multiple sclerosis: Results from an epidemiological survey in north east scotland. J. Neurol. Neurosurg. Psychiatry 1987, 50, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Kingwell, E.; Leray, E.; Zhu, F.; Petkau, J.; Edan, G.; Oger, J.; Tremlett, H. Multiple sclerosis: Effect of beta interferon treatment on survival. Brain 2019, 142, 1324–1333. [Google Scholar] [CrossRef]
- Barten, L.J.; Allington, D.R.; Procacci, K.A.; Rivey, M.P. New approaches in the management of multiple sclerosis. Drug Des. Dev. Ther. 2010, 4, 343–366. [Google Scholar]
- Song, L.; Zhou, Q.-H.; Wang, H.-L.; Liao, F.-J.; Hua, L.; Zhang, H.-F.; Huang, L.-B.; Lin, Y.; Zheng, G.-Q. Chinese herbal medicine adjunct therapy in patients with acute relapse of multiple sclerosis: A systematic review and meta-analysis. Compl. Med. 2017, 31, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-P.; Lee, C.T.-C. Impact of disease-modifying therapies on the survival of patients with multiple sclerosis in taiwan, 1997–2008. Clin. Drug Investig. 2013, 33, 647–652. [Google Scholar] [CrossRef]
- Lai, C.-H.; Tseng, H.-F. Population-based epidemiological study of neurological diseases in taiwan: I. Creutzfeldt-jakob disease and multiple sclerosis. Neuroepidemiology 2009, 33, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.-W.; Wang, H.-P.; Chen, H.-M.; Lin, J.-W.; Lin, W.-S. Epidemiology and comorbidities of adult multiple sclerosis and neuromyelitis optica in taiwan, 2001–2015. Mult. Scler. Relat. Disord. 2020, 45, 102425. [Google Scholar] [CrossRef]
- Hsu, C.-Y.; Ro, L.-S.; Chen, L.-J.; Chang, C.-W.; Chang, K.-H.; Wu, I.-H.; Lin, A.; Hsiao, F.-Y. Epidemiology, treatment patterns and healthcare utilizations in multiple sclerosis in taiwan. Sci. Rep. 2021, 11, 7727. [Google Scholar] [CrossRef]
- Liao, C.-M.; Kuo, W.-Y.; Kung, P.-T.; Chuan, C.-H.; Tsai, W.-C. Epidemiological investigation of multiple sclerosis and related medical utilisation in taiwan. Mult. Scler. J. 2022, 28, 1198–1208. [Google Scholar] [CrossRef]
- Health Promotion Administration. National Health Insurance Act. Ministry of Health and Welfare. ROC (Taiwan): Ministry of Health and Welfare; 2015. Available online: https://law.moj.gov.tw/ENG/LawClass/LawAll.aspx?pcode=L0060001 (accessed on 10 January 2022).
- Ministry of Health and Welfare. The National Health Insurance Statistics, 2016. Ministry of Health and Welfare; 2016. Available online: https://www.nhi.gov.tw/english/Content_List.aspx?n=17BC32CF4F3F289E&topn=616B97F8DF2C3614 (accessed on 14 January 2022).
- Huang, S.-K. 2015–2016 National Health Insurance Annual Report; National Health Insurance Administration, Ministry of Health: Taiwan, China, December 2015.
- Health Promotion Administration. Prevention of Rare Diseases and Orphan Drug Act. Health Promotion Administration Ministry of Health and Welfare; 2015. Available online: https://www.hpa.gov.tw/EngPages/Detail.aspx?nodeid=1058&pid=6031 (accessed on 14 January 2022).
- Sharma, A.; Jacob, A.; Tandon, M.; Kumar, D. Orphan drug: Development trends and strategies. J. Pharm. Bioallied Sci. 2010, 2, 290–299. Available online: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2996062/ (accessed on 9 April 2023). [CrossRef] [PubMed]
- Hsu, J.C.; Wu, H.-C.; Feng, W.-C.; Chou, C.-H.; Lai, E.C.-C.; Lu, C.Y. Disease and economic burden for rare diseases in taiwan: A longitudinal study using taiwan’s national health insurance research database. PLoS ONE 2018, 13, e0204206. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Atlas: Multiple Sclerosis Resources in the World 2008; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- Lin, J.-D.; Lin, L.-P.; Hung, W.-J. Reported numbers of patients with rare diseases based on ten-year longitudinal national disability registries in taiwan. Res. Dev. Disabil. 2013, 34, 133–138. [Google Scholar] [CrossRef]
- National Health Insurance Administration. Scope of Catastrophically Ill of National Health Insurance. M.o.H. National Health Insurance Administration, Taiwan. ROC (Taiwan): National Health Insurance Administration, Ministry of Health; 2019. Available online: https://www.nhi.gov.tw/Resource/webdata/1059_2_10000406%E9%87%8D%E5%A4%A7%E5%82%B7%E7%97%85%E7%AF%84%E5%9C%8D%E8%A1%A8-%E7%BD%AE%E7%B6%B2%E7%AB%99.pdf (accessed on 14 January 2022).
- Duignan, S.; Brownlee, W.; Wassmer, E.; Hemingway, C.; Lim, M.; Ciccarelli, O.; Hacohen, Y. Paediatric multiple sclerosis: A new era in diagnosis and treatment. Dev. Med. Child Neurol. 2019, 61, 1039–1049. [Google Scholar] [CrossRef]
- Liu, C.-Y.; Hung, Y.-T.; Chuang, Y.-L.; Chen, Y.-J.; Weng, W.-S.; Liu, J.-S.; Liang, K. Incorporating development stratification of taiwan townships into sampling design of large scale health interview survey. J. Health Manag. 2006, 4, 1–22. [Google Scholar]
- Deyo, R.A.; Cherkin, D.C.; Ciol, M.A. Adapting a clinical comorbidity index for use with icd-9-cm administrative databases. J. Clin. Epidemiol. 1992, 45, 613–619. [Google Scholar] [CrossRef]
- Shrive, F.M.; Stuart, H.; Quan, H.; Ghali, W.A. Dealing with missing data in a multi-question depression scale: A comparison of imputation methods. BMC Med. Res. Methodol. 2006, 6, 57. [Google Scholar] [CrossRef] [PubMed]
- Nakai, M.; Chen, D.-G.; Nishimura, K.; Miyamoto, Y. Comparative study of four methods in missing value imputations under missing completely at random mechanism. Open J. Stat. 2014, 4, 42574. [Google Scholar] [CrossRef]
- Food and Drug Administration. 2017 Rare Disease Prevention and Treatment and Medication Act Drug Annual Report. Food and Drug Administration Ministry of Health and Welfare; 2017. Available online: https://www.hpa.gov.tw/EngPages/EngTopicList.aspx?nodeid=1072 (accessed on 9 April 2023).
- Hsieh, C.-Y.; Su, C.-C.; Shao, S.-C.; Sung, S.-F.; Lin, S.-J.; Yang, Y.-H.K.; Lai, E.C.-C. Taiwan’s national health insurance research database: Past and future. Clin. Epidemiol. 2019, 11, 349. [Google Scholar] [CrossRef]
- Lin, L.-Y.; Warren-Gash, C.; Smeeth, L.; Chen, P.-C. Data resource profile: The national health insurance research database (nhird). Epidemiol. Health 2018, 40, e2018062. [Google Scholar] [CrossRef]
- Smestad, C.; Sandvik, L.; Celius, E. Excess mortality and cause of death in a cohort of norwegian multiple sclerosis patients. Mult. Scler. J. 2009, 15, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Koch-Henriksen, N.; Brønnum-Hansen, H.; Stenager, E. Underlying cause of death in danish patients with multiple sclerosis: Results from the danish multiple sclerosis registry. J. Neurol. Neurosurg. Psychiatry 1998, 65, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Hirst, C.; Swingler, R.; Compston, D.; Ben-Shlomo, Y.; Robertson, N.P. Survival and cause of death in multiple sclerosis: A prospective population-based study. J. Neurol. Neurosurg. Psychiatry 2008, 79, 1016–1021. [Google Scholar] [CrossRef] [PubMed]
- Koriem, K.M.M. Corrigendum to ‘multiple sclerosis: New insights and trends’. Asian Pac. J. Trop. Biomed. 2017, 7, 493–504. [Google Scholar] [CrossRef]
- Liang, Y.-W.; Chen, W.-Y.; Lee, J.-L.; Huang, L.-C. Nurse staffing, direct nursing care hours and patient mortality in taiwan: The longitudinal analysis of hospital nurse staffing and patient outcome study. BMC Health Serv. Res. 2012, 12, 44. [Google Scholar] [CrossRef] [PubMed]
- Kronek, L.-P.; Reddy, A. Logical analysis of survival data: Prognostic survival models by detecting high-degree interactions in right-censored data. Bioinformatics 2008, 24, i248–i253. [Google Scholar] [CrossRef]
- Gerds, T.A.; Schumacher, M. Consistent estimation of the expected brier score in general survival models with right-censored event times. Biom. J. 2006, 48, 1029–1040. [Google Scholar] [CrossRef]
- Rosenbaum, P.R.; Rubin, D.B. The central role of the propensity score in observational studies for causal effects. Biometrika 1983, 70, 41–55. [Google Scholar] [CrossRef]
| Total | 2001~2005 a | 2006~2010 a | 2011~2015 a | p Value b | |||||
|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | ||
| All subjects | 1444 | 100.00 | 488 | 33.80 | 508 | 35.18 | 448 | 31.02 | |
| Survival status c | <0.001 | ||||||||
| censor | 1254 | 86.84 | 372 | 76.23 | 450 | 88.58 | 432 | 96.43 | |
| death | 190 | 13.16 | 116 | 23.77 | 58 | 11.42 | 16 | 3.57 | |
| Sex | 0.00 | 0.00 | 0.00 | 0.00 | 0.845 | ||||
| Female | 1113 | 77.08 | 372 | 76.23 | 395 | 77.76 | 346 | 77.23 | |
| Male | 331 | 22.92 | 116 | 23.77 | 113 | 22.24 | 102 | 22.77 | |
| Age at diagnosis d | 0.263 | ||||||||
| 15–24 | 298 | 20.64 | 87 | 17.83 | 104 | 20.47 | 107 | 23.88 | |
| 25–34 | 424 | 29.36 | 142 | 29.10 | 140 | 27.56 | 142 | 31.70 | |
| 35–44 | 323 | 22.37 | 116 | 23.77 | 115 | 22.64 | 92 | 20.54 | |
| 45–54 | 236 | 16.34 | 86 | 17.62 | 91 | 17.91 | 59 | 13.17 | |
| 55–64 | 115 | 7.96 | 38 | 7.79 | 40 | 7.87 | 37 | 8.26 | |
| ≥65 | 48 | 3.32 | 19 | 3.89 | 18 | 3.54 | 11 | 2.46 | |
| Marital status d | 0.001 | ||||||||
| Unmarried | 633 | 43.84 | 191 | 39.14 | 210 | 41.42 | 232 | 51.67 | |
| Married | 691 | 47.85 | 260 | 53.28 | 249 | 49.11 | 182 | 40.53 | |
| Other | 117 | 8.10 | 36 | 7.38 | 48 | 9.47 | 33 | 7.35 | |
| Unknown | 3 | 0.21 | 1 | 0.20 | 0 | 0.00 | 2 | 0.45 | |
| Education level d | <0.001 | ||||||||
| Elementary school and under | 185 | 12.81 | 100 | 20.41 | 55 | 11.14 | 30 | 6.52 | |
| Junior high school | 256 | 17.73 | 91 | 18.57 | 90 | 18.22 | 75 | 16.30 | |
| Senior high/vocational school | 604 | 41.83 | 202 | 41.22 | 220 | 44.53 | 182 | 39.57 | |
| Junior college/university and above | 363 | 25.14 | 85 | 17.35 | 120 | 24.29 | 158 | 34.35 | |
| Unknown | 36 | 2.49 | 12 | 2.45 | 9 | 1.82 | 15 | 3.26 | |
| Monthly salary (TWD) d,e | 0.014 | ||||||||
| ≤22,800 | 843 | 58.38 | 304 | 62.30 | 303 | 59.65 | 236 | 52.68 | |
| 22,801–45,800 | 439 | 30.40 | 143 | 29.30 | 143 | 28.15 | 153 | 34.15 | |
| ≥45,801 | 162 | 11.22 | 41 | 8.40 | 62 | 12.20 | 59 | 13.17 | |
| Urbanization level of residence area d | 0.932 | ||||||||
| Level 1 | 455 | 31.51 | 156 | 32.16 | 154 | 30.26 | 145 | 32.23 | |
| Level 2 | 503 | 34.83 | 158 | 32.58 | 195 | 38.30 | 150 | 33.34 | |
| Level 3 | 219 | 15.17 | 74 | 15.26 | 76 | 14.93 | 69 | 15.33 | |
| Level 4 | 170 | 11.77 | 60 | 12.37 | 55 | 10.81 | 55 | 12.22 | |
| Level 5 | 14 | 0.97 | 5 | 1.03 | 4 | 0.79 | 5 | 1.11 | |
| Level 6 | 41 | 2.84 | 15 | 3.09 | 13 | 2.55 | 13 | 2.89 | |
| Level 7 | 38 | 2.63 | 16 | 3.30 | 11 | 2.16 | 11 | 2.44 | |
| Unknown | 4 | 0.28 | 1 | 0.21 | 1 | 0.20 | 2 | 0.44 | |
| CCI d,f | <0.001 | ||||||||
| 0 | 920 | 63.71 | 287 | 58.81 | 329 | 64.76 | 304 | 67.86 | |
| 1 | 265 | 18.35 | 80 | 16.39 | 99 | 19.49 | 86 | 19.20 | |
| ≥2 | 259 | 17.94 | 121 | 24.80 | 80 | 15.75 | 58 | 12.95 | |
| Other catastrophic illnesses d,g | <0.001 | ||||||||
| No | 1192 | 82.55 | 368 | 75.41 | 430 | 84.65 | 394 | 87.95 | |
| Yes | 252 | 17.45 | 120 | 24.59 | 78 | 15.35 | 54 | 12.05 | |
| DMTs h | 0.858 | ||||||||
| No | 659 | 45.64 | 293 | 60.04 | 197 | 38.78 | 169 | 37.72 | |
| Yes | 785 | 54.36 | 195 | 39.96 | 311 | 61.22 | 279 | 62.28 | |
| Chinese medicine treatment | 0.927 | ||||||||
| No | 989 | 68.49 | 337 | 69.06 | 345 | 67.91 | 307 | 68.53 | |
| Yes | 455 | 31.51 | 151 | 30.94 | 163 | 32.09 | 141 | 31.47 | |
| Physiotherapy treatment | 0.363 | ||||||||
| No | 1379 | 95.50 | 470 | 96.31 | 480 | 94.49 | 429 | 95.76 | |
| Yes | 65 | 4.50 | 18 | 3.69 | 28 | 5.51 | 19 | 4.24 | |
| Ownership of hospital d | 0.027 | ||||||||
| Public | 855 | 59.21 | 311 | 63.99 | 294 | 57.76 | 250 | 55.67 | |
| Private | 585 | 40.51 | 174 | 35.80 | 214 | 42.04 | 197 | 43.88 | |
| Unknown | 4 | 0.28 | 1 | 0.21 | 1 | 0.20 | 2 | 0.45 | |
| Hospital level d | 0.328 | ||||||||
| Medical center | 1023 | 70.84 | 344 | 70.78 | 373 | 73.28 | 306 | 68.15 | |
| Regional hospital | 351 | 24.31 | 114 | 23.46 | 116 | 22.79 | 121 | 26.95 | |
| District hospital | 66 | 4.57 | 27 | 5.55 | 19 | 3.73 | 20 | 4.45 | |
| Unknown | 4 | 0.28 | 1 | 0.21 | 1 | 0.20 | 2 | 0.45 | |
| Pharmaceutical Ingredients | ATC a | 2005 | 2010 | 2015 | |||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Total frequency b | 210 | 100.00 | 331 | 100.00 | 341 | 100.00 | |
| Interferon beta-1a | L03AB07 | 145 | 69.05 | 199 | 60.12 | 221 | 64.81 |
| Fingolimod | L04AA27 | 0 | 0 | 5 | 1.51 | 56 | 16.42 |
| Interferon beta-1b | L03AB08 | 52 | 24.76 | 90 | 27.19 | 38 | 11.14 |
| Glatiramer acetate | L03AX13 | 13 | 6.19 | 35 | 10.57 | 25 | 7.33 |
| Natalizumab | L04AA23 | 0 | 0 | 0 | 0 | 1 | 0.29 |
| Mitoxantrone | L01DB07 | 0 | 0 | 2 | 0.60 | 0 | 0 |
| Causes of Death/Disease Category/Disease | People | % |
|---|---|---|
| Total of people | 190 | 100.00% |
| Non-disease deaths | 6 | 3.16% |
| Accidental deaths | 3 | 1.58% |
| Suicides | 3 | 1.58% |
| Disease deaths | 184 | 96.84% |
| Diseases of the nervous system | ||
| Multiple sclerosis | 83 | 43.68% |
| Intracranial and intraspinal phlebitis and thrombophlebitis | 4 | 2.11% |
| Diseases of the respiratory system | ||
| Pneumonia, unspecified organism | 11 | 5.79% |
| Certain infectious and parasitic diseases | ||
| Sepsis, unspecified | 6 | 3.16% |
| Neoplasms | ||
| Malignant neoplasm of unspecified site of unspecified female breast | 4 | 2.11% |
| Malignant neoplasm of bronchus or lung, unspecified, unspecified side | 2 | 1.05% |
| Symptoms, signs and abnormal clinical and laboratory findings, not elsewhere classified | ||
| Respiratory arrest | 3 | 1.58% |
| Other specified symptoms and signs involving the circulatory and respiratory systems | 2 | 1.05% |
| Diseases of the circulatory system | ||
| Atherosclerotic heart disease of native coronary artery without angina pectoris | 3 | 1.58% |
| Diseases of the genitourinary system | ||
| Urinary tract infection, site not specified | 3 | 1.58% |
| Diseases of the musculoskeletal system and connective tissue | ||
| Systemic lupus erythematosus, organ or system involvement unspecified | 3 | 1.58% |
| Endocrine, nutritional and metabolic diseases | ||
| Type 2 diabetes mellitus with other diabetic neurological complication | 3 | 1.58% |
| Other (57 disease) | 57 | 29.99% |
| Bivariate Analysis | Survival Analysis | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Censor a | Death a | Unadjusted | Adjusted | |||||||||
| N | % | N | % | N | % | p Value b | HR | p-Value | HR | 95% CI | p-Value c | ||
| All subjects | 1444 | 100.00 | 1254 | 86.84 | 190 | 15.15 | |||||||
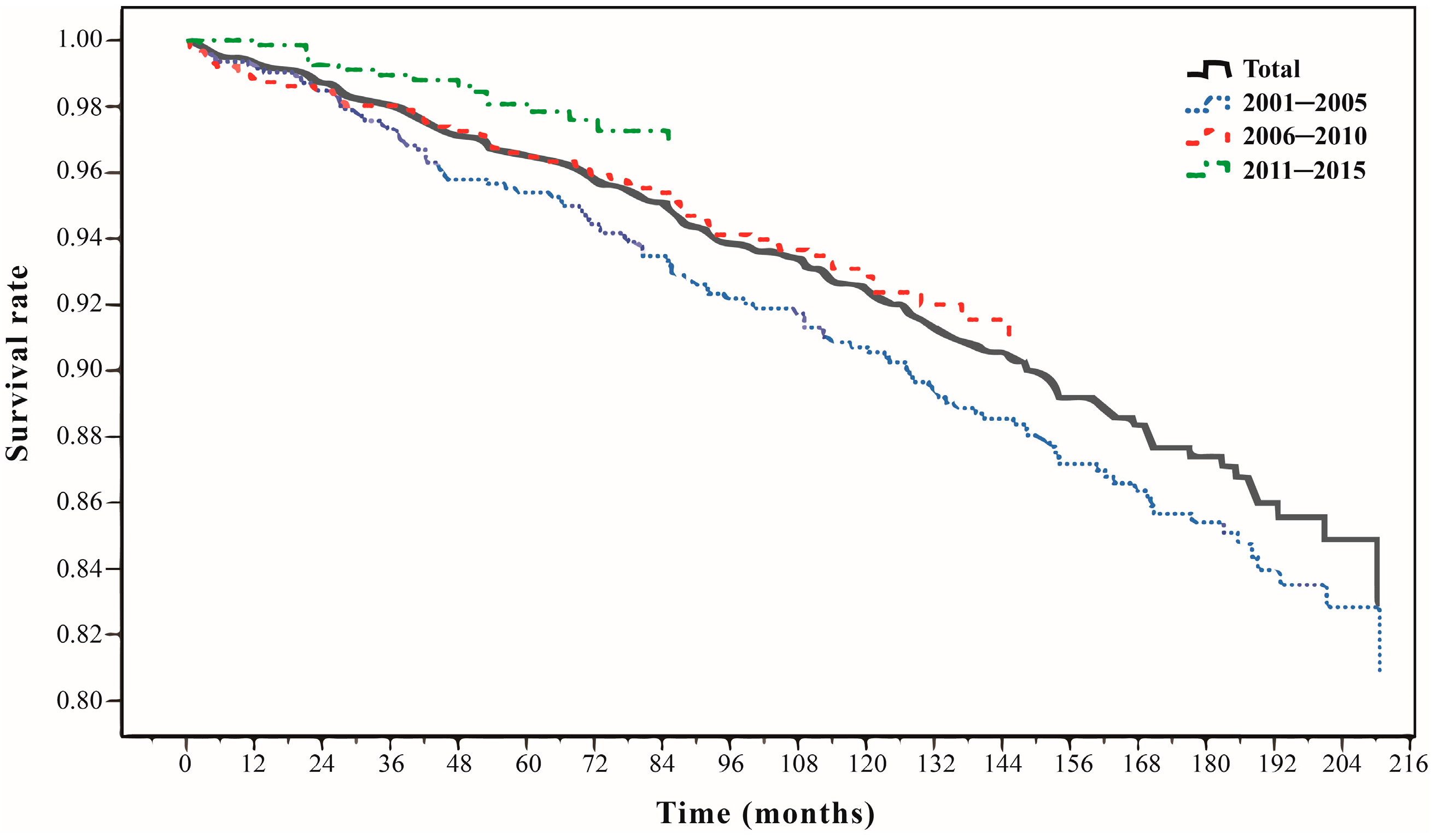
| Time periods d | <0.001 | ||||||||||||
| 2001~2005 (ref) | 488 | 33.80 | 372 | 76.23 | 116 | 23.77 | 1.00 | - | 1.00 | - | - | - | |
| 2006~2010 | 508 | 35.18 | 450 | 88.58 | 58 | 11.42 | 0.67 | 0.018 | 0.75 | 0.53 | 1.07 | 0.113 | |
| 2011~2015 | 448 | 31.02 | 432 | 96.43 | 16 | 3.57 | 0.37 | <0.001 | 0.43 | 0.24 | 0.76 | 0.004 | |
| Sex | 0.585 | ||||||||||||
| Female (ref) | 1113 | 77.08 | 956 | 76.20 | 144 | 75.80 | 1.00 | - | 1.00 | - | - | - | |
| Male | 331 | 22.92 | 298 | 23.80 | 46 | 24.20 | 1.10 | 0.585 | 1.36 | 0.95 | 1.94 | 0.089 | |
| Age at diagnosis e | <0.001 | ||||||||||||
| 15~24 (ref) | 298 | 20.64 | 287 | 96.31 | 11 | 3.69 | 1.00 | - | 1.00 | - | - | - | |
| 25~34 | 424 | 29.36 | 398 | 93.87 | 26 | 6.13 | 1.64 | 0.169 | 1.39 | 0.65 | 2.97 | 0.396 | |
| 35~44 | 323 | 22.37 | 281 | 87.00 | 42 | 13.00 | 3.47 | <0.001 | 2.52 | 1.15 | 5.51 | 0.021 | |
| 45~54 | 236 | 16.34 | 192 | 81.36 | 44 | 18.64 | 5.11 | <0.001 | 3.31 | 1.49 | 7.34 | 0.003 | |
| 55~64 | 115 | 7.96 | 74 | 64.35 | 41 | 35.65 | 11.85 | <0.001 | 7.26 | 3.18 | 16.55 | <0.001 | |
| ≥65 | 48 | 3.32 | 22 | 45.83 | 26 | 54.17 | 21.06 | <0.001 | 14.21 | 5.63 | 35.85 | <0.001 | |
| Marital status e | <0.001 | ||||||||||||
| Unmarried (ref) | 633 | 43.84 | 599 | 94.63 | 34 | 5.37 | 1.00 | - | 1.00 | - | - | - | |
| Married | 691 | 47.85 | 560 | 81.04 | 131 | 18.96 | 3.43 | <0.001 | 1.15 | 0.70 | 1.87 | 0.587 | |
| Other | 117 | 8.10 | 93 | 79.49 | 24 | 20.51 | 3.89 | <0.001 | 0.98 | 0.51 | 1.87 | 0.940 | |
| Unknown | 3 | 0.21 | 3 | 100.00 | 0 | 0.00 | |||||||
| Education level e | <0.001 | ||||||||||||
| Elementary school and under (ref) | 185 | 12.81 | 122 | 65.95 | 63 | 34.05 | 1.00 | - | 1.00 | - | - | - | |
| Junior high school | 256 | 17.73 | 210 | 82.03 | 46 | 17.97 | 0.54 | 0.002 | 1.24 | 0.79 | 1.94 | 0.347 | |
| Senior high/vocational school | 604 | 41.83 | 547 | 90.56 | 57 | 9.44 | 0.27 | <0.001 | 0.71 | 0.45 | 1.11 | 0.133 | |
| Junior college/university and above | 363 | 25.14 | 341 | 93.94 | 22 | 6.06 | 0.19 | <0.001 | 0.73 | 0.40 | 1.33 | 0.305 | |
| Unknown | 36 | 2.49 | 32 | 88.89 | 4 | 11.11 | |||||||
| Monthly salary (TWD) e,f | 0.004 | ||||||||||||
| ≤22,800 (ref) | 843 | 58.38 | 709 | 84.10 | 134 | 15.90 | 1.00 | - | 1.00 | - | - | - | |
| 22,801~45,800 | 439 | 30.40 | 394 | 89.75 | 45 | 10.25 | 0.65 | 0.011 | 0.76 | 0.52 | 1.09 | 0.131 | |
| ≥45,801 | 162 | 11.22 | 151 | 93.21 | 11 | 6.79 | 0.47 | 0.015 | 0.54 | 0.28 | 1.04 | 0.064 | |
| Urbanization level of residence area e | 0.022 | ||||||||||||
| Level 1 (ref) | 455 | 31.51 | 408 | 89.67 | 47 | 10.33 | 1.00 | - | 1.00 | - | - | - | |
| Level 2 | 503 | 34.83 | 432 | 85.88 | 71 | 14.12 | 1.43 | 0.056 | 1.40 | 0.96 | 2.06 | 0.083 | |
| Level 3 | 219 | 15.17 | 195 | 89.04 | 24 | 10.96 | 1.04 | 0.889 | 1.03 | 0.62 | 1.73 | 0.904 | |
| Level 4 | 170 | 11.77 | 138 | 81.18 | 32 | 18.82 | 1.94 | 0.004 | 1.36 | 0.84 | 2.21 | 0.213 | |
| Level 5 | 14 | 0.97 | 10 | 71.43 | 4 | 28.57 | 3.35 | 0.020 | 1.16 | 0.36 | 3.74 | 0.804 | |
| Level 6 | 41 | 2.84 | 37 | 90.24 | 4 | 9.76 | 0.95 | 0.922 | 0.66 | 0.23 | 1.91 | 0.442 | |
| Level 7 | 38 | 2.63 | 32 | 84.21 | 6 | 15.79 | 1.49 | 0.355 | 1.08 | 0.44 | 2.66 | 0.873 | |
| Unknown | 4 | 0.28 | 4 | 100.00 | 0 | 0.00 | |||||||
| CCI e,g | <0.001 | ||||||||||||
| 0 (ref) | 920 | 63.71 | 837 | 90.98 | 83 | 9.02 | 1.00 | - | 1.00 | - | - | - | |
| 1 | 265 | 18.35 | 227 | 85.66 | 38 | 14.34 | 1.70 | 0.007 | 1.04 | 0.68 | 1.57 | 0.869 | |
| ≥2 | 259 | 17.94 | 190 | 73.36 | 69 | 26.64 | 3.00 | <0.001 | 1.16 | 0.80 | 1.68 | 0.441 | |
| Other catastrophic illnesses e,h | <0.001 | ||||||||||||
| No (ref) | 1192 | 82.55 | 1083 | 90.86 | 109 | 9.14 | 1.00 | - | 1.00 | - | - | - | |
| Yes | 252 | 17.45 | 171 | 67.86 | 81 | 32.14 | 3.39 | <0.001 | 2.14 | 1.57 | 2.93 | <.0001 | |
| DMTs i | 0.112 | ||||||||||||
| No (ref) | 659 | 45.64 | 555 | 84.22 | 104 | 15.78 | 1.00 | - | 1.00 | - | - | - | |
| Yes | 785 | 54.36 | 699 | 89.04 | 86 | 10.96 | 0.79 | 0.114 | 1.17 | 0.85 | 1.60 | 0.335 | |
| Chinese medicine treatment | 0.278 | ||||||||||||
| No (ref) | 989 | 68.49 | 853 | 86.25 | 136 | 13.75 | 1.00 | - | 1.00 | - | - | - | |
| Yes | 455 | 31.51 | 401 | 88.13 | 54 | 11.87 | 0.84 | 0.279 | 0.85 | 0.60 | 1.18 | 0.327 | |
| Physiotherapy treatment | 0.003 | ||||||||||||
| No (ref) | 1379 | 95.50 | 1205 | 87.38 | 174 | 12.62 | 1.00 | - | 1.00 | - | - | - | |
| Yes | 65 | 4.50 | 49 | 75.38 | 16 | 24.62 | 2.13 | 0.004 | 1.16 | 0.68 | 2.00 | 0.581 | |
| Ownership of hospital e | 0.042 | ||||||||||||
| Public (ref) | 855 | 59.21 | 729 | 85.26 | 126 | 14.74 | 1.00 | - | 1.00 | - | - | - | |
| Private | 585 | 40.51 | 524 | 89.57 | 61 | 10.43 | 0.730 | 0.043 | 0.910 | 0.65 | 1.26 | 0.556 | 0.730 |
| Unknown | 4 | 0.28 | 4 | 100.00 | 0 | 0.00 | |||||||
| Hospital level e | <0.001 | ||||||||||||
| Medical center (ref) | 1023 | 70.84 | 906 | 88.56 | 117 | 11.44 | 1.00 | - | 1.00 | - | - | - | |
| Regional hospital | 351 | 24.31 | 299 | 85.19 | 52 | 14.81 | 1.38 | 0.052 | 1.09 | 0.76 | 1.58 | 0.634 | |
| District hospital | 66 | 4.57 | 48 | 72.73 | 18 | 27.27 | 2.66 | <0.001 | 2.31 | 1.36 | 3.91 | 0.002 | |
| Unknown | 4 | 0.28 | 4 | 100.00 | 0 | 0.00 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, C.-M.; Chen, C.-Y.; Kung, P.-T.; Kuo, W.-Y.; Chuang, H.-C.; Tsai, W.-C. Survival and Its Correlates in Multiple Sclerosis Patients under a Universal Health Insurance Program in Taiwan: An 18-Year Nationwide Cohort Study. Healthcare 2023, 11, 1551. https://doi.org/10.3390/healthcare11111551
Liao C-M, Chen C-Y, Kung P-T, Kuo W-Y, Chuang H-C, Tsai W-C. Survival and Its Correlates in Multiple Sclerosis Patients under a Universal Health Insurance Program in Taiwan: An 18-Year Nationwide Cohort Study. Healthcare. 2023; 11(11):1551. https://doi.org/10.3390/healthcare11111551
Chicago/Turabian StyleLiao, Chun-Ming, Chia-Yu Chen, Pei-Tseng Kung, Wei-Yin Kuo, Hui-Chuan Chuang, and Wen-Chen Tsai. 2023. "Survival and Its Correlates in Multiple Sclerosis Patients under a Universal Health Insurance Program in Taiwan: An 18-Year Nationwide Cohort Study" Healthcare 11, no. 11: 1551. https://doi.org/10.3390/healthcare11111551
APA StyleLiao, C.-M., Chen, C.-Y., Kung, P.-T., Kuo, W.-Y., Chuang, H.-C., & Tsai, W.-C. (2023). Survival and Its Correlates in Multiple Sclerosis Patients under a Universal Health Insurance Program in Taiwan: An 18-Year Nationwide Cohort Study. Healthcare, 11(11), 1551. https://doi.org/10.3390/healthcare11111551






