Functional MRI in Radiology—A Personal Review
Abstract
:1. Introduction
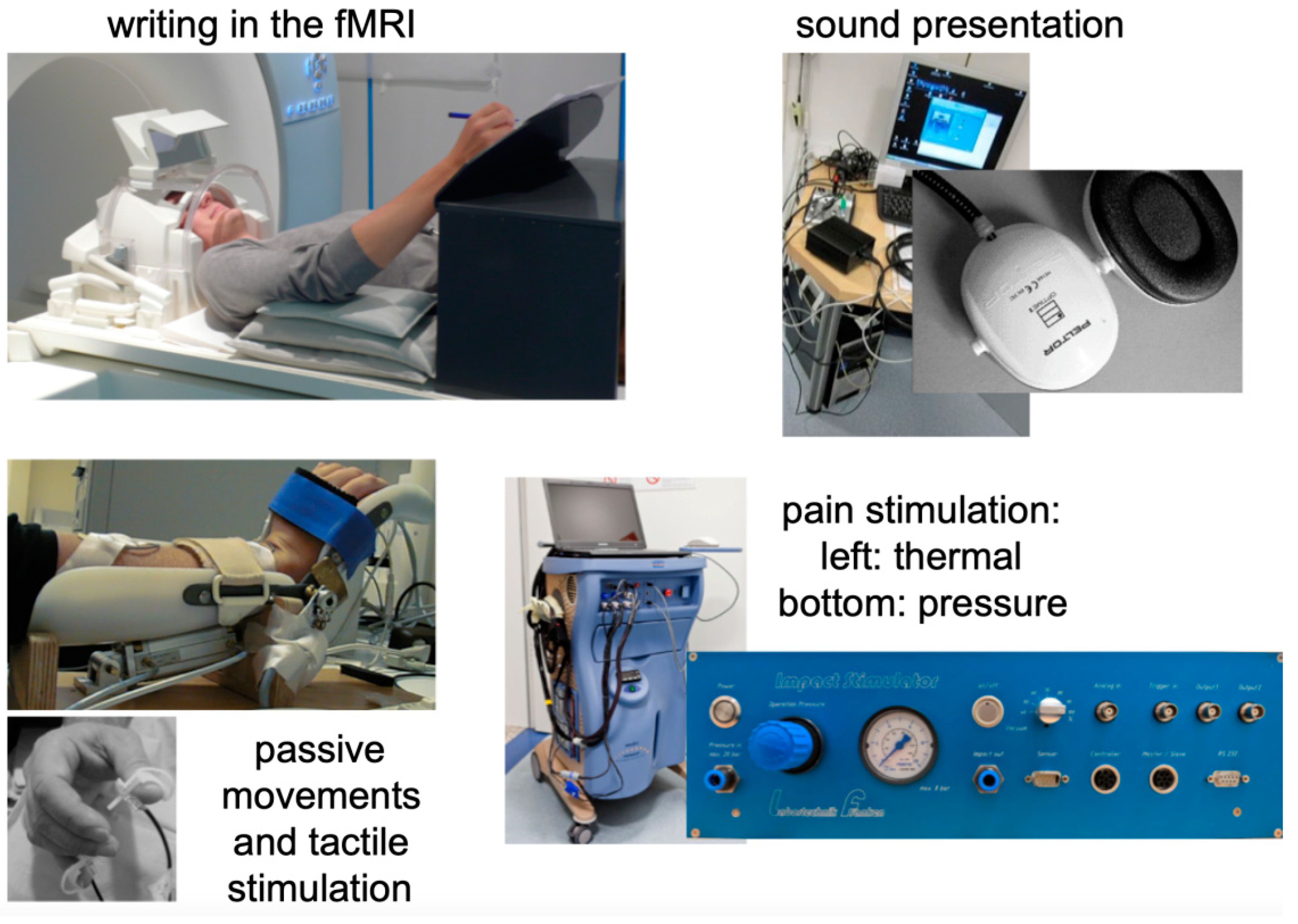
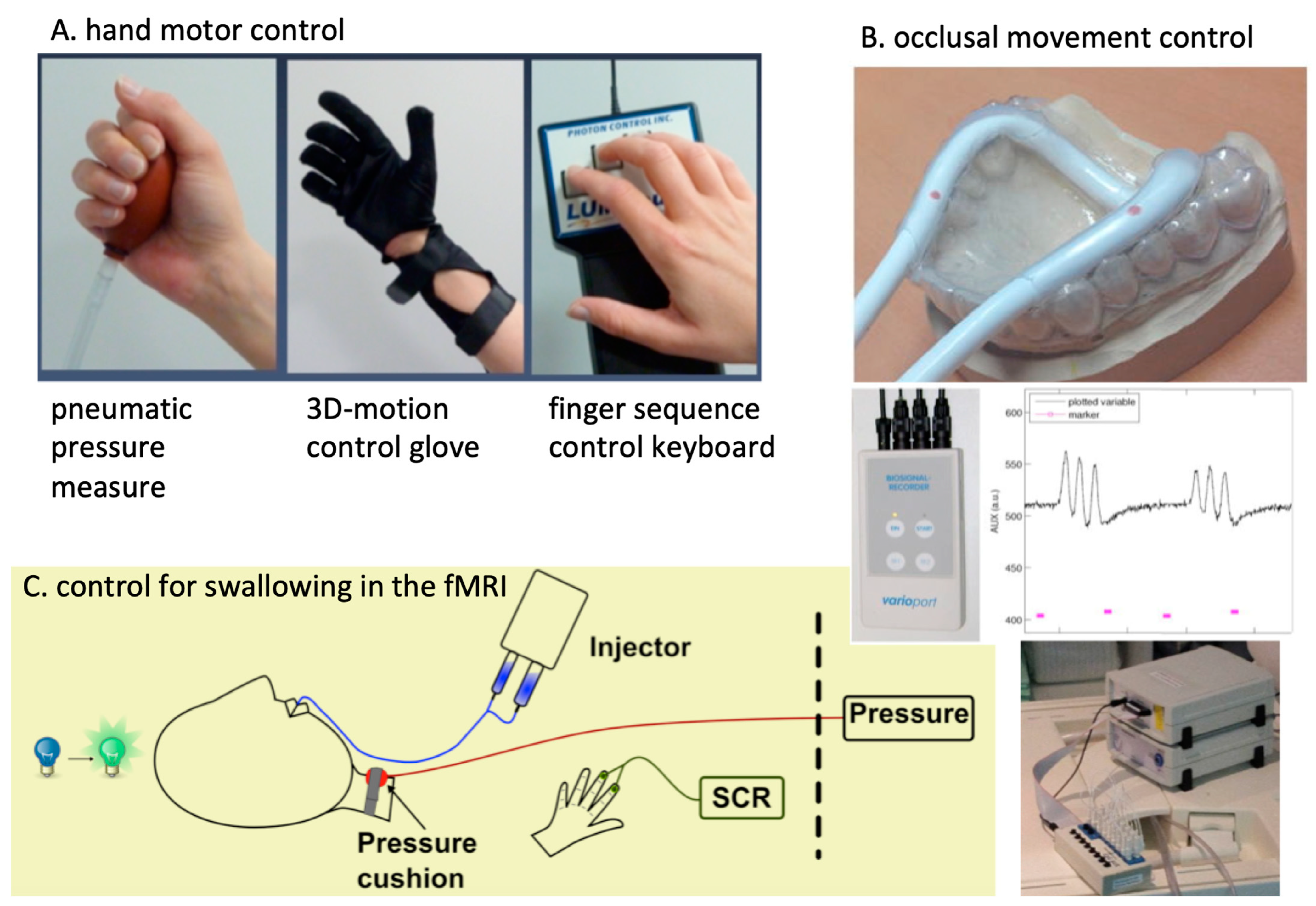
2. Methods and Material
Development of the fMRI Method
3. Results
3.1. fMRI in Tübingen (Personal View from 1996–2006)
3.2. Building up Research and Funding in the Greifswald fMRI Unit
3.3. Neurorehabilitation Studies
3.4. Cooperation Studies with Local Medical Partners
3.5. Cooperation with Psychology on fMRI in Emotional Processing
3.6. Pain Research
3.7. Methodological Developments
3.8. Funding Landscape and National and International Cooperation
4. Discussion
4.1. A Trend from Functional to Structural Big Data Evaluation
4.2. Functional MRI in Radiology—Pros and Cons
4.3. Limitations
4.4. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DARTEL | diffeomorphic anatomical registration using exponentiated Lie algebra |
| DTI | diffusion tensor imaging |
| FLASH | fast low-angle-shot |
| fMRI | functional magnetic resonance imaging |
| GMI | graded motor imagery |
| MEG | magnetoencephalography |
| MPRage | magnetization-prepared rapid gradient-echo imaging |
| OHBM | organization for human brain mapping |
| PET | positron emission tomography |
| rs fMRI | resting-state fMRI |
| SPM | statistical parametric mapping |
| TMD | temporomandibular disorder |
| TMS | transcranial magnetic stimulation |
References
- Bandettini, P.A. Twenty Years of Functional MRI: The Science and the Stories. NeuroImage 2012, 62, 575–588. [Google Scholar] [CrossRef] [PubMed]
- Hennig, J. Functional Spectroscopy to No-Gradient FMRI. NeuroImage 2012, 62, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Grodd, W.; Hülsmann, E.; Lotze, M.; Wildgruber, D.; Erb, M. Sensorimotor Mapping of the Human Cerebellum: FMRI Evidence of Somatotopic Organization: Sensorimotor Mapping of the Cerebellum. Hum. Brain Mapp. 2001, 13, 55–73. [Google Scholar] [CrossRef]
- Frahm, J.; Bruhn, H.; Merboldt, K.-D.; Hänicke, W. Dynamic MR Imaging of Human Brain Oxygenation during Rest and Photic Stimulation. J. Magn. Reson. Imaging 1992, 2, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S.; Lee, T.-M.; Nayak, A.S.; Glynn, P. Oxygenation-Sensitive Contrast in Magnetic Resonance Image of Rodent Brain at High Magnetic Fields. Magn. Reson. Med. 1990, 14, 68–78. [Google Scholar] [CrossRef]
- Thulborn, K.R. My Starting Point: The Discovery of an NMR Method for Measuring Blood Oxygenation Using the Transverse Relaxation Time of Blood Water. NeuroImage 2012, 62, 589–593. [Google Scholar] [CrossRef]
- Turner, B.O.; Paul, E.J.; Miller, M.B.; Barbey, A.K. Small Sample Sizes Reduce the Replicability of Task-Based FMRI Studies. Commun. Biol. 2018, 1, 62. [Google Scholar] [CrossRef]
- Kwong, K.K.; Belliveau, J.W.; Chesler, D.A.; Goldberg, I.E.; Weisskoff, R.M.; Poncelet, B.P.; Kennedy, D.N.; Hoppel, B.E.; Cohen, M.S.; Turner, R. Dynamic Magnetic Resonance Imaging of Human Brain Activity during Primary Sensory Stimulation. Proc. Natl. Acad. Sci. USA 1992, 89, 5675–5679. [Google Scholar] [CrossRef]
- Deiber, M.-P.; Passingham, R.E.; Colebatch, J.G.; Friston, K.J.; Nixon, P.D.; Frackowiak, R.S.J. Cortical Areas and the Selection of Movement: A Study with Positron Emission Tomography. Exp. Brain Res. 1991, 84, 393–402. [Google Scholar] [CrossRef]
- Bandettini, P.A.; Wong, E.C.; Jesmanowicz, A.; Hinks, R.S.; Hyde, J.S. Spin-Echo and Gradient-Echo Epi of Human Brain Activation Using Bold Contrast: A Comparative Study at 1.5 T. NMR Biomed. 1994, 7, 12–20. [Google Scholar] [CrossRef]
- Penny, W.D.; Friston, K.J.; Ashburner, J.T.; Kiebel, S.J.; Nichols, T.E. (Eds.) Statistical Parametric Mapping: The Analysis of Functional Brain Images; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Frahm, J.; Merboldt, K.D.; Hanicke, W. The Influence of the Slice-Selection Gradient on Functional MRI of Human Brain Activation. J. Magn. Reson. Ser. B 1994, 103, 91–93. [Google Scholar] [CrossRef]
- Deichmann, R.; Josephs, O.; Hutton, C.; Corfield, D.R.; Turner, R. Compensation of Susceptibility-Induced BOLD Sensitivity Losses in Echo-Planar FMRI Imaging. NeuroImage 2002, 15, 120–135. [Google Scholar] [CrossRef] [PubMed]
- Menon, R.S.; Kim, S.-G. Spatial and Temporal Limits in Cognitive Neuroimaging with FMRI. Trends Cogn. Sci. 1999, 3, 207–216. [Google Scholar] [CrossRef]
- Logothetis, N.K.; Pauls, J.; Augath, M.; Trinath, T.; Oeltermann, A. Neurophysiological Investigation of the Basis of the FMRI Signal. Nature 2001, 412, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Logothetis, N.K. Intracortical Recordings and FMRI: An Attempt to Study Operational Modules and Networks Simultaneously. NeuroImage 2012, 62, 962–969. [Google Scholar] [CrossRef] [PubMed]
- Wildgruber, D.; Ackermann, H.; Klose, U.; Kardatzki, B.; Grodd, W. Functional Lateralization of Speech Production at Primary Motor Cortex: A FMRI Study. Neuroreport 1996, 7, 2791–2795. [Google Scholar] [CrossRef]
- Lotze, M.; Erb, M.; Flor, H.; Huelsmann, E.; Godde, B.; Grodd, W. FMRI Evaluation of Somatotopic Representation in Human Primary Motor Cortex. NeuroImage 2000, 11, 473–481. [Google Scholar] [CrossRef]
- Birbaumer, N.; Veit, R.; Lotze, M.; Erb, M.; Hermann, C.; Grodd, W.; Flor, H. Deficient Fear Conditioning in Psychopathy: A Functional Magnetic Resonance Imaging Study. Arch. Gen. Psychiatry 2005, 62, 799–805. [Google Scholar] [CrossRef]
- Heun, R.; Klose, U.; Jessen, F.; Erb, M.; Papassotiropoulos, A.; Lotze, M.; Grodd, W. Functional MRI of Cerebral Activation during Encoding and Retrieval of Words. Hum. Brain Mapp. 1999, 8, 157–169. [Google Scholar] [CrossRef]
- Schneider, F.; Habel, U.; Kessler, C.; Posse, S.; Grodd, W.; Müller-Gärtner, H.W. Functional Imaging of Conditioned Aversive Emotional Responses in Antisocial Personality Disorder. Neuropsychobiology 2000, 42, 192–201. [Google Scholar] [CrossRef]
- Staudt, M.; Grodd, W.; Gerloff, C.; Erb, M.; Stitz, J.; Krägeloh-Mann, I. Two Types of Ipsilateral Reorganization in Congenital Hemiparesis: A TMS and FMRI Study. Brain 2002, 125, 2222–2237. [Google Scholar] [CrossRef] [PubMed]
- Lotze, M. Cortical Reorganization and Motor Learning 2002 Universität Tübingen, PHD-works and Habilitationen. Available online: https://www.researchgate.net/publication/34200854_Cortical_reorganization_and_motor_learning (accessed on 18 August 2022).
- Lotze, M.; Montoya, P.; Erb, M.; Hülsmann, E.; Flor, H.; Klose, U.; Birbaumer, N.; Grodd, W. Activation of Cortical and Cerebellar Motor Areas during Executed and Imagined Hand Movements: An FMRI Study. J. Cogn. Neurosci. 1999, 11, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Anders, S.; Lotze, M.; Erb, M.; Grodd, W.; Birbaumer, N. Brain Activity Underlying Emotional Valence and Arousal: A Response-Related FMRI Study. Hum. Brain Mapp. 2004, 23, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Anders, S.; Weiskopf, N.; Lule, D.; Birbaumer, N. Infrared Oculography—Validation of a New Method to Monitor Startle Eyeblink Amplitudes during FMRI. NeuroImage 2004, 22, 767–770. [Google Scholar] [CrossRef] [PubMed]
- Anders, S.; Birbaumer, N.; Sadowski, B.; Erb, M.; Mader, I.; Grodd, W.; Lotze, M. Parietal Somatosensory Association Cortex Mediates Affective Blindsight. Nat. Neurosci. 2004, 7, 339–340. [Google Scholar] [CrossRef]
- Anders, S.; Verrel, J.; Haynes, J.-D.; Ethofer, T. Pseudo-Hyperscanning Shows Common Neural Activity during Face-to-Face Communication of Affect to Be Associated with Shared Affective Feelings but Not with Mere Emotion Recognition. Cortex 2020, 131, 210–220. [Google Scholar] [CrossRef]
- Wild, B.; Rodden, F.A.; Grodd, W.; Ruch, W. Neural Correlates of Laughter and Humour. Brain 2003, 126, 2121–2138. [Google Scholar] [CrossRef]
- Sitaram, R.; Caria, A.; Veit, R.; Gaber, T.; Ruiz, S.; Birbaumer, N. Volitional Control of the Anterior Insula in Criminal Psychopaths Using Real-Time FMRI Neurofeedback: A Pilot Study. Front. Behav. Neurosci. 2014, 8, 344. [Google Scholar] [CrossRef]
- Karim, A.A.; Schneider, M.; Lotze, M.; Veit, R.; Sauseng, P.; Braun, C.; Birbaumer, N. The Truth about Lying: Inhibition of the Anterior Prefrontal Cortex Improves Deceptive Behavior. Cereb. Cortex 2010, 20, 205–213. [Google Scholar] [CrossRef]
- Lotze, M.; Veit, R.; Anders, S.; Birbaumer, N. Evidence for a Different Role of the Ventral and Dorsal Medial Prefrontal Cortex for Social Reactive Aggression: An Interactive FMRI Study. NeuroImage 2007, 34, 470–478. [Google Scholar] [CrossRef]
- Lotze, M. The Role of Multiple Contralesional Motor Areas for Complex Hand Movements after Internal Capsular Lesion. J. Neurosci. 2006, 26, 6096–6102. [Google Scholar] [CrossRef] [PubMed]
- Sitaram, R.; Veit, R.; Stevens, B.; Caria, A.; Gerloff, C.; Birbaumer, N.; Hummel, F. Acquired Control of Ventral Premotor Cortex Activity by Feedback Training: An Exploratory Real-Time FMRI and TMS Study. Neurorehabil. Neural Repair 2012, 26, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Lotze, M.; Scheler, G.; Tan, H.-R.M.; Braun, C.; Birbaumer, N. The Musician’s Brain: Functional Imaging of Amateurs and Professionals during Performance and Imagery. NeuroImage 2003, 20, 1817–1829. [Google Scholar] [CrossRef] [PubMed]
- Kleber, B.; Birbaumer, N.; Veit, R.; Trevorrow, T.; Lotze, M. Overt and Imagined Singing of an Italian Aria. NeuroImage 2007, 36, 889–900. [Google Scholar] [CrossRef]
- Kleber, B.; Veit, R.; Birbaumer, N.; Gruzelier, J.; Lotze, M. The Brain of Opera Singers: Experience-Dependent Changes in Functional Activation. Cereb. Cortex 2010, 20, 1144–1152. [Google Scholar] [CrossRef] [PubMed]
- Strauss, S.; Barby, S.; Härtner, J.; Pfannmöller, J.P.; Neumann, N.; Moseley, G.L.; Lotze, M. Graded Motor Imagery Modifies Movement Pain, Cortical Excitability and Sensorimotor Function in Complex Regional Pain Syndrome. Brain Commun. 2021, 3, fcab216. [Google Scholar] [CrossRef]
- Birbaumer, N.; Grodd, W.; Diedrich, O.; Klose, U.; Erb, M.; Lotze, M.; Schneider, F.; Weiss, U.; Flor, H. FMRI Reveals Amygdala Activation to Human Faces in Social Phobics: NeuroReport. NeuroReport 1998, 9, 1223–1226. [Google Scholar] [CrossRef]
- Weiskopf, N.; Veit, R.; Erb, M.; Mathiak, K.; Grodd, W.; Goebel, R.; Birbaumer, N. Physiological Self-Regulation of Regional Brain Activity Using Real-Time Functional Magnetic Resonance Imaging (FMRI): Methodology and Exemplary Data. NeuroImage 2003, 19, 577–586. [Google Scholar] [CrossRef]
- Caria, A.; Veit, R.; Sitaram, R.; Lotze, M.; Weiskopf, N.; Grodd, W.; Birbaumer, N. Regulation of Anterior Insular Cortex Activity Using Real-Time FMRI. NeuroImage 2007, 35, 1238–1246. [Google Scholar] [CrossRef]
- Anders, S.; Beck, C.; Domin, M.; Lotze, M. Empathic Responses to Unknown Others Are Modulated by Shared Behavioural Traits. Sci. Rep. 2020, 10, 1938. [Google Scholar] [CrossRef] [Green Version]
- Saulin, A.; Horn, U.; Lotze, M.; Kaiser, J.; Hein, G. The Neural Computation of Human Prosocial Choices in Complex Motivational States. NeuroImage 2022, 247, 118827. [Google Scholar] [CrossRef] [PubMed]
- Lotze, M.; Beutling, W.; Loibl, M.; Domin, M.; Platz, T.; Schminke, U.; Byblow, W.D. Contralesional Motor Cortex Activation Depends on Ipsilesional Corticospinal Tract Integrity in Well-Recovered Subcortical Stroke Patients. Neurorehabil. Neural Repair 2012, 26, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Platz, T. Impairment-Oriented Training (IOT)–Scientific Concept and Evidence-Based Treatment Strategies. Restor. Neurol. Neurosci. 2004, 22, 301–315. [Google Scholar] [PubMed]
- Platz, T.; Van Kaick, S.; Mehrholz, J.; Leidner, O.; Eickhof, C.; Pohl, M. Best Conventional Therapy versus Modular Impairment-Oriented Training for Arm Paresis after Stroke: A Single-Blind, Multicenter Randomized Controlled Trial. Neurorehabilit. Neural Repair 2009, 23, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Platz, T.; Lotze, M. Arm Ability Training (AAT) Promotes Dexterity Recovery After a Stroke—s Review of Its Design, Clinical Effectiveness, and the Neurobiology of the Actions. Front. Neurol. 2018, 9, 1082. [Google Scholar] [CrossRef]
- Walz, A.D.; Doppl, K.; Kaza, E.; Roschka, S.; Platz, T.; Lotze, M. Changes in Cortical, Cerebellar and Basal Ganglia Representation after Comprehensive Long Term Unilateral Hand Motor Training. Behav. Brain Res. 2015, 278, 393–403. [Google Scholar] [CrossRef]
- Grothe, M.; Doppl, K.; Roth, C.; Roschka, S.; Platz, T.; Lotze, M. Changes in Motor Cortex Excitability for the Trained and Non-Trained Hand after Long-Term Unilateral Motor Training. Neurosci. Lett. 2017, 647, 117–121. [Google Scholar] [CrossRef]
- Platz, T.; Schüttauf, J.; Aschenbach, J.; Mengdehl, C.; Lotze, M. Effects of Inhibitory Theta Burst TMS to Different Brain Sites Involved in Visuospatial Attention—A Combined Neuronavigated CTBS and Behavioural Study. Restor. Neurol. Neurosci. 2016, 34, 271–285. [Google Scholar] [CrossRef]
- Lotze, M.; Ladda, A.M.; Roschka, S.; Platz, T.; Dinse, H.R. Priming Hand Motor Training with Repetitive Stimulation of the Fingertips; Performance Gain and Functional Imaging of Training Effects. Brain Stimul. 2017, 10, 139–146. [Google Scholar] [CrossRef]
- Horn, U.; Roschka, S.; Eyme, K.; Walz, A.-D.; Platz, T.; Lotze, M. Increased Ventral Premotor Cortex Recruitment after Arm Training in an FMRI Study with Subacute Stroke Patients. Behav. Brain Res. 2016, 308, 152–159. [Google Scholar] [CrossRef]
- Lindow, J.; Domin, M.; Grothe, M.; Horn, U.; Eickhoff, S.B.; Lotze, M. Connectivity-Based Predictions of Hand Motor Outcome for Patients at the Subacute Stage After Stroke. Front. Hum. Neurosci. 2016, 10, 101. [Google Scholar] [CrossRef] [PubMed]
- Horn, U.; Grothe, M.; Lotze, M. MRI Biomarkers for Hand-Motor Outcome Prediction and Therapy Monitoring Following Stroke. Neural Plast. 2016, 2016, 9265621. [Google Scholar] [CrossRef] [PubMed]
- Mihai, P.G.; von Bohlen und Halbach, O.; Lotze, M. Differentiation of Cerebral Representation of Occlusion and Swallowing with FMRI. Am. J. Physiol.-Gastrointest. Liver Physiol. 2013, 304, G847–G854. [Google Scholar] [CrossRef] [PubMed]
- Mihai, P.G.; Otto, M.; Platz, T.; Eickhoff, S.B.; Lotze, M. Sequential Evolution of Cortical Activity and Effective Connectivity of Swallowing Using FMRI: Cortical Activity and Effective Connectivity of Swallowing. Hum. Brain Mapp. 2014, 35, 5962–5973. [Google Scholar] [CrossRef]
- Mihai, P.G.; Otto, M.; Domin, M.; Platz, T.; Hamdy, S.; Lotze, M. Brain Imaging Correlates of Recovered Swallowing after Dysphagic Stroke: A FMRI and DWI Study. NeuroImage: Clin. 2016, 12, 1013–1021. [Google Scholar] [CrossRef]
- Domin, M.; Mihai, G.P.; Platz, T.; Lotze, M. Swallowing Function in the Chronic Stage Following Stroke Is Associated with White Matter Integrity of the Callosal Tract between the Interhemispheric S1 Swallowing Representation Areas. NeuroImage: Clin. 2022, 35, 103093. [Google Scholar] [CrossRef]
- Loibl, M.; Beutling, W.; Kaza, E.; Lotze, M. Non-Effective Increase of FMRI-Activation for Motor Performance in Elder Individuals. Behav. Brain Res. 2011, 223, 280–286. [Google Scholar] [CrossRef]
- Windel, A.-S.; Mihai, P.G.; Lotze, M. Neural Representation of Swallowing Is Retained with Age. A Functional Neuroimaging Study Validated by Classical and Bayesian Inference. Behav. Brain Res. 2015, 286, 308–317. [Google Scholar] [CrossRef]
- Davids, S.; Lauffer, H.; Thoms, K.; Jagdhuhn, M.; Hirschfeld, H.; Domin, M.; Hamm, A.; Lotze, M. Increased Dorsolateral Prefrontal Cortex Activation in Obese Children during Observation of Food Stimuli. Int. J. Obes. 2010, 34, 94–104. [Google Scholar] [CrossRef]
- Fritz, H.-C.; Wittfeld, K.; Schmidt, C.O.; Domin, M.; Grabe, H.J.; Hegenscheid, K.; Hosten, N.; Lotze, M. Current Smoking and Reduced Gray Matter Volume—A Voxel-Based Morphometry Study. Neuropsychopharmacology 2014, 39, 2594–2600. [Google Scholar] [CrossRef] [Green Version]
- Wendt, J.; Lotze, M.; Weike, A.I.; Hosten, N.; Hamm, A.O. Brain Activation and Defensive Response Mobilization during Sustained Exposure to Phobia-Related and Other Affective Pictures in Spider Phobia. Psychophysiology 2008, 45, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, F.; McAuley, J.H.; Parkitny, L.; Lotze, M.; Wand, B.M.; Moseley, G.L.; Stanton, T.R. Primary Somatosensory Cortex Function in Complex Regional Pain Syndrome: A Systematic Review and Meta-Analysis. J. Pain 2013, 14, 1001–1018. [Google Scholar] [CrossRef] [PubMed]
- Friebel, U.; Eickhoff, S.B.; Lotze, M. Coordinate-based meta-analysis of experimentally induced and chronic persistent neuropathic pain. NeuroImage 2011, 58, 1070–1080. [Google Scholar] [CrossRef] [PubMed]
- Shah, C.; Erhard, K.; Ortheil, H.-J.; Kaza, E.; Kessler, C.; Lotze, M. Neural Correlates of Creative Writing: An FMRI Study. Hum. Brain Mapp. 2013, 34, 1088–1101. [Google Scholar] [CrossRef] [PubMed]
- Fritz, H.C.; McAuley, J.H.; Wittfeld, K.; Hegenscheid, K.; Schmidt, C.O.; Langner, S.; Lotze, M. Chronic Back Pain Is Associated With Decreased Prefrontal and Anterior Insular Gray Matter: Results From a Population-Based Cohort Study. J. Pain 2016, 17, 111. [Google Scholar] [CrossRef]
- Lotze, M.; Domin, M.; Gerlach, F.H.; Gaser, C.; Lueders, E.; Schmidt, C.O.; Neumann, N. Novel Findings from 2,838 Adult Brains on Sex Differences in Gray Matter Brain Volume. Sci. Rep. 2019, 9, 1671. [Google Scholar] [CrossRef]
- Holtz, K.; Pané-Farré, C.A.; Wendt, J.; Lotze, M.; Hamm, A.O. Brain Activation during Anticipation of Interoceptive Threat. NeuroImage 2012, 61, 857–865. [Google Scholar] [CrossRef]
- Wattendorf, E.; Westermann, B.; Fiedler, K.; Kaza, E.; Lotze, M.; Celio, M.R. Exploration of the neural correlates of ticklish laughter by functional magnetic resonance imaging. Cereb. Cortex 2013, 23, 1280–1289. [Google Scholar] [CrossRef]
- Erhard, K.; Kessler, F.; Neumann, N.; Ortheil, H.-J.J.; Lotze, M. Professional Training in Creative Writing Is Associated with Enhanced Fronto-Striatal Activity in a Literary Text Continuation Task. NeuroImage 2014, 100, 15–23. [Google Scholar] [CrossRef]
- Langner, S.; Kromrey, M.-L.; Kuehn, J.-P.; Grothe, M.; Domin, M. Repeated Intravenous Administration of Gadobutrol Does Not Lead to Increased Signal Intensity on Unenhanced T1-Weighted Images—A Voxel-Based Whole Brain Analysis. Eur. Radiol. 2017, 27, 3687–3693. [Google Scholar] [CrossRef]
- Kanda, T.; Ishii, K.; Kawaguchi, H.; Kitajima, K.; Takenaka, D. High Signal Intensity in the Dentate Nucleus and Globus Pallidus on Unenhanced T1-Weighted MR Images: Relationship with Increasing Cumulative Dose of a Gadolinium-Based Contrast Material. Radiology 2014, 270, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Wendt, J.; Löw, A.; Weymar, M.; Lotze, M.; Hamm, A.O. Active Avoidance and Attentive Freezing in the Face of Approaching Threat. NeuroImage 2017, 158, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lueken, U.; Richter, J.; Hamm, A.; Wittmann, A.; Konrad, C.; Ströhle, A.; Pfleiderer, B.; Herrmann, M.J.; Lang, T.; et al. Effect of CBT on Biased Semantic Network in Panic Disorder: A Multicenter FMRI Study Using Semantic Priming. Am. J. Psychiatry 2020, 177, 254–264. [Google Scholar] [CrossRef]
- Klepzig, K.; Horn, U.; König, J.; Holtz, K.; Wendt, J.; Hamm, A.O.; Lotze, M. Brain Imaging of Chill Reactions to Pleasant and Unpleasant Sounds. Behav. Brain Res. 2020, 380, 112417. [Google Scholar] [CrossRef]
- Usichenko, T.; Hacker, H.; Lotze, M. Transcutaneous Auricular Vagal Nerve Stimulation (TaVNS) Might Be a Mechanism behind the Analgesic Effects of Auricular Acupuncture. Brain Stimul. 2017, 10, 1042–1044. [Google Scholar] [CrossRef] [PubMed]
- Lickteig, R.; Lotze, M.; Kordass, B. Successful Therapy for Temporomandibular Pain Alters Anterior Insula and Cerebellar Representations of Occlusion. Cephalalgia 2013, 33, 1248–1257. [Google Scholar] [CrossRef]
- Ernst, M.; Schenkenberger, A.E.; Domin, M.; Kordass, B.; Lotze, M. Effects of Centric Mandibular Splint Therapy on Orofacial Pain and Cerebral Activation Patterns. Clin. Oral Investig. 2020, 24, 2005–2013. [Google Scholar] [CrossRef]
- Lotze, M.; Grodd, W.; Birbaumer, N.; Erb, M.; Huse, E.; Flor, H. Does Use of a Myoelectric Prosthesis Prevent Cortical Reorganization and Phantom Limb Pain? Nat. Neurosci. 1999, 2, 501–502. [Google Scholar] [CrossRef]
- Strauss, S.; Grothe, M.; Usichenko, T.; Neumann, N.; Byblow, W.D.; Lotze, M. Inhibition of the Primary Sensorimotor Cortex by Topical Anesthesia of the Forearm in Patients with Complex Regional Pain Syndrome. Pain 2015, 156, 2556–2561. [Google Scholar] [CrossRef]
- Lebon, F.; Horn, U.; Domin, M.; Lotze, M. Motor Imagery Training: Kinesthetic Imagery Strategy and Inferior Parietal FMRI Activation. Hum. Brain Mapp. 2018, 39, 1805–1813. [Google Scholar] [CrossRef] [Green Version]
- Ladda, A.M.; Lebon, F.; Lotze, M. Using Motor Imagery Practice for Improving Motor Performance—A Review. Brain Cogn. 2021, 150, 105705. [Google Scholar] [CrossRef] [PubMed]
- Lotze, M.; Roschka, S.; Domin, M.; Platz, T. Predicting Training Gain for a 3 Week Period of Arm Ability Training in the Subacute Stage After Stroke. Front. Neurol. 2018, 9, 854. [Google Scholar] [CrossRef] [PubMed]
- Lotze, M.; Moseley, G.L. Clinical and Neurophysiological Effects of Progressive Movement Imagery Training for Pathological Pain. J. Pain 2022, in press. [Google Scholar] [CrossRef] [PubMed]
- Domin, M.; Lotze, M. Parcellation of Motor Cortex-Associated Regions in the Human Corpus Callosum on the Basis of Human Connectome Project Data. Brain Struct. Funct. 2019, 224, 1447–1455. [Google Scholar] [CrossRef]
- Pfannmöller, J.P.; Greiner, M.; Balasubramanian, M.; Lotze, M. High-Resolution FMRI Investigations of the Fingertip Somatotopy and Variability in BA3b and BA1 of the Primary Somatosensory Cortex. Neuroscience 2016, 339, 667–677. [Google Scholar] [CrossRef]
- Pfannmöller, J.; Strauss, S.; Langner, I.; Usichenko, T.; Lotze, M. Investigations on Maladaptive Plasticity in the Sensorimotor Cortex of Unilateral Upper Limb CRPS i Patients. Restor. Neurol. Neurosci. 2019, 37, 143–153. [Google Scholar] [CrossRef]
- Härtner, J.; Strauss, S.; Pfannmöller, J.; Lotze, M. Tactile Acuity of Fingertips and Hand Representation Size in Human Area 3b and Area 1 of the Primary Somatosensory Cortex. Neuroimage 2021, 232, 117912. [Google Scholar] [CrossRef]
- Lotze, M.; Moseley, G.L. Role of Distorted Body Image in Pain. Curr. Rheumatol. Rep. 2007, 9, 488–496. [Google Scholar] [CrossRef]
- Lotze, M.; Moseley, G.L. Theoretical Considerations for Chronic Pain Rehabilitation. Phys. Ther. 2015, 95, 1316–1320. [Google Scholar] [CrossRef]
- Lotze, M.; Domin, M.; Schmidt, C.O.; Hosten, N.; Grabe, H.J.; Neumann, N. Income Is Associated with Hippocampal/Amygdala and Education with Cingulate Cortex Grey Matter Volume. Sci. Rep. 2020, 10, 18786. [Google Scholar] [CrossRef]
- Eyme, K.M.; Domin, M.; Gerlach, F.H.; Hosten, N.; Schmidt, C.O.; Gaser, C.; Flöel, A.; Lotze, M. Physically Active Life Style Is Associated with Increased Grey Matter Brain Volume in a Medial Parieto-Frontal Network. Behav. Brain Res. 2019, 359, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Hosten, N.; Bülow, R.; Völzke, H.; Domin, M.; Schmidt, C.O.; Teumer, A.; Ittermann, T.; Nauck, M.; Felix, S.; Dörr, M.; et al. SHIP-MR and Radiology: 12 Years of Whole-Body Magnetic Resonance Imaging in a Single Center. Healthcare 2021, 10, 33. [Google Scholar] [CrossRef] [PubMed]
- Stippich, C.; Heiland, S.; Tronnier, V.; Mohr, A.; Sartor, K. Klinische Funktionelle Magnetresonanztomographie (FMRT): Physiologische Grundlagen, Technische Aspekte Und Anforderungen Für Die Klinische Anwendung. Rofo Fortschr. Auf Dem Geb. Der Röntgenstrahlen Und Der Bildgeb. Verfahr. 2002, 174, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Stinear, C.M.; Byblow, W.D.; Ackerley, S.J.; Smith, M.-C.; Borges, V.M.; Barber, P.A. PREP2: A Biomarker-Based Algorithm for Predicting Upper Limb Function after Stroke. Ann. Clin. Transl. Neurol. 2017, 4, 811–820. [Google Scholar] [CrossRef]
- Barkhof, F.; Haller, S.; Rombouts, S.A.R.B. Resting-State Functional MR Imaging: A New Window to the Brain. Radiology 2014, 272, 29–49. [Google Scholar] [CrossRef]
- Lotze, M.; Langner, R. Editorial for the Special Issue “Resting-State FMRI and Cognition” in Brain and Cognition. Brain Cogn. 2019, 131, 1–3. [Google Scholar] [CrossRef]
- Cash, R.F.H.; Cocchi, L.; Lv, J.; Wu, Y.; Fitzgerald, P.B.; Zalesky, A. Personalized Connectivity-guided DLPFC-TMS for Depression: Advancing Computational Feasibility, Precision and Reproducibility. Hum. Brain Mapp. 2021, 42, 4155–4172. [Google Scholar] [CrossRef]
- Mansour, A.; Baria, A.T.; Tetreault, P.; Vachon-Presseau, E.; Chang, P.-C.; Huang, L.; Apkarian, A.V.; Baliki, M.N. Global Disruption of Degree Rank Order: A Hallmark of Chronic Pain. Sci. Rep. 2016, 6, 34853. [Google Scholar] [CrossRef]
- Pfannmöller, J.; Lotze, M. Review on Biomarkers in the Resting-State Networks of Chronic Pain Patients. Brain Cogn. 2019, 131, 4–9. [Google Scholar] [CrossRef]
- Di Pietro, F.; McAuley, J.H.; Parkitny, L.; Lotze, M.; Wand, B.M.; Moseley, G.L.; Stanton, T.R. Primary Motor Cortex Function in Complex Regional Pain Syndrome: A Systematic Review and Meta-Analysis. J. Pain 2013, 14, 1270–1288. [Google Scholar] [CrossRef]
- Koch, P.J.; Park, C.-H.; Girard, G.; Beanato, E.; Egger, P.; Evangelista, G.G.; Lee, J.; Wessel, M.J.; Morishita, T.; Koch, G.; et al. The Structural Connectome and Motor Recovery after Stroke: Predicting Natural Recovery. Brain 2021, 144, 2107–2119. [Google Scholar] [CrossRef] [PubMed]
- Krause, M.; Wendt, J.; Dressel, A.; Berneiser, J.; Kessler, C.; Hamm, A.O.; Lotze, M. Prefrontal Function Associated with Impaired Emotion Recognition in Patients with Multiple Sclerosis. Behav. Brain Res. 2009, 205, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Liew, S.-L.; Zavaliangos-Petropulu, A.; Jahanshad, N.; Lang, C.E.; Hayward, K.S.; Lohse, K.R.; Juliano, J.M.; Assogna, F.; Baugh, L.A.; Bhattacharya, A.K.; et al. The ENIGMA Stroke Recovery Working Group: Big Data Neuroimaging to Study Brain-Behavior Relationships after Stroke. Hum. Brain Mapp. 2022, 43, 129–148. [Google Scholar] [CrossRef] [PubMed]
- Zavaliangos-Petropulu, A.; Lo, B.; Donnelly, M.; Schweighofer, N.; Lohse, K.; Jahanshad, N.; Barisano, G.; Banaj, N.; Borich, M.; Boyd, L.; et al. Chronic Stroke Sensorimotor Impairment Is Related to Smaller Hippocampal Volumes: An ENIGMA Analysis. J. Am. Heart Assoc. 2022, 11, e025109. [Google Scholar] [CrossRef]
- Liew, S.-L.; Lo, B.; Donnelly, M.R.; Zavaliangos-Petropulu, A.; Jeong, J.N.; Barisano, G.; Hutton, A.; Simon, J.P.; Juliano, J.M.; Suri, A.; et al. A Large, Curated, Open-Source Stroke Neuroimaging Dataset to Improve Lesion Segmentation Algorithms. Sci. Data 2022, 9, 320. [Google Scholar] [CrossRef]
| Area of Research Referenced in (Web of Science) in 7/22 | Partner | Topic | First Author, Year, Citation | Title |
|---|---|---|---|---|
| Cross-sectional fMRI study on children (112) | Pediatrics UMG | Obesity | Davids S, 2010, [61] | “Increased dorsolateral prefrontal cortex activation in obese children during observation of food stimuli” |
| Voxel-based morphometry from SHIP data (95) | Community Medicine UMG | Life Style factors, brain atrophy | Fritz HC, 2014 [62] | “Current Smoking and Reduced Gray Matter Volume-a Voxel-Based Morphometry Study” |
| fMRI on phobia-related stimuli (95) | Psychology University | Emotion processing | Wendt J, 2008 [63] | “Brain activation and defensive response mobilization during sustained exposure to phobia-related and other affective pictures in spider phobia” |
| Review (88) | External (Australia) | Biomarkers in CRPS | DiPietro F, 2013 [64] | “Primary Somatosensory Cortex Function in Complex Regional Pain Syndrome: A Systematic Review and Meta-Analysis” |
| ALE meta-analysis (83) | Jülich | Pain biomarkers | Friebel U, 2011 [65] | “Coordinate-based meta-analysis of experimentally induced and chronic persistent neuropathic pain” |
| Functional MRI on creative writing (83) | Neurology, University of Hildesheim | Verbal Creativity | Shah C, 2013 [66] | “Neural correlates of creative writing: An fMRI Study” |
| Voxel-based morphometry from SHIP data (73) | Community Medicine UMG | Chronic back pain; brain atrophy | Fritz HC, 2016 [67] | “Chronic Back Pain Is Associated With Decreased Prefrontal and Anterior Insular Gray Matter: Results From a Population-Based Cohort Study” |
| Voxel-based morphometry from SHIP data (70) | Community Medicine UMG | Gender identification, paper 1 | Lotze M, 2019 [68] | “Novel findings from 2838 Adult Brains on Sex Differences in Gray Matter Brain Volume” |
| fMRI, MRI, and TMS on chronic stroke (61) | Neurology UMG, Auckland NZ | Biomarkers on upper limb function | Lotze M, 2016 [44] | “Contralesional Motor Cortex Activation Depends on Ipsilesional Corticospinal Tract Integrity in Well-Recovered Subcortical Stroke Patients” |
| fMRI on phobia-related stimuli (55) | Psychology University | Emotion processing | Holtz K, 2012 [69] | “Brain activation during anticipation of interoceptive threat” |
| fMRI on emotional processing (53) | Fribourg, Switzerland | Ticklish laughter | Wattendorf E, 2013 [70] | “Exploration of the Neural Correlates of Ticklish Laughter by Functional Magnetic Resonance Imaging” |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lotze, M.; Domin, M.; Langner, S.; Platz, T. Functional MRI in Radiology—A Personal Review. Healthcare 2022, 10, 1646. https://doi.org/10.3390/healthcare10091646
Lotze M, Domin M, Langner S, Platz T. Functional MRI in Radiology—A Personal Review. Healthcare. 2022; 10(9):1646. https://doi.org/10.3390/healthcare10091646
Chicago/Turabian StyleLotze, Martin, Martin Domin, Sönke Langner, and Thomas Platz. 2022. "Functional MRI in Radiology—A Personal Review" Healthcare 10, no. 9: 1646. https://doi.org/10.3390/healthcare10091646
APA StyleLotze, M., Domin, M., Langner, S., & Platz, T. (2022). Functional MRI in Radiology—A Personal Review. Healthcare, 10(9), 1646. https://doi.org/10.3390/healthcare10091646






