A Brief mHealth-Based Psychological Intervention in Emotion Regulation to Promote Positive Subjective Well-Being in Cardiovascular Disease Patients: A Non-Randomized Controlled Trial
Abstract
:1. Introduction
1.1. Brief Psychological Interventions
1.2. Emotion Regulation and Positive Well-Being
1.3. mHealth
1.4. The Present Study
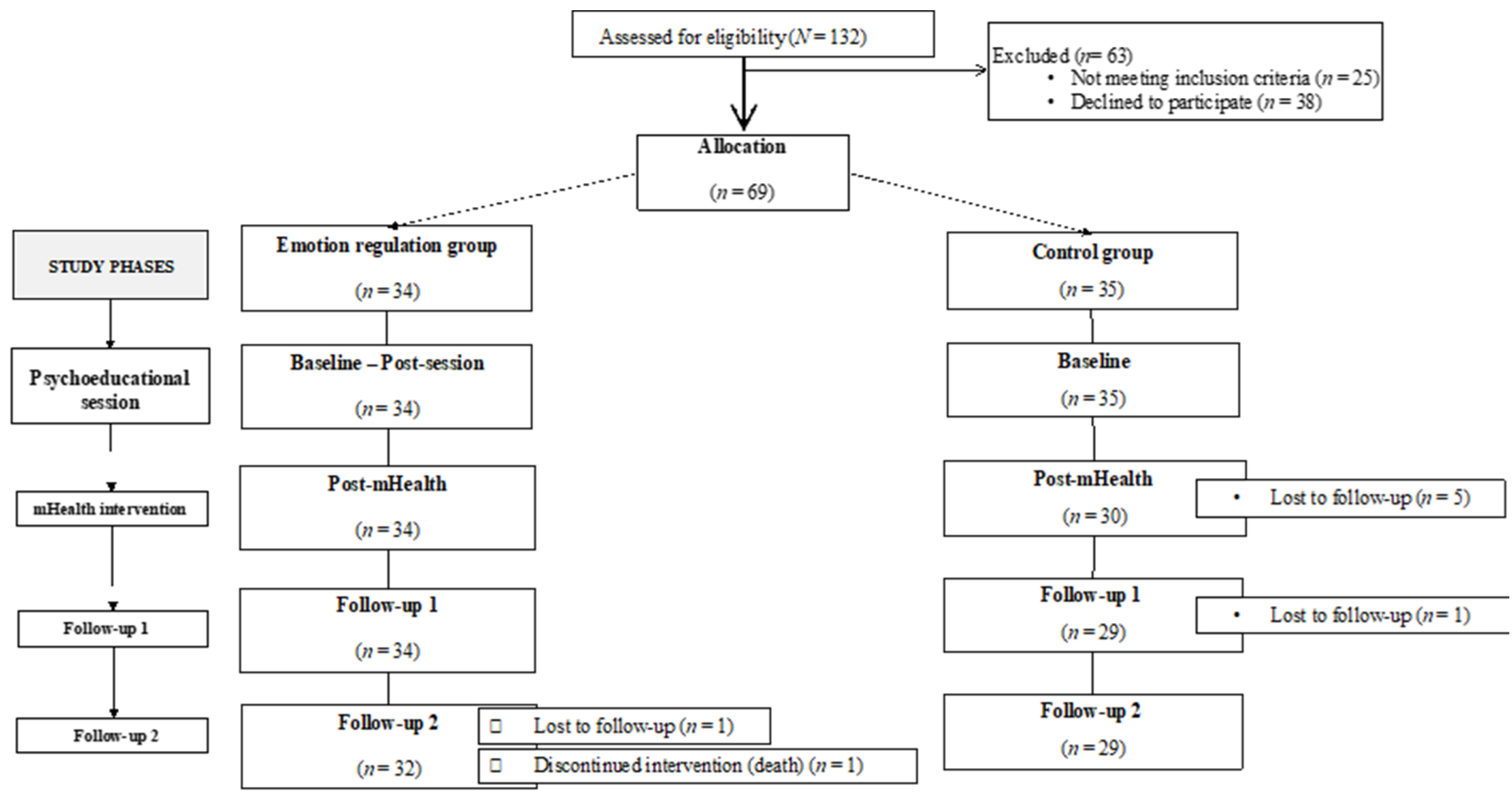
2. Methods
2.1. Study Design
2.2. Participants
2.3. Procedure
2.3.1. Experimental Group
2.3.2. Control Group
2.4. Outcome Measures
2.4.1. Participant Characteristics
2.4.2. Primary Outcome
Positive Subjective Well-Being (PSWB)
2.4.3. Secondary Outcome
2.5. Data Analysis
3. Results
| Total (N = 69) | Experimental Group (n = 34) | Control Group (n = 35) | Statistical Significance | |
|---|---|---|---|---|
| Age (M, SD) | 63.7 (11.5) | 61.24 (11.1) | 66.1 (11.6) | t(67) = 1.77, p = 0.081 a |
| Sex, n (%) | χ2(1) = 1.95, p = 0.163 b | |||
| Male | 54 (78.3%) | 29 | 25 | |
| Female | 15 (21.7%) | 5 | 10 | |
| Marital status, n (%) | p = 0.924 c | |||
| Single | 2 (2.9%) | 1 | 1 | |
| Single with partner | 1 (1.4%) | 1 | 0 | |
| Married | 57 (82.6%) | 28 | 29 | |
| Separated | 2 (2.9%) | 1 | 1 | |
| Divorced | 3 (4.3%) | 2 | 1 | |
| Widowed | 4 (5.8%) | 1 | 3 | |
| Employment status, n (%) | p = 0.004 c | |||
| Retired | 40 (58%) | 13 | 27 | |
| Full-time work | 21 (30.4%) | 15 | 6 | |
| Unemployed | 6 (8.7%) | 5 | 1 | |
| Home care | 2 (2.9%) | 1 | 1 | |
| Educational level, n (%) | p = 0.119 c | |||
| Basic primary school | 54 (78.3%) | 24 | 30 | |
| High school or higher | 15 (21.7%) | 10 | 5 | |
| Type of CVD, n (%) | p = 0.677 c | |||
| Angina pectoris | 8 (11.6%) | 3 | 5 | |
| Myocardial infarction | 33 (47.8%) | 17 | 16 | |
| Heart failure | 5 (7.3%) | 1 | 4 | |
| Arrhythmia | 5 (7.3%) | 2 | 3 | |
| Other | 11 (15.9%) | 7 | 4 | |
| More than one of the above | 7 (10.1) | 4 | 3 | |
| Level of limitation of ADLs, n (%) | ||||
| Level 1 | 29 (42%) | 17 | 12 | |
| Level 2 | 22 (31.9%) | 10 | 12 | |
| Level 3 | 14 (20.3%) | 4 | 10 | |
| Level 4 | 4 (5.8%) | 3 | 1 | |
| HADS (M, SD) | 1.84 (0.49) | 1.95 (0.44) | 1.73 (0.52) | t(65) = −1.88, p = 0.065 a |
| P-scale (M, SD) | 3.94 (0.82) | 3.89 (0.69) | 3.99 (0.93) | t(67) = 0.50, p = 0.614 a |
3.1. Psychoeducational Session
3.2. mHealth Intervention
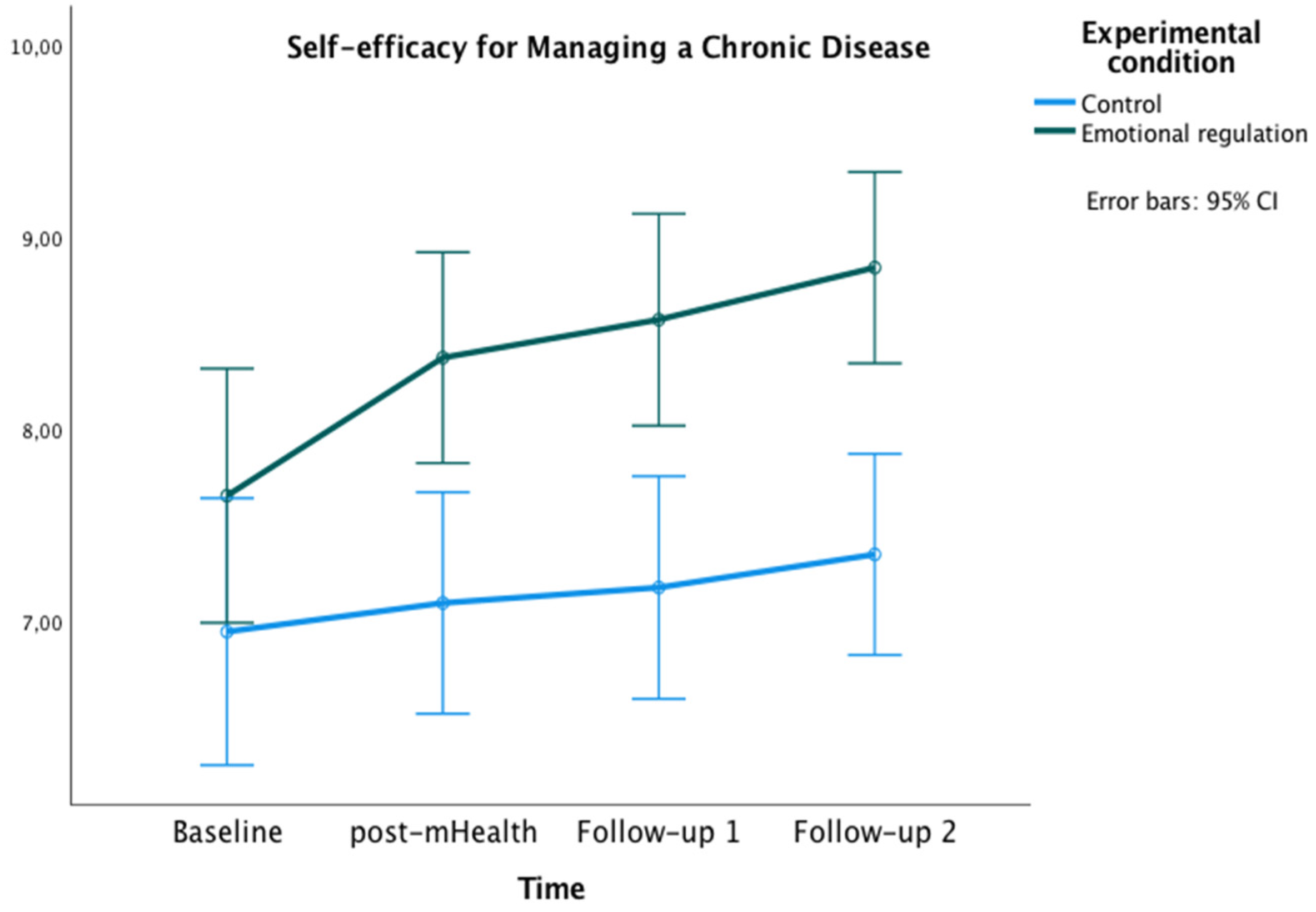
3.2.1. Primary Outcome
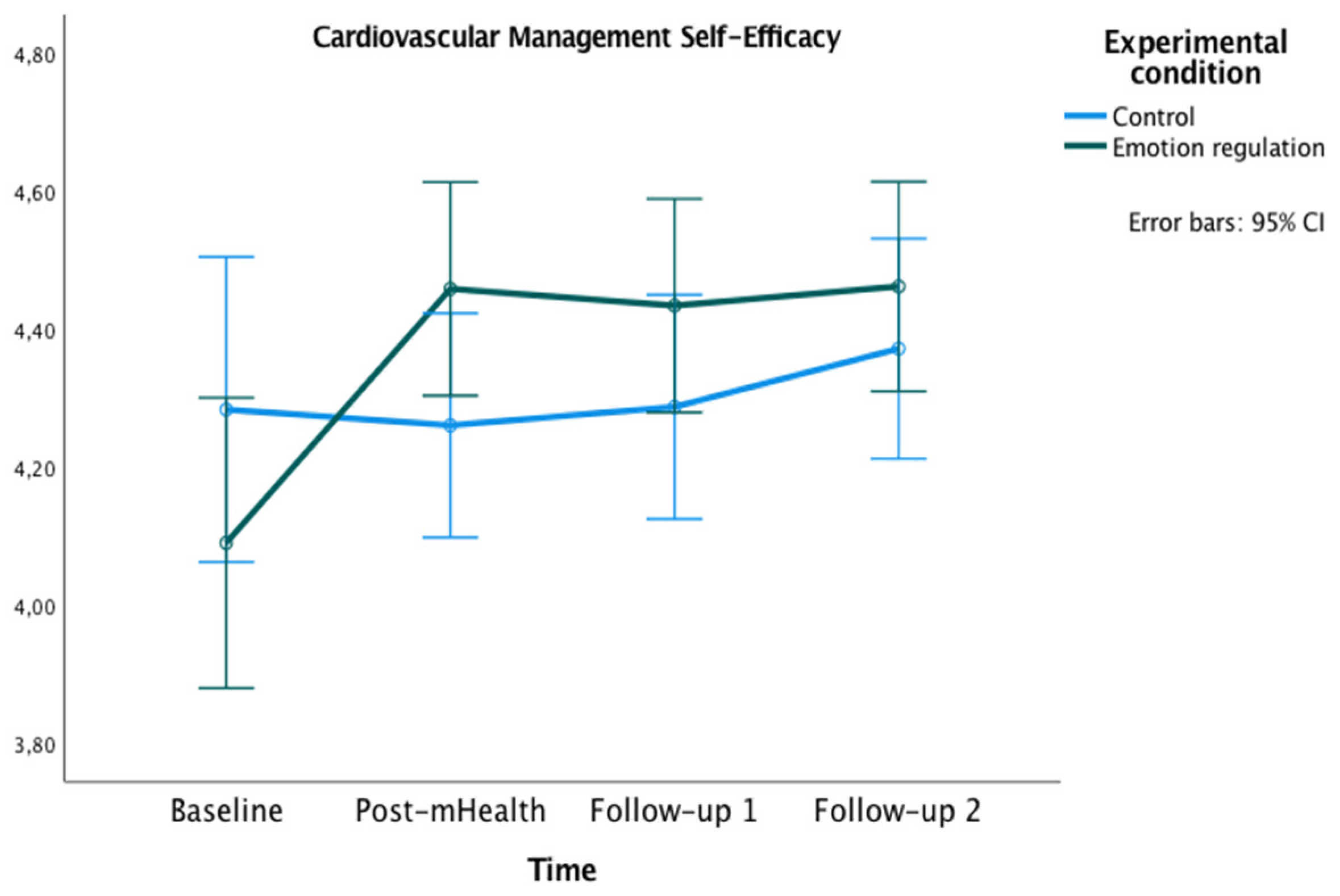
3.2.2. Secondary Outcome
Self-Efficacy for Managing the Disease
3.3. Subjective Evaluation of the Intervention
4. Discussion
4.1. Limitations
4.2. Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roth, G.A.; Johnson, C.; Abajobir, A.; Abd-Allah, F.; Abera, S.F.; Abyu, G.; Ahmed, M.; Aksut, B.; Alam, T.; Alam, K.; et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J. Am. Coll. Cardiol. 2017, 70, 1–25. [Google Scholar] [CrossRef]
- Cardiovascular Diseases. Available online: https://www.who.int/health-topics/cardiovascular-diseases/#tab=tab_1 (accessed on 30 June 2022).
- Patel, S.; Saha, A.; Poojary, P.; Pandya, D.; Pawar, S.; Patel, J.; Mahajan, K.; Mondal, P.; Agarwal, S.; Hollander, G.; et al. Trends and impact of psychosocial factors in adults with congenital heart disease in the United States. J. Am. Coll. Cardiol. 2018, 71, A561. [Google Scholar] [CrossRef]
- Larkin, K.T.; Chantler, P.D. Chapter 1—Stress, depression, and cardiovascular disease. In Cardiovascular Implications of Stress and Depression; Chantler, P.D., Larkin, K.T., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 1–12. ISBN 978-0-12-815015-3. [Google Scholar]
- Grewal, K.; Gravely-Witte, S.; Stewart, D.E.; Grace, S.L. A simultaneous test of the relationship between identified psychosocial risk factors and recurrent events in coronary artery disease patients. Anxiety Stress Coping 2011, 24, 463–475. [Google Scholar] [CrossRef]
- Grenier, S.; Potvin, O.; Hudon, C.; Boyer, R.; Préville, M.; Desjardins, L.; Bherer, L. Twelve-month prevalence and correlates of subthreshold and threshold anxiety in community-dwelling older adults with cardiovascular diseases. J. Affect. Disord. 2012, 136, 724–732. [Google Scholar] [CrossRef]
- Chen, H.; Wang, X.; Huang, Y.; Li, G.; Liu, Z.; Li, Y.; Geng, H. Prevalence, risk factors and multi-group latent class analysis of lifetime anxiety disorders comorbid depressive symptoms. J. Affect. Disord. 2019, 243, 360–365. [Google Scholar] [CrossRef]
- Fernandes, A.C.; McIntyre, T.; Coelho, R.; Prata, J.; Maciel, M.J. Impact of a brief psychological intervention on lifestyle, risk factors and disease knowledge during phase i of cardiac rehabilitation after acute coronary syndrome. Rev. Port. Cardiol. Engl. Ed. 2019, 38, 361–368. [Google Scholar] [CrossRef]
- Fernandes, A.C.; McIntyre, T.; Coelho, R.; Prata, J.; Maciel, M.J. Brief psychological intervention in phase I of cardiac rehabilitation after acute coronary syndrome. Rev. Port. Cardiol. 2017, 36, 641–649. [Google Scholar] [CrossRef]
- Armitage, C.J. A brief psychological intervention to protect subjective well-being in a community sample. Qual. Life Res. 2016, 25, 385–391. [Google Scholar] [CrossRef]
- Cohen, G.L.; Sherman, D.K. The psychology of change: Self-affirmation and social psychological intervention. Annu. Rev. Psychol. 2014, 65, 333–371. [Google Scholar] [CrossRef]
- Garcia, J.; Cohen, G.L. A social psychological approach to educational intervention. In The Behavioral Foundations of Public Policy; Princeton University Press: Princeton, NJ, USA, 2013; pp. 329–347. ISBN 978-0-691-13756-8. [Google Scholar]
- Abreu, A. Breve intervenção psicológica em doentes internados após síndrome coronária aguda: Essencial ou acessória? Rev. Port. Cardiol. 2017, 36, 651–654. [Google Scholar] [CrossRef]
- Mikkelsen, M.E.; Gaieski, D.F.; Goyal, M.; Miltiades, A.N.; Munson, J.C.; Pines, J.M.; Fuchs, B.D.; Shah, C.V.; Bellamy, S.L.; Christie, J.D. Factors associated with nonadherence to early goal-directed therapy in the ED. Chest 2010, 138, 551–558. [Google Scholar] [CrossRef]
- Van Der Laan, D.M.; Elders, P.J.M.; Boons, C.C.L.M.; Nijpels, G.; Hugtenburg, J.G. Factors associated with nonadherence to cardiovascular medications: A cross-sectional study. J. Cardiovasc. Nurs. 2019, 34, 344–352. [Google Scholar] [CrossRef]
- Appleton, A.A.; Loucks, E.B.; Buka, S.L.; Kubzansky, L.D. Divergent associations of antecedent- and response-focused emotion regulation strategies with midlife cardiovascular disease risk. Ann. Behav. Med. 2014, 48, 246–255. [Google Scholar] [CrossRef]
- Bolier, L.; Haverman, M.; Westerhof, G.J.; Riper, H.; Smit, F.; Bohlmeijer, E. Positive psychology interventions: A meta-analysis of randomized controlled studies. BMC Public Health 2013, 13, 119. [Google Scholar] [CrossRef]
- Boehm, J.K.; Chen, Y.; Koga, H.; Mathur, M.B.; Vie, L.L.; Kubzansky, L.D. Is optimism associated with healthier cardiovascular-related behavior? Meta-analyses of 3 health behaviors. Circ. Res. 2018, 122, 1119–1134. [Google Scholar] [CrossRef]
- Boehm, J.K.; Kubzansky, L.D. The heart’s content: The association between positive psychological well-being and cardiovascular health. Psychol. Bull. 2012, 138, 655–691. [Google Scholar] [CrossRef]
- Lambiase, M.J.; Kubzansky, L.D.; Thurston, R.C. Positive psychological health and stroke risk: The benefits of emotional vitality. Health Psychol. 2015, 34, 1043–1046. [Google Scholar] [CrossRef]
- Kim, E.S.; Sun, J.K.; Park, N.; Kubzansky, L.D.; Peterson, C. Purpose in life and reduced risk of myocardial infarction among older U.S. adults with coronary heart disease: A two-year follow-up. J. Behav. Med. 2013, 36, 124–133. [Google Scholar] [CrossRef]
- Sin, N.L. The protective role of positive well-being in cardiovascular disease: Review of current evidence, mechanisms, and clinical implications. Curr. Cardiol. Rep. 2016 1811 2016, 18, 106. [Google Scholar] [CrossRef]
- Brummett, B.H.; Boyle, S.H.; Kuhn, C.M.; Siegler, I.C.; Williams, R.B. Positive affect is associated with cardiovascular reactivity, norepinephrine level, and morning rise in salivary cortisol. Psychophysiology 2009, 46, 862–869. [Google Scholar] [CrossRef] [Green Version]
- Dockray, S.; Steptoe, A. Positive affect and psychobiological processes. Neurosci. Biobehav. Rev. 2010, 35, 69–75. [Google Scholar] [CrossRef]
- Steptoe, A.; Leigh Gibson, E.; Hamer, M.; Wardle, J. Neuroendocrine and cardiovascular correlates of positive affect measured by ecological momentary assessment and by questionnaire. Psychoneuroendocrinology 2007, 32, 56–64. [Google Scholar] [CrossRef]
- Huffman, J.C.; Beale, E.E.; Celano, C.M.; Beach, S.R.; Belcher, A.M.; Moore, S.V.; Suarez, L.; Motiwala, S.R.; Gandhi, P.U.; Gaggin, H.K.; et al. Effects of optimism and gratitude on physical activity, biomarkers, and readmissions after an acute coronary syndrome: The gratitude research in acute coronary events study. Circ. Cardiovasc. Qual. Outcomes 2016, 9, 55–63. [Google Scholar] [CrossRef]
- Mavaddat, N.; Ross, S.; Dobbin, A.; Williams, K.; Graffy, J.; Mant, J. Training in positivity for stroke? A qualitative study of acceptability of use of positive mental training (PosMT) as a tool to assist stroke survivors with post-stroke psychological problems and in coping with rehabilitation. NeuroRehabilitation 2017, 40, 259–270. [Google Scholar] [CrossRef]
- Sanjuán, P.; Montalbetti, T.; Pérez-García, A.M.; Bermúdez, J.; Arranz, H.; Castro, A. A randomised trial of a positive intervention to promote well-being in cardiac patients. Appl. Psychol. Health Well-Being 2016, 8, 64–84. [Google Scholar] [CrossRef]
- Gross, J.J. The emerging field of emotion regulation: An integrative review. Rev. Gen. Psychol. 1998, 2, 271–299. [Google Scholar] [CrossRef]
- Gross, J.J. Emotion regulation: Taking stock and moving forward. Emotion 2013, 13, 359–365. [Google Scholar] [CrossRef]
- Spitznagel, M.B.; Potter, V.; Miller, L.A.; Roberts Miller, A.N.; Hughes, J.; Rosneck, J.; Gunstad, J. Ability to regulate emotion is predicted by depressive symptoms and cognitive function in a cardiac sample. J. Cardiovasc. Nurs. 2013, 28, 453–459. [Google Scholar] [CrossRef]
- Haedtke, C.; Smith, M.; Vanburen, J.; Klein, D.; Turvey, C. The relationships among pain, depression, and physical activity in patients with heart failure. J. Cardiovasc. Nurs. 2017, 32, E21–E25. [Google Scholar] [CrossRef]
- Bichara, V.M.; Santillán, J.; de Rosa, R.; Estofan, L. Depresión en insuficiencia cardíaca crónica: Causa o consecuencia. Insuf Card 2016, 11, 173–200. [Google Scholar]
- Cruz-Ramos, N.A.; Alor-Hernández, G.; Colombo-Mendoza, L.O.; Sánchez-Cervantes, J.L.; Rodríguez-Mazahua, L.; Guarneros-Nolasco, L.R. MHealth apps for self-management of cardiovascular diseases: A scoping review. Healthcare 2022, 10, 322. [Google Scholar] [CrossRef]
- Malvey, D.; Slovensky, D.J. MHealth: Transforming Healthcare; Springer: Berlin/Heidelberg, Germany, 2014; ISBN 978-1-4899-7457-0. [Google Scholar]
- Scherrenberg, M.; Wilhelm, M.; Hansen, D.; Völler, H.; Cornelissen, V.; Frederix, I.; Kemps, H.; Dendale, P. The future is now: A call for action for cardiac telerehabilitation in the COVID-19 pandemic from the secondary prevention and rehabilitation section of the european association of preventive cardiology. Eur. J. Prev. Cardiol. 2021, 28, 524–540. [Google Scholar] [CrossRef]
- Chowdhury, R.; Khan, H.; Heydon, E.; Shroufi, A.; Fahimi, S.; Moore, C.; Stricker, B.; Mendis, S.; Hofman, A.; Mant, J.; et al. Adherence to cardiovascular therapy: A meta-analysis of prevalence and clinical consequences. Eur. Heart J. 2013, 34, 2940–2948. [Google Scholar] [CrossRef]
- Klimis, H.; Thakkar, J.; Chow, C.K. Breaking barriers: Mobile health interventions for cardiovascular disease. Can. J. Cardiol. 2018, 34, 905–913. [Google Scholar] [CrossRef]
- Adler, A.J.; Martin, N.; Mariani, J.; Tajer, C.D.; Serrano, N.C.; Casas, J.P.; Perel, P. Mobile phone text messaging to improve adherence to cardiovascular disease secondary prevention interventions. Cochrane Database Syst. Rev. 2015, 2015. [Google Scholar] [CrossRef]
- Palmer, M.J.; Barnard, S.; Perel, P.; Free, C. Mobile phone-based interventions for improving adherence to medication prescribed for the primary prevention of cardiovascular disease in adults. Cochrane Database Syst. Rev. 2017, 2017. [Google Scholar] [CrossRef]
- Hamine, S.; Gerth-Guyette, E.; Faulx, D.; Green, B.B.; Ginsburg, A.S. Impact of mhealth chronic disease management on treatment adherence and patient outcomes: A systematic review. J. Med. Internet Res. 2015, 17, e3951. [Google Scholar] [CrossRef]
- Kebapci, A.; Ozkaynak, M.; Lareau, S.C. Effects of Ehealth-based interventions on adherence to components of cardiac rehabilitation: A systematic review. J. Cardiovasc. Nurs. 2020, 35, 74–85. [Google Scholar] [CrossRef]
- Rathbone, A.L.; Prescott, J. The use of mobile apps and sms messaging as physical and mental health interventions: Systematic review. J. Med. Internet Res. 2017, 19, e7740. [Google Scholar] [CrossRef]
- Firth, J.; Torous, J.; Nicholas, J.; Carney, R.; Pratap, A.; Rosenbaum, S.; Sarris, J. The efficacy of smartphone-based mental health interventions for depressive symptoms: A meta-analysis of randomized controlled trials. World Psychiatry 2017, 16, 287–298. [Google Scholar] [CrossRef]
- Leahy, R.L.; Tirch, D.; Napolitano, L.A. Emotion Regulation in Psychotherapy: A Practitioner’s Guide; Guilford Press: New York, NY, USA, 2011; ISBN 978-1-4625-0237-0. [Google Scholar]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [Green Version]
- Terol, M.C.; López-Roig, S.; Rodríguez-Marín, J.; Martín-Aragón, M.; Pastor, M.A.; Reig, M.T. Propiedades psicométricas de la escala Hospitalaria de Ansiedad y Depresión (HAD) en población española. [Hospital Anxiety and Depression Scale (HAD): Psychometric Properties in Spanish Population.]. Ansiedad Estrés 2007, 13, 163–176. [Google Scholar]
- Caprara, G.V.; Alessandri, G.; Eisenberg, N.; Kupfer, A.; Steca, P.; Caprara, M.G.; Yamaguchi, S.; Fukuzawa, A.; Abela, J. The positivity scale. Psychol. Assess. 2012, 24, 701–712. [Google Scholar] [CrossRef]
- Watson, D.; Clark, L.A.; Tellegen, A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J. Pers. Soc. Psychol. 1988, 54, 1063–1070. [Google Scholar] [CrossRef]
- Tabernero, C.; Chambel, M.J.; Curral, L.; Arana, J.M. The role of task-oriented versus relationshiporiented leadership on normative contract and group performance. Soc. Behav. Personal. 2009, 37, 1391–1404. [Google Scholar] [CrossRef]
- Ritter, P.L.; Lorig, K. The english and spanish self-efficacy to manage chronic disease scale measures were validated using multiple studies. J. Clin. Epidemiol. 2014, 67, 1265–1273. [Google Scholar] [CrossRef]
- Steca, P.; Greco, A.; Cappelletti, E.; Monzani, D.; Pancani, L.; Ferrari, G.; Politi, A.; Gestra, R.; Malfatto, G.; Parati, G.; et al. Cardiovascular management self-efficacy: Psychometric properties of a new scale and its usefulness in a rehabilitation context. Ann. Behav. Med. 2015, 49, 660–674. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.-G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef]
- Huffman, J.C.; Feig, E.H.; Millstein, R.A.; Freedman, M.; Healy, B.C.; Chung, W.-J.; Amonoo, H.L.; Malloy, L.; Slawsby, E.; Januzzi, J.L.; et al. Usefulness of a positive psychology-motivational interviewing intervention to promote positive affect and physical activity after an acute coronary syndrome. Am. J. Cardiol. 2019, 123, 1906–1914. [Google Scholar] [CrossRef]
- Meyerowitz-Katz, G.; Ravi, S.; Arnolda, L.; Feng, X.; Maberly, G.; Astell-Burt, T. Rates of attrition and dropout in app-based interventions for chronic disease: Systematic review and meta-analysis. J. Med. Internet Res. 2020, 22, e20283. [Google Scholar] [CrossRef]
- Gao, Z.; Chen, Z.; Sun, A.; Deng, X. Gender differences in cardiovascular disease. Med. Nov. Technol. Devices 2019, 4, 100025. [Google Scholar] [CrossRef]
- Appleton, A.A.; Buka, S.L.; Loucks, E.B.; Gilman, S.E.; Kubzansky, L.D. Divergent associations of adaptive and maladaptive emotion regulation strategies with inflammation. Health Psychol. 2013, 32, 748–756. [Google Scholar] [CrossRef] [Green Version]
- Gross, J.J.; John, O.P. Individual differences in two emotion regulation processes: Implications for affect, relationships, and well-being. J. Pers. Soc. Psychol. 2003, 85, 348–362. [Google Scholar] [CrossRef]
- Kubzansky, L.D.; Park, N.; Peterson, C.; Vokonas, P.; Sparrow, D. Healthy psychological functioning and incident coronary heart disease: The importance of self-regulation. Arch. Gen. Psychiatry 2011, 68, 400–408. [Google Scholar] [CrossRef]
- Charlson, M.E.; Wells, M.T.; Peterson, J.C.; Boutin-Foster, C.; Ogedegbe, G.O.; Mancuso, C.A.; Hollenberg, J.P.; Allegrante, J.P.; Jobe, J.; Isen, A.M. Mediators and moderators of behavior change in patients with chronic cardiopulmonary disease: The impact of positive affect and self-affirmation. Transl. Behav. Med. 2014, 4, 7–17. [Google Scholar] [CrossRef]
- Nsamenang, S.A.; Hirsch, J.K. Positive psychological determinants of treatment adherence among primary care patients. Prim. Health Care Res. Dev. 2015, 16, 398–406. [Google Scholar] [CrossRef]
- Hoen, P.W.; Denollet, J.; De Jonge, P.; Whooley, M.A. Positive affect and survival in patients with stable coronary heart disease: Findings from the heart and soul study. J. Clin. Psychiatry 2013, 74, 14722. [Google Scholar] [CrossRef]
- DuBois, C.M.; Lopez, O.V.; Beale, E.E.; Healy, B.C.; Boehm, J.K.; Huffman, J.C. Relationships between positive psychological constructs and health outcomes in patients with cardiovascular disease: A systematic review. Int. J. Cardiol. 2015, 195, 265–280. [Google Scholar] [CrossRef]
- Tabernero, C.; Gutiérrez-Domingo, T.; Vecchione, M.; Cuadrado, E.; Castillo-Mayén, R.; Rubio, S.; Arenas, A.; Delgado-Lista, J.; Pérez-Martínez, P.; Luque, B. Correction: A longitudinal study on perceived health in cardiovascular patients: The role of conscientiousness, subjective wellbeing and cardiac self-efficacy. PLoS ONE 2020, 15, e0229582. [Google Scholar] [CrossRef]
- Gomis-Pastor, M.; Mirabet Perez, S.; Roig Minguell, E.; Brossa Loidi, V.; Lopez Lopez, L.; Ros Abarca, S.; Galvez Tugas, E.; Mas-Malagarriga, N.; Mangues Bafalluy, M.A. Mobile health to improve adherence and patient experience in heart transplantation recipients: The MHeart trial. Healthcare 2021, 9, 463. [Google Scholar] [CrossRef]
- Mohammadi, N.; Aghayousefi, A.; Nikrahan, G.R.; Adams, C.N.; Alipour, A.; Sadeghi, M.; Roohafza, H.; Celano, C.M.; Huffman, J.C. A Randomized trial of an optimism training intervention in patients with heart disease. Gen. Hosp. Psychiatry 2018, 51, 46–53. [Google Scholar] [CrossRef]
- Nikrahan, G.R.; Eshaghi, L.; Massey, C.N.; Hemmat, A.; Amonoo, H.L.; Healy, B.; Huffman, J.C. Randomized controlled trial of a well-being intervention in cardiac patients. Gen. Hosp. Psychiatry 2019, 61, 116–124. [Google Scholar] [CrossRef]
- Samayoa, L.; Grace, S.L.; Gravely, S.; Scott, L.B.; Marzolini, S.; Colella, T.J.F. Sex differences in cardiac rehabilitation enrollment: A meta-analysis. Can. J. Cardiol. 2014, 30, 793–800. [Google Scholar] [CrossRef]
- Resurrección, D.M.; Motrico, E.; Rigabert, A.; Rubio-Valera, M.; Conejo-Cerón, S.; Pastor, L.; Moreno-Peral, P. Barriers for nonparticipation and dropout of women in cardiac rehabilitation programs: A systematic review. J. Womens Health 2002 2017, 26, 849–859. [Google Scholar] [CrossRef]

| Experimental Group (n = 34) | Control Group (n = 35) | ||||
|---|---|---|---|---|---|
| M | SD | M | SD | p | |
| PSWB | 3.28 | 0.76 | 3.36 | 0.85 | 0.674 |
| SEMCD | 7.71 | 1.79 | 6.85 | 1.92 | 0.059 |
| CMSES | 4.11 | 0.66 | 4.31 | 0.47 | 0.144 |
| Baseline | Post-Session (Face-to-Face) | Baseline–Post-Session Emotion Regulation | |||||
|---|---|---|---|---|---|---|---|
| M | SD | M | SD | t(33) | p | d | |
| PSWB | 3.28 | 0.76 | 3.93 | 0.68 | −6.60 | <0.001 | 0.57 |
| SEMCD | 7.71 | 1.79 | 8.02 | 2.00 | −1.40 | 0.170 | 1.31 |
| CMSES | 4.11 | 0.66 | 4.26 | 0.67 | −2.42 | 0.021 | 0.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farhane-Medina, N.Z.; Castillo-Mayén, R.; Luque, B.; Rubio, S.J.; Gutiérrez-Domingo, T.; Cuadrado, E.; Arenas, A.; Tabernero, C. A Brief mHealth-Based Psychological Intervention in Emotion Regulation to Promote Positive Subjective Well-Being in Cardiovascular Disease Patients: A Non-Randomized Controlled Trial. Healthcare 2022, 10, 1640. https://doi.org/10.3390/healthcare10091640
Farhane-Medina NZ, Castillo-Mayén R, Luque B, Rubio SJ, Gutiérrez-Domingo T, Cuadrado E, Arenas A, Tabernero C. A Brief mHealth-Based Psychological Intervention in Emotion Regulation to Promote Positive Subjective Well-Being in Cardiovascular Disease Patients: A Non-Randomized Controlled Trial. Healthcare. 2022; 10(9):1640. https://doi.org/10.3390/healthcare10091640
Chicago/Turabian StyleFarhane-Medina, Naima Z., Rosario Castillo-Mayén, Bárbara Luque, Sebastián J. Rubio, Tamara Gutiérrez-Domingo, Esther Cuadrado, Alicia Arenas, and Carmen Tabernero. 2022. "A Brief mHealth-Based Psychological Intervention in Emotion Regulation to Promote Positive Subjective Well-Being in Cardiovascular Disease Patients: A Non-Randomized Controlled Trial" Healthcare 10, no. 9: 1640. https://doi.org/10.3390/healthcare10091640
APA StyleFarhane-Medina, N. Z., Castillo-Mayén, R., Luque, B., Rubio, S. J., Gutiérrez-Domingo, T., Cuadrado, E., Arenas, A., & Tabernero, C. (2022). A Brief mHealth-Based Psychological Intervention in Emotion Regulation to Promote Positive Subjective Well-Being in Cardiovascular Disease Patients: A Non-Randomized Controlled Trial. Healthcare, 10(9), 1640. https://doi.org/10.3390/healthcare10091640








