Lip and Oral Cavity Cancer Burden and Related Risk Factors in China: Estimates and Forecasts from 1990 to 2049
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Data Source
2.2. Statistical Analysis
2.3. Software
3. Results
3.1. Lip and Oral Cavity Cancer Burden in China
3.2. Joinpoint Regression Analysis of the Disease Burden of Lip and Oral Cavity Cancer in China
3.3. Difference in Attributable Risk Factors
3.4. APC Model Analysis of Lip and Oral Cavity Cancer Incidence in China
3.5. Prediction Based on the Bayesian Age Cohort Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B
Appendix C
References
- Siakholak, F.R.; Ghoncheh, M.; Pakzad, R.; Gandomani, H.S.; Ghorat, F.; Salehiniya, H. Epidemiology, incidence and mortality of oral cavity and lips cancer and their relationship with the human development index in the world. Biomed. Res. Ther. 2016, 3, 872–888. [Google Scholar] [CrossRef]
- Mathavan, S.; Kue, C.S.; Kumar, S. Identification of potential candidate genes for lip and oral cavity cancer using network analysis. Genom. Inform. 2021, 19, e4. [Google Scholar] [CrossRef]
- Mathavan, S.; Kumar, S. Identification of potential biomarkers for lip and oral cavity cancer using systems biology approach. In Proceedings of the Asian Regional Conference on Systems Biology (ARCSB2020), Langkawi, Malaysia, 2–4 March 2020. [Google Scholar]
- Pereira, M.C.; Oliveira, D.T.; Landman, G.; Kowalski, L.P. Histologic subtypes of oral squamous cell carcinoma: Prognostic relevance. J. Can. Dent. Assoc. 2007, 73, 339–344. [Google Scholar] [PubMed]
- Stnga, A.C.; Mrgritescu, O.; Stng, A.S.; Pirici, D.; Cruce, M. VEGFR1 and VEGFR2 immunohistochemical expression in oral squamous cell carcinoma: A morphometric study. Rom. J. Morphol. Embryol. 2011, 52, 1269. [Google Scholar]
- Miranda-Filho, A.; Bray, F. Global patterns and trends in cancers of the lip, tongue and mouth. Oral Oncol. 2020, 102, 104551. [Google Scholar] [CrossRef]
- Shrestha, A.D.; Vedsted, P.; Kallestrup, P.; Neupane, D. Prevalence and incidence of oral cancer in low- and middle-income countries: A scoping review. Eur. J. Cancer Care 2019, 29, e13207. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Dyba, T.; Randi, G.; Bettio, M.; Gavin, A.; Visser, O.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur. J. Cancer 2018, 103, 356–387. [Google Scholar] [CrossRef]
- Warnakulasuriya, S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009, 45, 309–316. [Google Scholar] [CrossRef]
- Priya, M.; Lando, H.A. Tobacco control: An issue twinned with oral cancer control. Int. Dent. J. 2015, 64, 229–232. [Google Scholar] [CrossRef]
- Salehiniya, H.; Raei, M. Oral cavity and lip cancer in the world: An epidemiological review. Biomed. Res. Ther. 2020, 7, 3898–3905. [Google Scholar] [CrossRef]
- Gupta, B.; Bray, F.; Kumar, N.; Johnson, N.W. Associations between oral hygiene habits, diet, tobacco and alcohol and risk of oral cancer: A case–control study from India. Cancer Epidemiol. 2017, 51, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.R.; Johnson, N.W.; Pierce, A.M.; Wilson, D.F. The epidemiology of lip cancer: A review of global incidence and aetiology. Oral Dis. 2010, 5, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Radoi, L.; Luce, D. A review of risk factors for oral cavity cancer: The importance of a standardized case definition. Community Dent. Oral Epidemiol. 2012, 41, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Merchant, A.; Husain, S.; Hosain, M.; Fikree, F.F.; Saeed, S.A. Paan without tobacco: An independent risk factor for oral cancer. Int. J. Cancer 2000, 86, 592–593. [Google Scholar] [CrossRef]
- Ribeiro, I.L.A.; de Medeiros, J.J.; Rodrigues, L.V.; Valença, A.M.G.; Neto, E.D.A.L. Factors associated with lip and oral cavity cancer. Rev. Bras. De Epidemiol. 2015, 18, 618–629. [Google Scholar] [CrossRef]
- Freedman, N.D.; Park, Y.; Subar, A.F.; Albert, R. Fruit and vegetable intake and head and neck cancer risk in a large United States prospective cohort study. Int. J. Cancer 2008, 122, 2330–2336. [Google Scholar] [CrossRef]
- Bravi, F.; Bosetti, C.; Filomeno, M.; Levi, F.; Garavello, W.; Galimberti, S.; Negri, E.; LaVecchia, C. Foods, nutrients and the risk of oral and pharyngeal cancer. Br. J. Cancer 2013, 109, 2904–2910. [Google Scholar] [CrossRef]
- Franceschi, S.; Favero, A.; Conti, E.; Talamini, R.; Volpe, R.; Negri, E.; Barzan, L.; Vecchia, C.L. Food groups, oils and butter, and cancer of the oral cavity and pharynx. Br. J. Cancer 1999, 80, 614–620. [Google Scholar] [CrossRef]
- Hashibe, M.; Sankaranarayanan, R.; Thomas, G.; Kuruvilla, B.; Mathew, B.; Somanathan, T.; Parkin, D.M.; Zhang, Z.F. Alcohol drinking, body mass index and the risk of oral leukoplakia in an Indian population. Int. J. Cancer 2000, 88, 129–134. [Google Scholar] [CrossRef]
- Visscher, J.; Waal, I. Etiology of cancer of the lip. Int. J. Oral Maxillofac. Surg. 1998, 27, 199–203. [Google Scholar] [CrossRef]
- Brown, L.M.; Gridley, G.; Diehl, S.R.; Winn, D.M.; Harty, L.C.; Otero, E.B.; Fraumeni, J.F.; Hayes, R.B. Family cancer history and susceptibility to oral carcinoma in Puerto Rico. Cancer 2001, 92, 2102–2108. [Google Scholar] [CrossRef]
- Bräutigam, K.; Meier, S.; Meneder, S.; Proppe, L.; Stroschein, K.; Polack, S.; Köster, F.; Rody, A.; Baum, S. Distribution of HPV Subtypes in Diverse Anogenital and Oral Samples from Women and Correlation of Infections with Neoplasia of the Cervix. Cancers 2022, 14, 3136. [Google Scholar] [CrossRef] [PubMed]
- Rosenblatt, K.A. Marijuana Use and Risk of Oral Squamous Cell Carcinoma. Cancer Res. 2004, 64, 4049–4054. [Google Scholar] [CrossRef] [PubMed]
- Kreimer, A.R.; Viscidi, R.; Pawlita, M.; Fakhry, C.; Koch, W.M.; Westra, W.H.; Gillison, M.L. Case-control study of human papillomavirus and oropharyngeal cancer. Mass. Med. Soc. 2007, 356, 1944–1956. [Google Scholar]
- Giovenco, D.P.; Spillane, T.E.; Maggi, R.M.; Lee, E.Y.; Philbin, M.M. Multi-level drivers of tobacco use and purchasing behaviors during COVID-19 “lockdown”: A qualitative study in the United States. Int. J. Drug Policy 2021, 94, 103175. [Google Scholar] [CrossRef]
- Grossman, E.R.; Benjamin-Neelon, S.E.; Sonnenschein, S. Alcohol Consumption during the COVID-19 Pandemic: A Cross-Sectional Survey of US Adults. Int. J. Environ. Res. Public Health 2020, 17, 9189. [Google Scholar] [CrossRef]
- Robinson, E.; Boyland, E.; Chisholm, A.; Harrold, J.; Maloney, N.G.; Marty, L.; Mead, B.R.; Noonan, R.; Hardman, C.A. Obesity, eating behavior and physical activity during COVID-19 lockdown: A study of UK adults—ScienceDirect. Appetite 2020, 156, 104853. [Google Scholar] [CrossRef]
- Ruiz-Roso, M.B.; Padilha, P.; Mantilla-Escalante, D.C.; Ulloa, N.; Dávalos, A. Covid-19 Confinement and Changes of Adolescent’s Dietary Trends in Italy, Spain, Chile, Colombia and Brazil. Nutrients 2020, 12, 1807. [Google Scholar] [CrossRef]
- Nath, B.S.; Ferreira, B.J.; McVicar, B.A.; Oshilaja, B.T.; Dds, B.S. Rise in oral cancer risk factors associated with the COVID-19 pandemic mandates a more diligent approach to oral cancer screening and treatment. J. Am. Dent. Assoc. 2022, 153, 495–499. [Google Scholar] [CrossRef]
- Tiyuri, A.; Mohammadian-Hafshejani, A.; Iziy, E.; Gandomani, H.S.; Salehiniya, H. The incidence and mortality of lip and oral cavity cancer and its relationship to the 2012 Human Development Index of Asia. Biomed. Res. Ther. 2017, 4, 1147. [Google Scholar] [CrossRef]
- Li, N.; Deng, Y.; Zhou, L.; Tian, T.; Yang, S.; Wu, Y.; Zheng, Y.; Zhai, Z.; Hao, Q.; Song, D.; et al. Global burden of breast cancer and attributable risk factors in 195 countries and territories, from 1990 to 2017: Results from the Global Burden of Disease Study 2017. J. Hematol. Oncol. 2019, 12, 1–12. [Google Scholar] [CrossRef]
- Zhai, Z.; Zheng, Y.; Li, N.; Deng, Y.; Wang, Z. Incidence and disease burden of prostate cancer from 1990 to 2017: Results from the Global Burden of Disease Study 2017: Global disease burden of prostate cancer. Cancer 2020, 126, 1969–1978. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Deng, Y.; Li, N.; Zheng, Y.; Dai, Z. Global, regional, and national burden of Hodgkin lymphoma from 1990 to 2017: Estimates from the 2017 Global Burden of Disease study. J. Hematol. Oncol. 2019, 12, 107. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Wang, M.; Zhou, L.; Zheng, Y.; Dai, Z. Global burden of larynx cancer, 1990-2017: Estimates from the global burden of disease 2017 study. Aging 2020, 12, 2545–2583. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Wang, T.; Yang, J.; Zhang, J.; Lyu, J. GBD database application and data extraction methods and processes. Chin. J. Evid.-Based Cardiovasc. Med. 2019, 11, 1043–1046. [Google Scholar]
- Dereje, N. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar]
- GBD 2019 Risk Factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2021, 396, 1223–1249. [Google Scholar]
- United Nations. World Population Prospects 2019 Highlights. Available online: https://population.un.org/wpp/Download/Standard/CSV/ (accessed on 19 May 2022).
- Kim, H.J.; Fay, M.P.; Feuer, E.J.; Midthune, D.N. Permutation tests for joinpoint regression with applications to cancer rates. Stat. Med. 2000, 19, 335–351. [Google Scholar] [CrossRef]
- Zhu, W.; Wang, Y.; Li, T.; Chen, W.; Wang, W. Gap to End-TB targets in eastern China: A joinpoint analysis from population-based notification data in Zhejiang Province, China, 2005–2018. Int. J. Infect. Dis. 2021, 104, 407–414. [Google Scholar] [CrossRef]
- Bramajo, O.N.; Valk, H. An Age-Period-Cohort Approach to Analyse Late-Life Depression Prevalence in Six European Countries, 2004–2016. Eur. J. Popul. 2022, 38, 223–245. [Google Scholar] [CrossRef]
- Luo, G.; Zhang, Y.; Guo, P.; Wang, L.; Huang, Y.; Li, K. Global patterns and trends in stomach cancer incidence: Age, period and birth cohort analysis. Int. J. Cancer 2017, 141, 1333–1344. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, K.; Righolt, C.H.; Elliott, L.J.; Fanella, S.; Mahmud, S.M. The impact of pertussis vaccine programme changes on pertussis disease burden in Manitoba, 1992–2017—An age-period-cohort analysis. Int. J. Epidemiol. 2022, 51, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Riebler, A.; Held, L. Projecting the future burden of cancer: Bayesian age–period–cohort analysis with integrated nested Laplace approximations. Biom. J. 2017, 59, 531. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zhang, W. China’s Changing Population Structure and Its Implications for US Agricultural Exports; Center for Agricultural and Rural Development (CARD) Publications: Ames, IA, USA, 2021. [Google Scholar]
- Hussein, A.A.; Helder, M.N.; de Visscher, J.G.; Leemans, C.R.; Braakhuis, B.J.; de Vet, H.C.; Forouzanfar, T. Global incidence of oral and oropharynx cancer in patients younger than 45 years versus older patients: A systematic review. Eur. J. Cancer 2017, 82, 115–127. [Google Scholar] [CrossRef]
- Kishore, C.; Prashanti, B. 1375 Age-standardization to world population and under-estimation of oral cancer burden. Int. J. Epidemiol. 2021, 50 (Suppl. S1), dyab168.119. [Google Scholar] [CrossRef]
- Batchelor, P. The changing epidemiology of oral diseases in the elderly, their growing importance for care and how they can be managed. Age Ageing 2015, 44, 1064–1070. [Google Scholar] [CrossRef][Green Version]
- Gómez, I.; Seoane, J.; Varela-Centelles, P.; Diz, P.; Takkouche, B. Is diagnostic delay related to advanced-stage oral cancer? A meta-analysis. Eur. J. Oral Sci. 2010, 117, 541–546. [Google Scholar] [CrossRef]
- Varela-Centelles, P. Early Diagnosis and Diagnostic Delay in Oral Cancer. Cancers 2022, 14, 1758. [Google Scholar] [CrossRef]
- Feng, X.F.; Huang, H.T.; Wang, R. Study on the Patient Delay among Oral Cancer Patients. J. Oral Sci. Res. 2016, 32, 4. [Google Scholar]
- Luo, Y.; Su, B.; Zheng, X. Trends and Challenges for Population and Health During Population Aging—China, 2015–2050. China CDC Wkly. 2021, 3, 7. [Google Scholar] [CrossRef]
- Yang, Y.; Land, K.C. A mixed models approach to the age-period-cohort analysis of repeated cross-section surveys, with an application to data on trends in verbal test scores. Sociol. Methodol. 2006, 36, 75–97. [Google Scholar] [CrossRef]
- Geng, Y.; Zhao, L.; Wang, Y.; Jiang, Y.; Meng, K.; Zheng, D.; Lin, C.P. Competency model for dentists in China: Results of a Delphi study. PLoS ONE 2018, 13, e0194411. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, P.K.; Mathur, P.; Nandakumar, A.; Fitzmaurice, C.; Kumar, G.A.; Mehrotra, R.; Shukla, D.K.; Rath, G.K.; Gupta, P.C.; Swaminathan, R.; et al. The burden of cancers and their variations across the states of India: The Global Burden of Disease Study 1990–2016. Lancet Oncol. 2018, 19, 1289–1306. [Google Scholar] [CrossRef]
- Shield, K.D.; Ferlay, J.; Jemal, A.; Sankaranarayanan, R.; Chaturvedi, A.K.; Bray, F.; Soerjomataram, I. The global incidence of lip, oral cavity, and pharyngeal cancers by subsite in 2012. CA Cancer J. Clin. 2017, 67, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Keyuri, P.; McQueen, K.; Feeley, T.W. The Global Burden of Cancer 2013. JAMA Oncol. 2015, 1, 505. [Google Scholar]
- Ren, Z.; Hu, C.; Lyu, J.; Ji, T. Global, Regional, and National Burdens of Oral Cancer, 1990 to 2017: Results from the Global Burden of Disease Study. Cancer Commun. 2019, 40, 81–92. [Google Scholar] [CrossRef]
- Gu, J.; Song, J.W.; Liu, Y.; Hui, R.; Liu, Y. Diease burden and trend of oral cancer in China from 1990–2019. Chin. Prev. Med. 2022, 23, 457–461. [Google Scholar] [CrossRef]
- Ren, Z.H.; Hu, C.Y.; He, H.; Li, Y.; Lyu, J. Global and regional burden of oral cancer from 1990 to 2017: A report on the Global Burden of Disease Study. Chin. J. Cancer Res. 2020, 39, 159–171. [Google Scholar] [CrossRef]
- Song, S.W.; Xie, L.; Huang, P. Analysis and prediction of mortality risk of lip and oral cavity cancer in China, 1990–2019. Mod. Prev. Med. 2022, 49, 789–793. [Google Scholar]
- Park, J.-O.; Nam, I.-C.; Kim, C.-S.; Park, S.-J.; Lee, D.-H.; Kim, H.-B.; Han, K.-D.; Joo, Y.-H. Sex Differences in the Prevalence of Head and Neck Cancers: A 10-Year Follow-Up Study of 10 Million Healthy People. Cancers 2022, 14, 2521. [Google Scholar] [CrossRef]
- Mehrtash, H.; Duncan, K.; Parascandola, M.; David, A.; Gritz, E.R.; Gupta, P.C.; Mehrotra, R.; Nordin, A.S.A.; Pearlman, P.C.; Warnakulasuriya, S.; et al. Defining a global research and policy agenda for betel quid and areca nut. Lancet Oncol. 2017, 18, e767–e775. [Google Scholar] [CrossRef]
- Warnakulasuriya, S. Causes of oral cancer—An appraisal of controversies. Br. Dent. J. 2009, 207, 471–475. [Google Scholar] [CrossRef]
- Winn, D.M.; Lee, Y.A.; Hashibe, M.; Boffetta, P.; The INHANCE Consortium. The INHANCE consortium: Toward a better understanding of the causes and mechanisms of head and neck cancer. Oral Dis. 2015, 21, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Sung, W.W.; Hsu, Y.C.; Dong, C.; Chen, Y.C.; Chen, C.J. Favorable Lip and Oral Cancer Mortality-to-Incidence Ratios in Countries with High Human Development Index and Expenditures on Health. Int. J. Environ. Res. Public Health 2021, 18, 6012. [Google Scholar] [CrossRef] [PubMed]
- Gupta, B.; Johnson, N.; Kumar, N. Global Epidemiology of Head and Neck Cancers: A Continuing Challenge. Oncology 2016, 91, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.J.; Chung, C.Y.; Kuo, C.C.; Ho, C.K. and Kawachi, I. The influence of work nature and workplace subculture on individual drinking behavior: An exploratory pilot study. Kaohsiung J. Med. Sci. 1996, 12, 339–347. [Google Scholar] [PubMed]
- Clegg, D.; Hevener, A.L.; Moreau, K.L.; Morselli, E.; Criollo, A.; Van Pelt, R.E.; Vieira-Potter, V.J. Sex Hormones and Cardiometabolic Health: Role of Estrogen and Estrogen Receptors. Endocrinology 2017, 158, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Kujan, O.; Oliver, R.; Thakker, N.; Sloan, P.; Glenny, A.-M. Screening programmes for the early detection and prevention of oral cancer. Aust. Dent. J. 2009, 54, 170–172. [Google Scholar] [CrossRef]
- Warnakulasuriya, S.; Kerr, A.R. Oral Cancer Screening: Past, Present, and Future. J. Dent. Res. 2021, 100, 1313–1320. [Google Scholar] [CrossRef]
- Peng, Y.; Liu, J.; Xu, K.; Liu, X.; Wang, J.; Liao, X. Incidence and Mortality of Oral Cancer in Registered Regions of Hunan in 2009–2015. China Cancer 2019, 28, 680–688. [Google Scholar] [CrossRef]
- Liu, G.; Yang, L.; Fu, Z.; Xu, A.; Guo, X. Incidence and mortality of oral cancer in Shandong Province in 2014. J. Shandong Univ. 2019, 57, 102–107. [Google Scholar]
- Bai, X.; Bai, X.; Zhao, D.; Zhang, J. Analysis of primary oral squamous cell carcinoma in Beijing inhabitants: A 5-year continuous study of a single center. J. Pract. Stomatol. 2017, 33, 657–660. [Google Scholar]

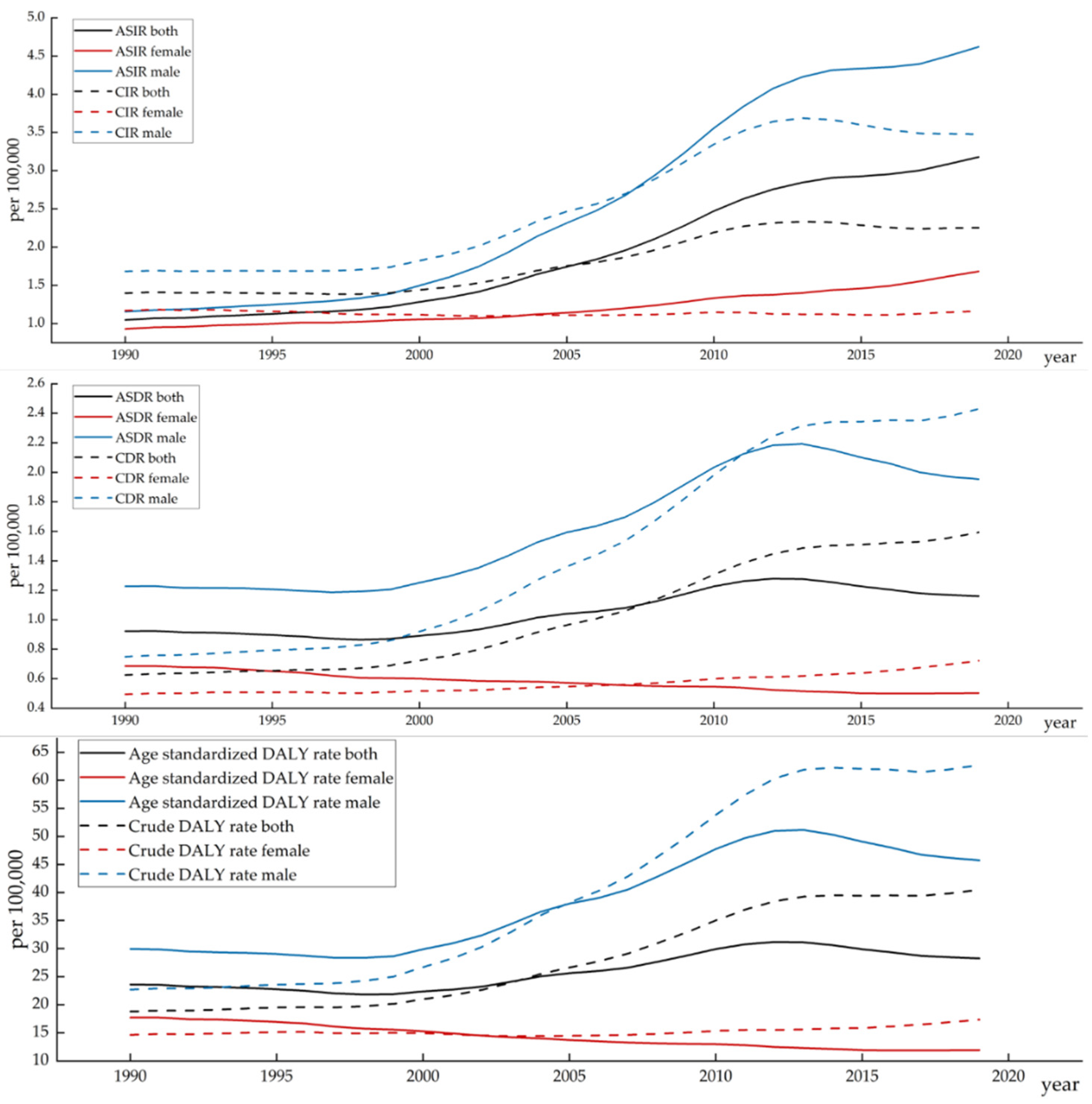

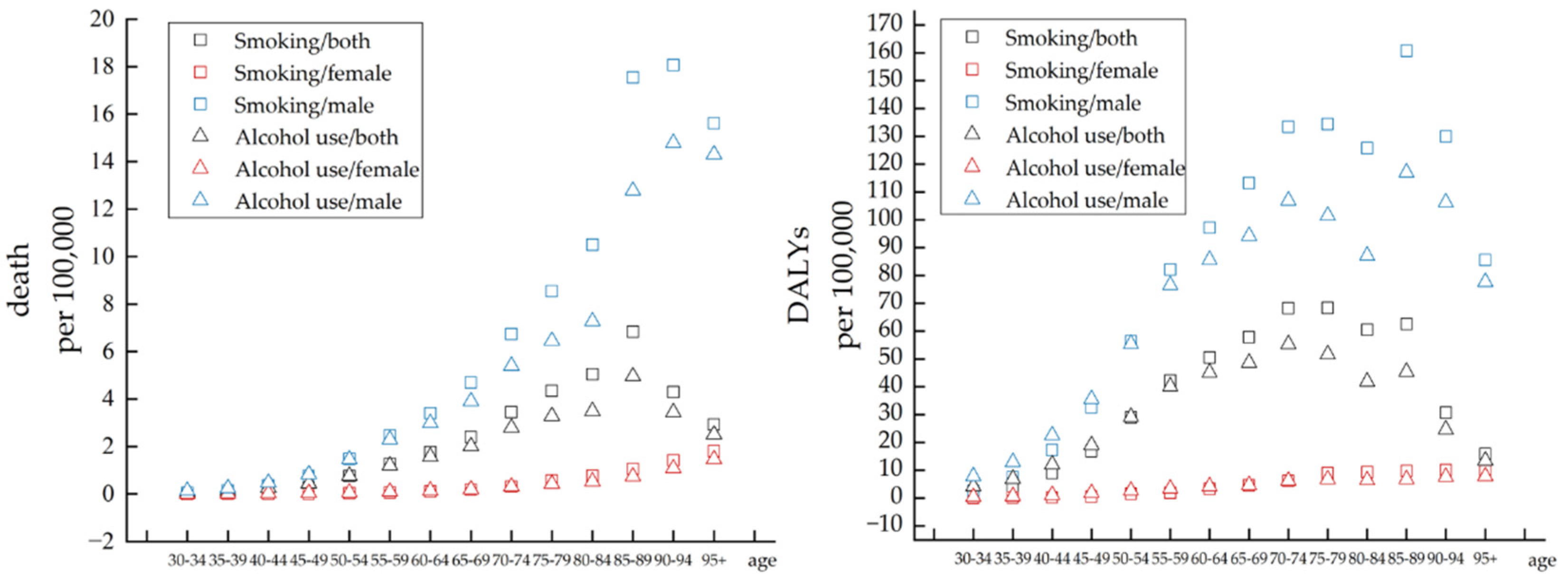

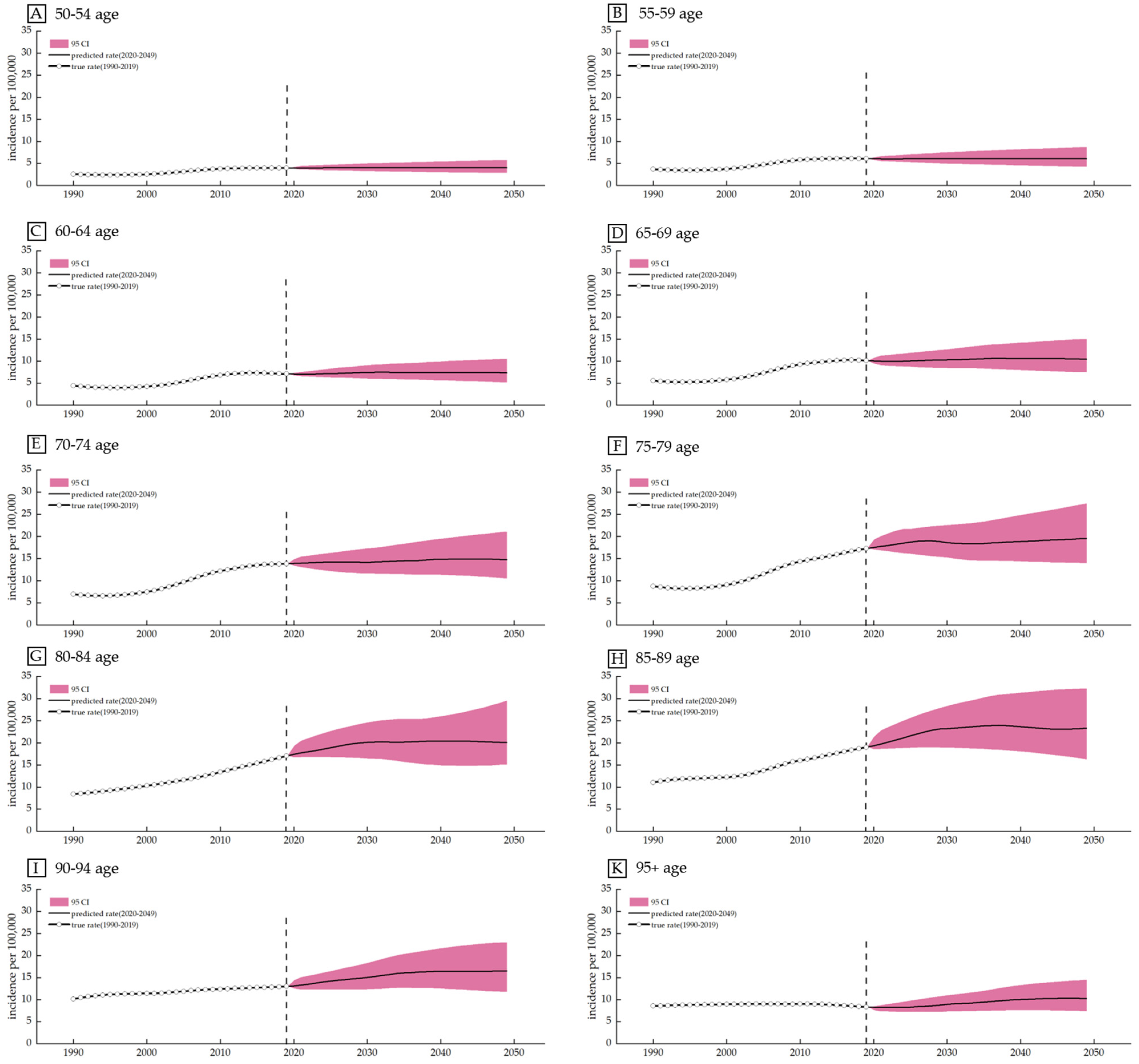
| Measure | Sex | Trend 1 | Trend 2 | Trend 3 | Trend 4 | Trend 5 | Trend 6 | 1900–2019 AAPC (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Years | APC | Years | APC | Years | APC | Years | APC | Years | APC | Years | APC | |||
| Age-Standardized incidence Rate | Both | 1990–1998 | −0.2 | 1998–2001 | 2.1 | 2001–2012 | 4.3 * | 2012–2019 | −0.7 * | NA | NA | NA | NA | 1.6 * (1.3–1.9) |
| Female | 1990–1993 | 0.1 | 1993–2001 | −0.8 * | 2001–2007 | 0.1 | 2007–2010 | 1.0 | 2010–2016 | −0.5 * | 2016–2019 | 1.6 * | −0.0(−0.2–0.2) | |
| Male | 1990–1999 | 0.2 | 1999–2012 | 6.1 * | 2012–2019 | −1.0 * | NA | NA | NA | NA | NA | NA | 2.5 * (2.4–2.6) | |
| Age-Standardized death Rate | Both | 1990–1999 | −0.9 * | 1999–2012 | 3.1 * | 2012–2019 | −1.6 * | NA | NA | NA | NA | NA | NA | 0.7 * (0.6–0.9) |
| Female | 1990–1993 | −0.5 | 1993–1998 | −2.1 * | 1998–2011 | −1.0 * | 2011–2015 | −1.8 * | 2015–2019 | 0.1 | NA | NA | −1.1 * (−1.2~−0.9) | |
| Male | 1990–1999 | −0.4 * | 1999–2012 | 4.9 * | 2012–2019 | −1.8 * | NA | NA | NA | NA | NA | NA | 1.6 * (1.5–1.7) | |
| Age-Standardized DALY Rate | Both | 1990–1999 | −1.0 * | 1999–2007 | 2.7 * | 2007–2012 | 3.3 * | 2012–2019 | −1.7 * | NA | NA | NA | NA | 0.6 * (0.4–0.7) |
| Female | 1990–1994 | −0.8 * | 1994–2006 | −2.1 * | 2006–2010 | −0.9 * | 2010–2015 | −1.7 * | 2015–2019 | 0.0 | NA | NA | −1.4 * (−1.5~−1.2) | |
| Male | 1990–1999 | −0.6 * | 1999–2012 | 4.7 * | 2012–2019 | −1.9 * | NA | NA | NA | NA | NA | NA | 1.5 * (1.3–1.6) | |
| Incidence | Female | Male | ||
|---|---|---|---|---|
| Coef. (95% CI) | p > z | Coef. (95% CI) | p > z | |
| Age (years) | ||||
| 50–54 | −3.039 (−3.129, −2.949) | 0.000 | −6.695 (−7.221, −6.169) | 0.000 |
| 55–59 | −2.442 (−2.525, −2.36) | 0.000 | −6.068 (−6.552, −5.583) | 0.000 |
| 60–64 | −2.144 (−2.228, −2.06) | 0.000 | −6.405 (−6.898, −5.912) | 0.000 |
| 65–69 | −1.384 (−1.469, −1.298) | 0.000 | −4.337 (−4.837, −3.838) | 0.000 |
| 70–74 | −0.192 (−0.278, −0.107) | 0.000 | −1.248 (−1.749, −0.746) | 0.000 |
| 75–79 | 1.333 (1.247, 1.419) | 0.000 | 1.603 (1.101, 2.105) | 0.000 |
| 80–84 | 1.511 (1.426, 1.597) | 0.000 | 2.488 (1.987, 2.989) | 0.000 |
| 85–89 | 2.353 (2.269, 2.438) | 0.000 | 10.596 (10.1, 11.092) | 0.000 |
| 90–94 | 2.736 (2.653, 2.82) | 0.000 | 9.363 (8.875, 9.851) | 0.000 |
| 95+ | 1.268 (1.181, 1.355) | 0.000 | 0.704 (0.195, 1.213) | 0.007 |
| Period (year) | ||||
| 1992 | −0.531 (−0.592, −0.47) | 0.000 | −5.69 (−6.045, −5.334) | 0.000 |
| 1997 | −0.485 (−0.547, −0.423) | 0.000 | −4.993 (−5.357, −4.629) | 0.000 |
| 2002 | −0.172 (−0.234, −0.109) | 0.000 | −3.087 (−3.452, −2.721) | 0.000 |
| 2007 | 0.201 (0.139, 0.263) | 0.000 | 0.829 (0.466, 1.192) | 0.000 |
| 2012 | 0.424 (0.363, 0.485) | 0.000 | 6.245 (5.888, 6.602) | 0.000 |
| 2017 | 0.563 (0.499, 0.628) | 0.000 | 6.696 (6.318, 7.074) | 0.000 |
| Cohort (year) | ||||
| 1892–1896 | 1.458 (1.267, 1.648) | 0.000 | 4.091 (2.974, 5.208) | 0.000 |
| 1897–1901 | 1.267 (1.127, 1.407) | 0.000 | 2.162 (1.344, 2.98) | 0.000 |
| 1902–1906 | 1.201 (1.082, 1.32) | 0.000 | 1.108 (0.41, 1.805) | 0.002 |
| 1907–1911 | 0.76 (0.652, 0.867) | 0.000 | 0.654 (0.026, 1.283) | 0.041 |
| 1912–1916 | 0.353 (0.254, 0.452) | 0.000 | −0.059 (−0.639, 0.52) | 0.842 |
| 1917–1921 | 0.12 (0.029, 0.211) | 0.010 | 1.143 (0.609, 1.677) | 0.000 |
| 1922–1926 | 0.028 (−0.067, 0.122) | 0.564 | 2.307 (1.756, 2.859) | 0.000 |
| 1927–1931 | −0.013 (−0.108, 0.081) | 0.781 | 2.54 (1.986, 3.094) | 0.000 |
| 1932–1936 | −0.11 (−0.202, −0.017) | 0.020 | 2.236 (1.693, 2.778) | 0.000 |
| 1937–1941 | −0.248 (−0.336, −0.159) | 0.000 | 1.752 (1.235, 2.268) | 0.000 |
| 1942–1946 | −0.429 (−0.523, −0.335) | 0.000 | 0.729 (0.177, 1.28) | 0.010 |
| 1947–1951 | −0.721 (−0.823, −0.62) | 0.000 | −1.014 (−1.609, −0.42) | 0.001 |
| 1952–1956 | −1.011 (−1.124, −0.898) | 0.000 | −3.47 (−4.131, −2.809) | 0.000 |
| 1957–1961 | −1.212 (−1.346, −1.078) | 0.000 | −6.266 (−7.051, −5.481) | 0.000 |
| 1962–1966 | −1.442 (−1.665, −1.22) | 0.000 | −7.913 (−9.214, −6.612) | 0.000 |
| Constance | 5.795 (5.759, 5.83) | 0.000 | 13.704 (13.497, 13.91) | 0.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Lu, Y.; Li, H.; Zhang, N.; He, R.; Zhang, R.; Mao, Y.; Zhu, B. Lip and Oral Cavity Cancer Burden and Related Risk Factors in China: Estimates and Forecasts from 1990 to 2049. Healthcare 2022, 10, 1611. https://doi.org/10.3390/healthcare10091611
Zhang J, Lu Y, Li H, Zhang N, He R, Zhang R, Mao Y, Zhu B. Lip and Oral Cavity Cancer Burden and Related Risk Factors in China: Estimates and Forecasts from 1990 to 2049. Healthcare. 2022; 10(9):1611. https://doi.org/10.3390/healthcare10091611
Chicago/Turabian StyleZhang, Jingya, Yongbo Lu, Haoran Li, Ning Zhang, Rongxin He, Ruhao Zhang, Ying Mao, and Bin Zhu. 2022. "Lip and Oral Cavity Cancer Burden and Related Risk Factors in China: Estimates and Forecasts from 1990 to 2049" Healthcare 10, no. 9: 1611. https://doi.org/10.3390/healthcare10091611
APA StyleZhang, J., Lu, Y., Li, H., Zhang, N., He, R., Zhang, R., Mao, Y., & Zhu, B. (2022). Lip and Oral Cavity Cancer Burden and Related Risk Factors in China: Estimates and Forecasts from 1990 to 2049. Healthcare, 10(9), 1611. https://doi.org/10.3390/healthcare10091611







