Changes in the Epidemiology of Diabetic Retinopathy in Spain: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Material and Methods
2.1. Literature Research and Study Selection
2.2. Inclusion Criteria
- Studies of patients with T2DM in Spain published from 1 January 2001 to 31 December 2020;
- Population-based studies, cross-sectional or longitudinal type;
- Studies on screening of DR in Spain; Studies that have provided data on the incidence or prevalence of DR in Spain;
- The studies must have provided a clear definition of DM made by general practitioner or endocrinologist;
- The studies must have provided a clear definition of DR.
2.3. Exclusion Criteria
2.4. Data Extraction
2.5. Quality Assessment
2.6. Diabetic Retinopathy Definition and Assessment
2.7. Statistical Analysis
3. Results
3.1. Selection of Articles and Documents
3.2. Study of DR Prevalence
3.3. Statistical Analysis of Prevalence Studies
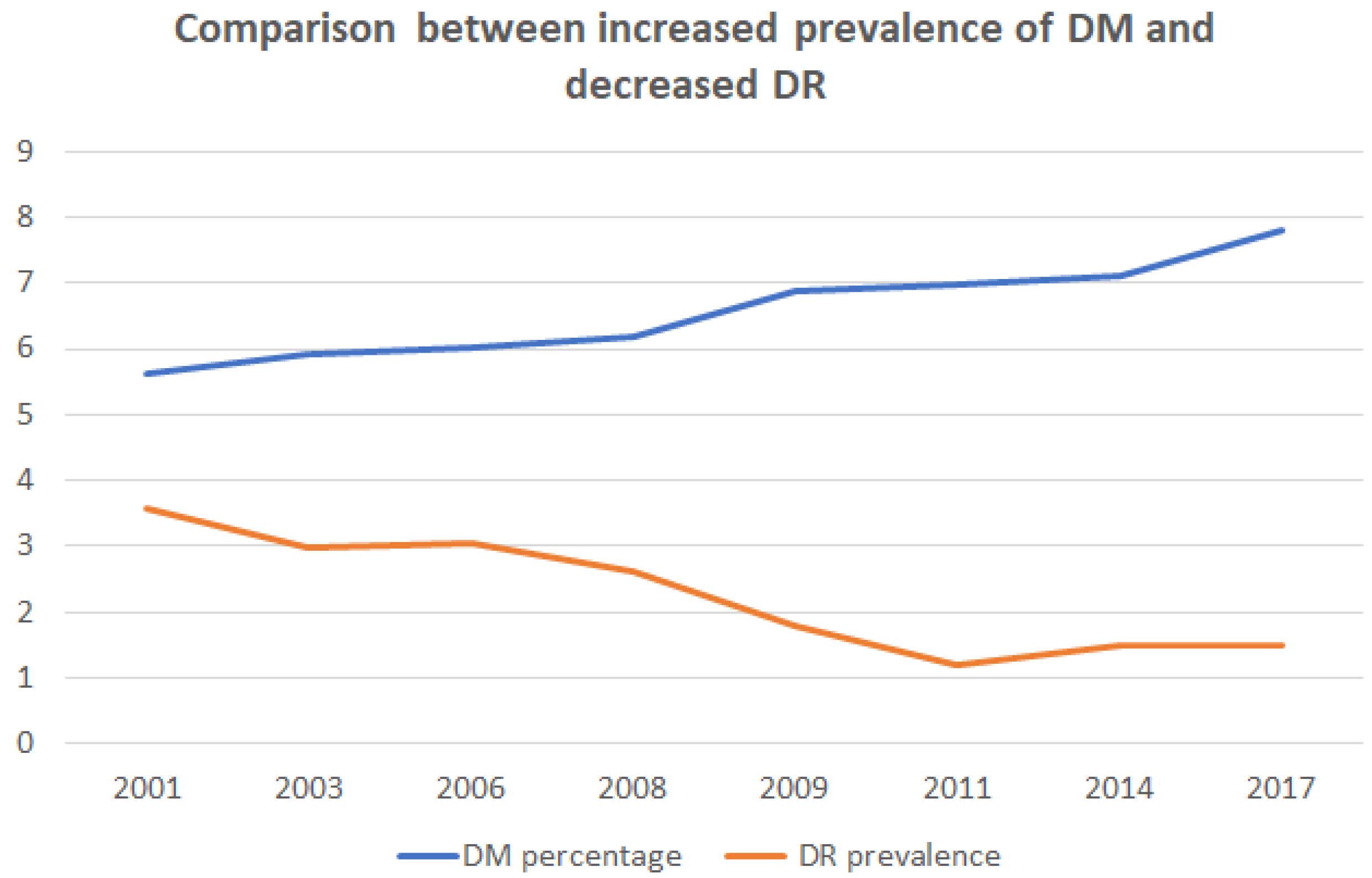
3.4. Relation between DR Prevalence and DM Diagnosis
3.5. Results with Longitudinal Studies of the Application of DR Screening Programs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Diabetes federation. IDF Diabetes Atlas, 10th ed.; 2021; Available online: https://diabetesatlas.org/atlas/tenth-edition/ (accessed on 10 July 2022).
- Soriguer, F.; Goday, A.; Bosch-Comas, A.; Bordiú, E.; Calle-Pascual, A.; Carmena, R.; Casamitjana, R.; Castaño, L.; Castell, C.; Catalá, M.; et al. Prevalence of diabetes mellitus and impaired glucose regulation in Spain: The Di@bet.es Study. Diabetologia 2012, 55, 88–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rojo-Martínez, G.; Valdés, S.; Soriguer, F.; Vendrell, J.; Urrutia, I.; Pérez, V.; Ortega, E.; Ocón, P.; Montanya, E.; Menéndez, E.; et al. Incidence of diabetes mellitus in Spain as results of the nation-wide cohort di@bet.es study. Sci. Rep. 2020, 10, 2765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourne, R.R.A.; Jonas, J.B.; Bron, A.M.; Cicinelli, M.V.; Das, A.; Flaxman, S.R.; Friedman, D.; Keeffe, J.E.; Kempen, J.H.; Leasher, J.; et al. Prevalence and causes of vision loss in high-income countries and in Eastern and Central Europe in 2015: Magnitude, temporal trends and projections. Br. J. Ophthalmol. 2018, 102, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.S. Diabetic retinopathy screening: A systematic review of the economic evidence. Diabet. Med. 2010, 27, 249–256. [Google Scholar]
- American Diabetes Association. Section 12, retinopathy, neuropathy, and foot care: Standards of medical care in diabetes. Diabetes Care 2022, 45, S185–S194. [Google Scholar] [CrossRef]
- Ghanchi, F. Diabetic Retinopathy Guidelines Working Group. The Royal College of Ophthalmologists’ clinical guidelines for diabetic retinopathy: A summary. Eye 2013, 7, 285–287. [Google Scholar] [CrossRef] [Green Version]
- Wong, T.Y.; Sun, J.; Kawasaki, R.; Ruamviboonsuk, P.; Gupta, N.; Lansingh, V.C.; Maia, M.; Mathenge, W.; Moreker, S.; Muqit, M.M.; et al. Guidelines on Diabetic Eye Care: The International Council of Ophthalmology Recommendations for Screening, Follow-up, Referral, and Treatment Based on Resource Settings. Ophthalmology 2018, 125, 1608–1622. [Google Scholar] [CrossRef] [Green Version]
- Sistema Nacional de Salud, Estrategia en Diabetes. Estrategia aprobada por el Consejo Interterritorial del Sistema Nacional de Salud el 11 de octubre de 2006. Plan de Calidad para el Sistema Nacional de Salud; Ministerio de Sanidad y Consumo: Madrid, Spain, 2007; Available online: https://www.sanidad.gob.es/organizacion/sns/planCalidadSNS/pdf/excelencia/cuidadospaliativos-. (accessed on 10 July 2022).
- Estrategia en Diabetes aprobada en El Consejo Interterritorial del Sistema Nacional de Salud, en el Pleno del 29 de febrero de 2012, Esta Estrategia en Diabetes del Sistema Nacional de Salud. Actualización. Año 2012. Available online: https://www.mscbs.gob.es/organizacion/sns/planCalidadSNS/pdf/excelencia/cuidadospaliativos-diabetes/DIABETES/Estrategia_en_diabetes_del_SNS_Accesible.pdf (accessed on 24 April 2022).
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 350, g7647. [Google Scholar] [CrossRef] [Green Version]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology [STROBE] statement: Guidelines for reporting observational studies. Gac. Sanit. 2008, 22, 144–150. [Google Scholar] [CrossRef] [Green Version]
- Early Treatment Diabetic Retinopathy Study Research Group. Grading diabetic retinopathy from stereoscopic color fundus photographsdan extension of the modified Airlie House classification. ETDRS report number 10. Ophthalmology 1991, 98, 786e806. [Google Scholar]
- Wilkinson, C.P.; Ferris, F.L., 3rd; Klein, R.E.; Lee, P.P.; Agardh, C.D.; Davis, M.; Dills, D.; Kampik, A.; Pararajasegaram, R.; Verdaguer, J.T.; et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 2003, 110, 1677–1682. [Google Scholar] [CrossRef]
- MetaXL, a Software Program for Meta-Analysis in Microsoft Excel. Available online: http://epigear.com/index_files/metaxl.html (accessed on 20 April 2022).
- Santos-Bueso, E.; Fernández-Pérez, C.; Macarro, A.; Fernández-Vigo, J. Prevalencia de retinopatía diabética en la ciudad de Badajoz 2002 (proyecto extremadura para prevención de la ceguera) [Prevalence of diabetic retinopathy in the city of Badajoz 2002 (Extremadura project to prevent blindness)]. Arch. Soc. Esp. Oftalmol. 2007, 82, 153–158. (In Spanish) [Google Scholar] [CrossRef] [Green Version]
- Santos Bueso, E.; Fernández-Vigo, J.; Fernández Pérez, C.; Macarro Merino, A.; Fernández Perianes, J. Prevalencia de retinopatía diabética en la Comunidad Autónoma de Extremadura. 1997-2001 (Proyecto Extremadura para Prevención de la Ceguera) [Prevalence of diabetic retinopathy in the Regional Comunity of Extremadura. 1997-2001 (Extremadura Project to Prevent Blindness)]. Arch. Soc. Esp. Oftalmol. 2005, 80, 187–194. (In Spanish) [Google Scholar] [CrossRef] [PubMed]
- Teruel Macias, C.; Fernández-Real, J.M.; Ricart, W.; Valent Ferrer, R.; Vallés Prats, M. Prevalencia de la retinopatía diabética en la población de diabéticos diagnosticados en las comarcas de Girona. Estudio de los factores asociados [Prevalence of diabetic retinopathy in the region of Girona. Study of related factors]. Arch. Soc. Esp. Oftalmol. 2005, 80, 85–91. (In Spanish) [Google Scholar] [CrossRef]
- Romero Aroca, P.; Sagarra Alamo, R.; Baget Bernaldiz, M.; Fernandez Ballart, J.; Mendez Marin, I. Prevalence and relationship between diabetic retinopathy and nephropathy, and its risk factors in the North-East of Spain, a population-based study. Ophthalmic Epidemiol. 2010, 17, 251–265. [Google Scholar] [CrossRef]
- Martínez Rubio, M.; Moya Moya, M.; Bellot Bernabé, A.; Belmonte Martínez, J. Cribado de retinopatía diabética y teleoftalmología [Diabetic retinopathy screening and teleophthalmology]. Arch. Soc. Esp. Oftalmol. 2012, 87, 392–395. (In Spanish) [Google Scholar] [CrossRef]
- Rodríguez Villa, S.; Alonso Álvarez, C.; de Dios Del Valle, R.; Salazar Méndez, R.; Cuesta García, M.; Ruiz García, M.J.; Cubillas Martín, M.; Rodríguez Vazquez, M. Five-year experience of tele-ophthalmology for diabetic retinopathy screening in a rural population. Arch. Soc. Esp. Oftalmol. 2016, 91, 426–430, (In English, Spanish). [Google Scholar] [CrossRef]
- López, M.; Cos, F.X.; Álvarez-Guisasola, F.; Fuster, E. Prevalence of diabetic retinopathy and its relationship with glomerular filtration rate and other risk factors in patients with type 2 diabetes mellitus in Spain. DM2 HOPE study. J. Clin. Transl. Endocrinol. 2017, 9, 61–65. [Google Scholar] [CrossRef]
- Castillo-Otí, J.M.; Cañal-Villanueva, J.; García-Unzueta, M.T.; Galván-Manso, A.I.; Callejas-Herrero, M.R.; Muñoz-Cacho, P. Prevalencia y factores de riesgo asociados a la retinopatía diabética en Santander. Norte de España [Prevalence and risk factors associated with diabetic retinopathy in Santander. Northern Spain]. Aten. Primaria 2020, 52, 29–37. (In Spanish) [Google Scholar] [CrossRef]
- Rufas-Ribas, C. Programa Para el Cribado Poblacional de la Retinopatía Diabética. Experiencia en el Sector Sanitario de Barbastro. Ph.D. Thesis, Medicine and Surgery for Universitat Autònoma de Barcelona, Barcelona, Spain, 2019. Available online: https://www.tdx.cat/handle/10803/669398?show=full (accessed on 10 July 2022).
- Valpuesta Martin, Y.; Pacheco Callirgos, G.E.; Maroto Martín, T.M.; Piriz Veloso, M.; Hernández Santamaría, S.; López Gálvez, M.I. Satisfaction of patients and primary care professionals with a teleophthalmology-based screening programme for diabetic retinopathy in a rural area in Castilla y León, Spain. Rural Remote Health 2020, 20, 5180. [Google Scholar] [CrossRef] [Green Version]
- Evolución de la Población con Diabetes en España. Available online: https://www.epdata.es/porcentaje-poblacion-mayor-quince-anos-diabetes-espana/2d866cbd-e61f-4be9-b183-332973c43dfc (accessed on 10 March 2022).
- Rodriguez-Acuña, R.; Mayoral, E.; Aguilar-Diosdado, M.; Rave, R.; Oyarzabal, B.; Lama, C.; Carriazo, A.; Martinez-Brocca, M.A. Andalusian program for early detection of diabetic retinopathy: Implementation and 15-year follow-up of a population-based screening program in Andalusia, Southern Spain. BMJ Open Diabetes Res. Care 2020, 8, e00162. [Google Scholar] [CrossRef] [PubMed]
- Retisalud programa de Detección y Seguimiento de la Retinopatía Diabética a Través de Telemedicina Proporciona Cuidados Oftalmológicos a Distancia. Available online: www.unidaddocentemfyclaspalmas.org.es (accessed on 11 March 2022).
- Guia de Pràctica Clínica per a L’abordatge de la Diabetes. Available online: https://portal.guiasalud.es/gpc/abordatge-de-la-diabetis-mellitus-tipus-2/ (accessed on 10 July 2022).
- Cartera de Servicios Estandarizados de Atención Primaria. Servicio Madrileño de Salud de la Comunidad de Madrid. Revisión 2009. Available online: http://www.madrid.org/cs/Satellite?c=CMPublicacionesFA&cid=1142521116585&idTema=1109265603988&language=es&pagename=ComunidadMarid%2FEstructura&pid=1109181527641&segmento=1&sm=111 (accessed on 10 July 2022).
- Andonegui, J.; Serrano, L.; Eguzkiza, A.; Berástegui, L.; Jiménez-Lasanta, L.; Aliseda, D.; Gaminde, I. Diabetic retinopathy screening using tele-ophthalmology in a primary care setting. J. Telemed. Telecare 2010, 16, 429–432. [Google Scholar] [CrossRef] [PubMed]
- Spain Statistics Institute. Population by Communities and Autonomous Cities and Size of Municipalities. 2021. Available online: https://www.ine.es/jaxiT3/Datos.htm?t=2915 (accessed on 17 April 2022).
- Department of Health. Modern Standards and Service Models: National Service Framework for Diabetes in England and Wales. London: DoH. 2001. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/198836/National_Service_Framework_for_Diabetes.pdf (accessed on 14 April 2022).
- Scanlon, P.H. The contribution of the English NHS Diabetic Eye Screening Programme to reductions in diabetes-related blindness, comparisons within Europe, and future challenges. Acta Diabetol. 2021, 58, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Romero-Aroca, P.; Sagarra-Alamo, R.; Pareja-Rios, A.; López, M. Importance of telemedicine in diabetes care: Relationships between family physicians and ophthalmologists. World J. Diabetes 2015, 6, 1005–1008. [Google Scholar] [CrossRef]
- Andonegui, J.; Zurutuza, A.; de Arcelus, M.P.; Serrano, L.; Eguzkiza, A.; Auzmendi, M.; Gaminde, I.; Aliseda, D. Diabetic retinopathy screening with non-mydriatic retinography by general practitioners: 2-year results. Prim. Care Diabetes 2012, 6, 201–205. [Google Scholar] [CrossRef]
- Vargas-Sánchez, C.; Maldonado-Valenzuela, J.J.; Pérez-Durillo, F.T.; González-Calvo, J.; Pérez-Milena, A. Cribado de retinopatía diabética mediante retinografía midriática en atención primaria [Coverage and results of a screening program for diabetic retinopathy using mydriatic retinography in primary health care]. Salud. Publica Mex. 2011, 53, 212–219. [Google Scholar]
- Romero, P.; Sagarra, R.; Ferrer, J.; Fernández-Ballart, J.; Baget, M. The incorporation of family physicians in the assessment of diabetic retinopathy by non-mydriatic fundus camera. Diabetes Res. Clin. Pract. 2010, 88, 184–188. [Google Scholar] [CrossRef]
- Romero-Aroca, P.; Sagarra-Alamo, R.; Basora-Gallisa, J.; Basora-Gallisa, T.; Baget-Bernaldiz, M.; Bautista-Perez, A. Prospective comparison of two methods of screening for diabetic retinopathy by nonmydriatic fundus camera. Clin. Ophthalmol. 2010, 4, 1481–1488. [Google Scholar] [CrossRef] [Green Version]
- Burgmann, K.; Fatio, S.; Jordi, B.; Rutishauser, J. Medical care of type 2 diabetes mellitus in light of international and national recommendations: A retrospective analysis. Swiss. Med. Wkly. 2013, 143, w13871. [Google Scholar] [CrossRef]
- Salinero-Fort, M.Á.; San Andrés-Rebollo, F.J.; de Burgos-Lunar, C.; Arrieta-Blanco, F.J.; Gómez-Campelo, P.; MADIABETES Group. Four-year incidence of diabetic retinopathy in a Spanish cohort: The MADIABETES study. PLoS ONE 2013, 8, e76417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Poncelas, A.; Miravet-Jiménez, S.; Casellas, A.; Barrot-De La Puente, J.F.; Franch-Nadal, J.; López-Simarro, F.; Mata-Cases, M.; Mundet-Tudurí, X. Prevalence of diabetic retinopathy in individuals with type 2 diabetes who had recorded diabetic retinopathy from retinal photographs in Catalonia (Spain). Br. J. Ophthalmol. 2015, 99, 1628–1633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pareja-Ríos, A.; Bonaque-González, S.; Serrano-García, M.; Cabrera-López, F.; Abreu-Reyes, P.; Marrero-Saavedra, M.D. Tele-ophthalmology for diabetic retinopathy screening: 8 years of experience. Arch. Soc. Esp. Oftalmol. 2017, 92, 63–70, (In English, Spanish). [Google Scholar] [CrossRef] [PubMed]
- Romero-Aroca, P.; de la Riva-Fernandez, S.; Valls-Mateu, A.; Sagarra-Alamo, R.; Moreno-Ribas, A.; Soler, N. Changes observed in diabetic retinopathy: Eight-year follow-up of a Spanish population. Br. J. Ophthalmol. 2016, 100, 1366–1371. [Google Scholar] [CrossRef] [Green Version]
- Huemer, J.; Wagner, S.K.; Sim, D.A. The Evolution of Diabetic Retinopathy Screening Programmes: A Chronology of Retinal Photography from 35 Mm Slides to Artificial Intelligence. Available online: https://www.dovepress.com/the-evolution-ofdiabetic-retinopathy-screening-programmes-a-chronolog-peer-reviewed-fulltext-article-OPTH (accessed on 18 February 2022).
- Scanlon, P.H. The English National Screening Programme for diabetic retinopathy 2003–2016. Acta Diabetol. 2017, 54, 515–525. [Google Scholar] [CrossRef] [Green Version]
- Thomas, R.L.; Dunstan, F.D.; Luzio, S.D.; Chowdhury, S.R.; North, R.V.; Hale, S.L.; Gibbins, R.L.; Owens, D.R. Prevalence of diabetic retinopathy within a national diabetic retinopathy screening service. Br. J. Ophthalmol. 2015, 99, 64–68. [Google Scholar] [CrossRef] [Green Version]
- Looker, H.C.; Nyangoma, S.O.; Cromie, D.T.; Olson, J.A.; Leese, G.P.; Black, M.W.; Doig, J.; Lee, N.; Lindsay, R.S.; McKnight, J.A.; et al. Rates of Referable Eye Disease in the Scottish National Diabetic Retinopathy Screening Programme. Br. J. Ophthalmol. 2017, 98, 790–795. Available online: https://bjo.bmj.com/content/98/6/790 (accessed on 18 February 2022). [CrossRef] [Green Version]
- Rubino, A.; Rousculp, M.D.; Davis, K.; Wang, J.; Girach, A. Diagnosed diabetic retinopathy in France, Italy, Spain, and the United Kingdom. Prim. Care Diabetes 2007, 1, 75–80. [Google Scholar] [CrossRef]
- Hristova, E.; Koseva, D.; Zlatarova, Z.; Dokova, K. Diabetic Retinopathy Screening and Registration in Europe-Narrative Review. Healthcare 2021, 9, 745. [Google Scholar] [CrossRef]
- Andersen, N.; Hjortdal, J.Ø.; Schielke, K.C.; Bek, T.; Grauslund, J.; Laugesen, C.S.; Lund-Andersen, H.; Cerqueira, C.; Andresen, J. The Danish Registry of Diabetic Retinopathy. Clin. Epidemiol. 2016, 8, 613–619. [Google Scholar] [CrossRef] [Green Version]
- Laatikainen, L.; Ojamo, M.; Rudanko, S.L.; Summanen, P.; Keinänen-Kiukaanniemi, S.; Tuomilehto, J.; Herrala, S.; Uusitalo, H. Improving visual prognosis of the diabetic patients during the past 30 years based on the data of the Finnish Register of Visual Impairment. Acta Ophthalmol. 2016, 94, 226–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- James, M.; Goodchild, C.; Bashir, S.; Mannix, M. Report on the creation of a diabetes register and retinopathy screening outcomes in the Mid-West of Ireland. Ir. J. Med. Sci. 2016, 185, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Martín-Merino, E.; Fortuny, J.; Rivero-Ferrer, E.; García-Rodríguez, L.A. Incidence of retinal complications in a cohort of newly diagnosed diabetic patients. PLoS ONE 2014, 9, e100283. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Q.; Welchowski, T.; Schmid, M.; Letow, J.; Wolpers, C.; Pascual-Camps, I.; Holz, F.G.; Finger, R.P. Prevalence, incidence and future projection of diabetic eye disease in Europe: A systematic review and meta-analysis. Eur. J. Epidemiol. 2020, 35, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.L.; Halim, S.; Gurudas, S.; Sivaprasad, S.; Owens, D.R. IDF Diabetes Atlas: A review of studies utilising retinal photography on the global prevalence of diabetes related retinopathy between 2015 and 2018. Diabetes Res. Clin. Pract. 2019, 157, 107840. [Google Scholar] [CrossRef] [PubMed]
- Dutra Medeiros, M.; Mesquita, E.; Gardete-Correia, L.; Moita, J.; Genro, V.; Papoila, A.L.; Amaral-Turkman, A.; Raposo, J.F. First Incidence and Progression Study for Diabetic Retinopathy in Portugal, the RETINODIAB Study: Evaluation of the Screening Program for Lisbon Region. Ophthalmology 2015, 122, 2473–2481. [Google Scholar] [CrossRef] [Green Version]
- Cheyne, C.P.; Burgess, P.I.; Broadbent, D.M.; García-Fiñana, M.; Stratton, I.M.; Criddle, T.; Wang, A.; Alshukri, A.; Rahni, M.M.; Vazquez-Arango, P.; et al. Incidence of sight-threatening diabetic retinopathy in an established urban screening programme: An 11-year cohort study. Diabet. Med. 2021, 38, e14583. [Google Scholar] [CrossRef]
- Nevill, C.R.; Stratton, I.M.; Maruti, S.S.; Massó-González, E.L.; Sivaprasad, S.; Bailey, C.; Ehrlich, M.; Chong, V.; Scanlon, P.H. Epidemi-ology of moderately severe and severe non-proliferative diabetic retinopathy in South West England. Eye 2022, 36, 433–440. [Google Scholar] [CrossRef]

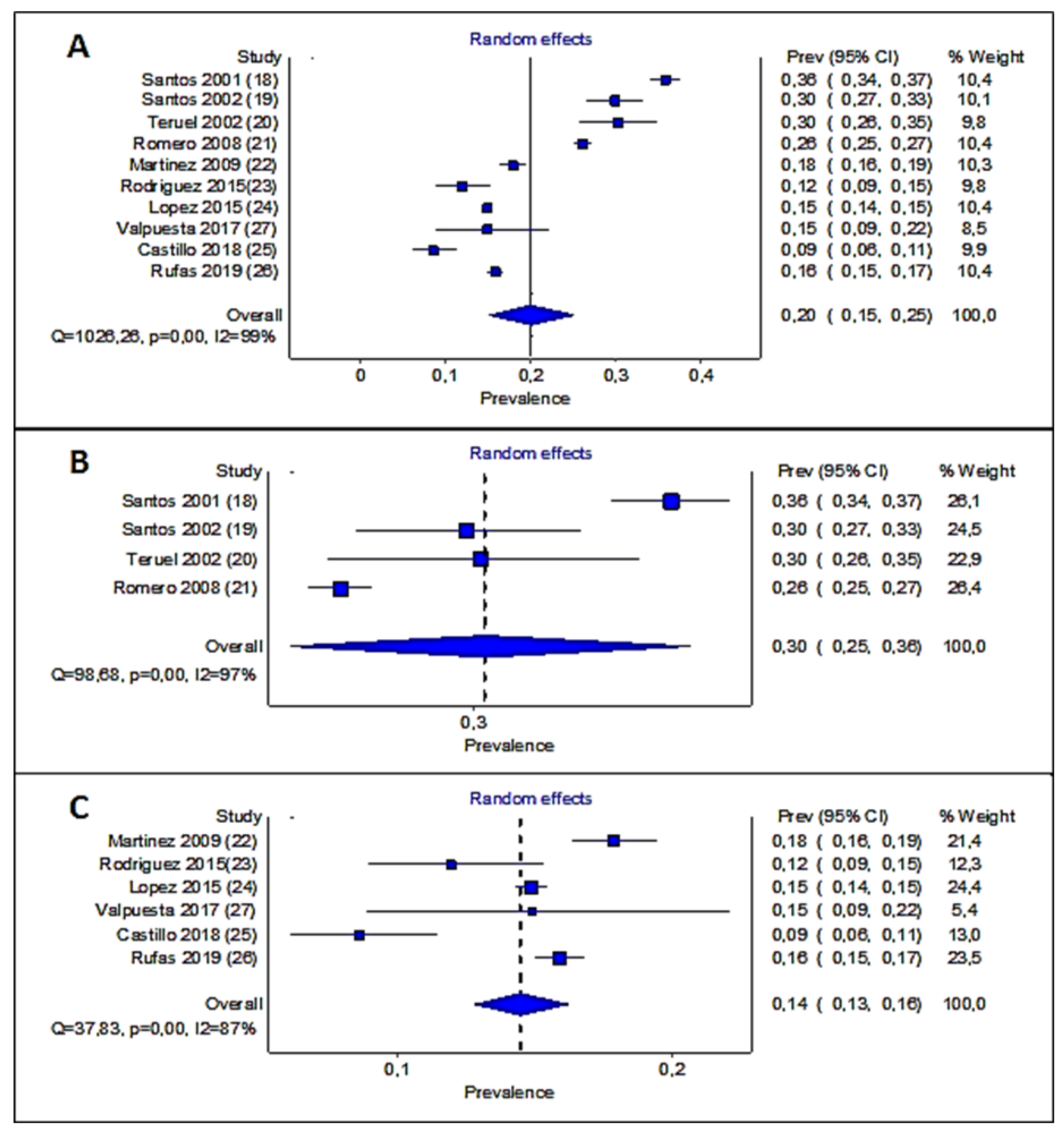

| Autor | Santos et al. | Santos et al. | Teruel et al. | Romero et al. | Martinez-Rubio M | Rodriguez-Villas et al. | Lopez et al. | Castillo et al. | Rufas et al. | Valpuesta et al. | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Year * | 2001 | 2002 | 2002 | 2008 | 2009 | 2015 | 2015 | 2015 | 2015 | 2019 | |
| Patients with DR/ Total patients | 1112/3114 | 226/762 | 121/401 | 2123/8187 | 436/2435 | 47/394 | 2126/ | 38/442 | 1001/6294 | 17/114 | |
| Patients with STDR/Total patients | 165/3114 | 37/762 | 10/401 | 162/8187 | 56/2435 | ND | ND | 8/442 | ND | 2/114 | |
| Mean age | 63.2 ± 13.4 | 66.2 ± 11.4 | ND | 64.6 ± 10.78 | ND | 70.4 ± 11.64 | 64.3 ± 11.2 | 70 ± 10.6 | 70.3 | 68.69 ± 9.85 | |
| Duration of DM in years | 12.75 ± 6.7 | 13.81 ± 5.4 | 14.54 ± 7.2 | 12.42 ± 6.3 | ND | 9.02 ± 2.08 | 9.0 ± 7,1 | 11.7 ± 7 | 11 | 9.8 ± 8.43 | |
| M/W % | 37.6/62.4 | 39.2/60.8 | 49/51 | 55.21/44.79 | 56.46/43.54 | 57.9/42.1 | 48.1/51.9 | 55.9/44.1 | 56/44 | 58.8/41.2 | |
| DM treatment | ND | ||||||||||
| Diet | 19.4 | 18.2 | 22 | 16.94 | 6.1 | 12.4 | 8.1 | 5.5 | |||
| Oral | 49.5 | 52.82 | 51.99 | 51.12 | 73.8 | 67.3 | 51.5 | 76.3 | |||
| IT / IT± oral | 31.1 | 29 | 32.01 | 31.54 | 20.1 | 20.3 | 40.8 | 18.4 | |||
| Arterial hypertension | 47.2 | 36 | 49 | 68.36 | ND | 71 | 74.1 | 78.8 | 69 | ND | |
| HbA1c | ND | ND | ND | 7.34 ± 1.23% | ND | 7.23 ± 1.34% | 7.30% | 6.92 ± 0.98% | 6.8 | ND | |
| Diabetic retinopathy | |||||||||||
| DR prevalence | 35.70% | 29.80% | 30.30% | 26.11% | 17.90% | 12.05% | 14.9 | 8.56% | 15.90% | 15% | |
| STDR prevalence | 5.30% | 4.80% | 2.50% | 1.98% | 2.29% | ND | ND | 1.81% | ND | 1.74%. | |
| 10-Year Follow-Up | 5-Year Follow-Up | 8-Year Follow-Up | ||||
|---|---|---|---|---|---|---|
| Author | Rodriguez-Acuña et al. | Salinero et al. | Rodriguez-Poncelas et al. | Rufas et al. | Pareja-Ríos et al. | Romero-Aroca et al. |
| Study dates * | 2008–2018 | 2007–2011 | 2008–2012 | 2010–2015 | 2011–2019 | 2008–2015 |
| Patients with DR / Total patients | / | 194/2405 | / | 808/4276 | / | 2462/ |
| DM duration at baseline (years) | 6.4 ± 6.9 | 7.7 | 7.6 ± 5.6 | 11 | ND ** | 7.37 ± 6.92 |
| Women/Men at baseline (%) | 54.6/45.4 | 39.2/60.8 | 43.8/56.2 | 56/44 | ND | 42.7/57.3 |
| Age at baseline (years) | 62.8 ± 12.8 | 67.5 ± 10.6 | 66.91 ± 11 | 70.3 | ND | 64.74 ± 12.39 |
| Diabetic retinopathy | ||||||
| Interval between visits | 2.9 | ND | ND | 2.8 | 2.7 | 2.5 |
| Cumulative incidence *** | 12.2% at 10 years | 8.1% at 5 years | 12.2% at 5 years | 15.9% at 5 years | 19.9% at 8 years | 16% at 8 years |
| Annual incidence of DR **** | 4.45% | 2.01% | 2.47% | 3.2% | 6.89 | 4.43% |
| Annual incidence of STDR | 0.45% | ND | 0.35% | ND | ND | 0.44% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero-Aroca, P.; López-Galvez, M.; Martinez-Brocca, M.A.; Pareja-Ríos, A.; Artola, S.; Franch-Nadal, J.; Fernandez-Ballart, J.; Andonegui, J.; Baget-Bernaldiz, M. Changes in the Epidemiology of Diabetic Retinopathy in Spain: A Systematic Review and Meta-Analysis. Healthcare 2022, 10, 1318. https://doi.org/10.3390/healthcare10071318
Romero-Aroca P, López-Galvez M, Martinez-Brocca MA, Pareja-Ríos A, Artola S, Franch-Nadal J, Fernandez-Ballart J, Andonegui J, Baget-Bernaldiz M. Changes in the Epidemiology of Diabetic Retinopathy in Spain: A Systematic Review and Meta-Analysis. Healthcare. 2022; 10(7):1318. https://doi.org/10.3390/healthcare10071318
Chicago/Turabian StyleRomero-Aroca, Pedro, Maribel López-Galvez, Maria Asuncion Martinez-Brocca, Alicia Pareja-Ríos, Sara Artola, Josep Franch-Nadal, Joan Fernandez-Ballart, José Andonegui, and Marc Baget-Bernaldiz. 2022. "Changes in the Epidemiology of Diabetic Retinopathy in Spain: A Systematic Review and Meta-Analysis" Healthcare 10, no. 7: 1318. https://doi.org/10.3390/healthcare10071318
APA StyleRomero-Aroca, P., López-Galvez, M., Martinez-Brocca, M. A., Pareja-Ríos, A., Artola, S., Franch-Nadal, J., Fernandez-Ballart, J., Andonegui, J., & Baget-Bernaldiz, M. (2022). Changes in the Epidemiology of Diabetic Retinopathy in Spain: A Systematic Review and Meta-Analysis. Healthcare, 10(7), 1318. https://doi.org/10.3390/healthcare10071318






