Effects of Early Initiation of High-Dose Dexamethasone Therapy on Pro-Inflammatory Cytokines and Mortality in LPS-Challenged Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Animals
2.2. Experimental Designs
2.2.1. Survival of LPS-Challenged Mice
2.2.2. Cytokine Analysis of LPS-Challenged Mice
2.3. Statistical Analysis
3. Results
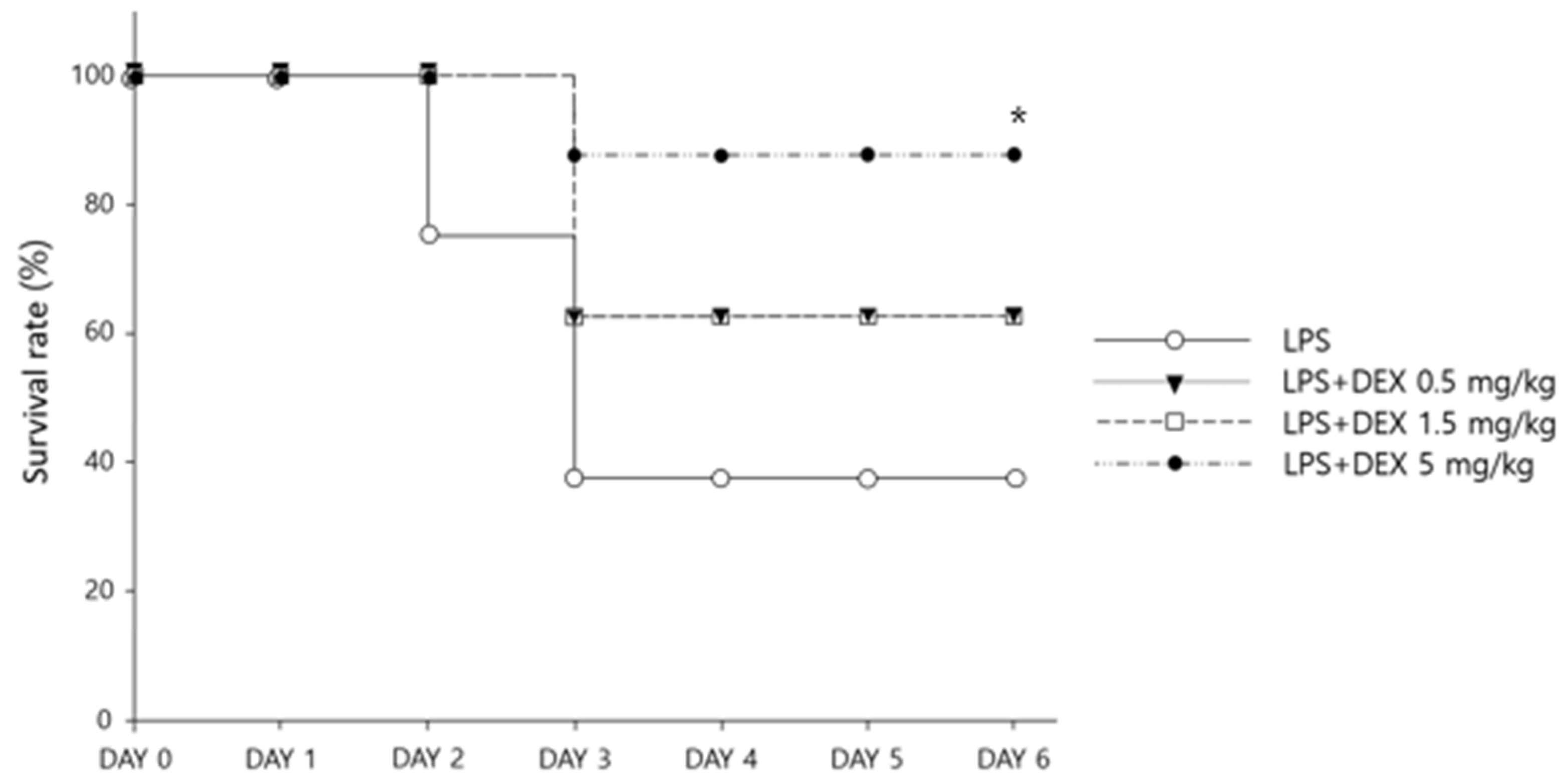
3.1. Survival and Weight Changes with Dexamethasone Treatment
3.2. Changes in Cytokine Levels with Dexamethasone Administration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hotchkiss, R.S.; Moldawer, L.L.; Opal, S.M.; Reinhart, K.; Turnbull, I.R.; Vincent, J.L. Sepsis and septic shock. Nat. Rev. Dis. Primers 2016, 2, 16045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martín, S.; Pérez, A.; Aldecoa, C. Sepsis and immunosenescence in the elderly patient: A review. Front. Med. 2017, 4, 20. [Google Scholar] [CrossRef] [PubMed]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [Green Version]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; McIntyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Crit. Care Med. 2021, 49, e1063–e1143. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthy, S.; Cidlowski, J.A. Corticosteroids: Mechanisms of action in health and disease. Rheum. Dis. Clin. N. Am. 2016, 42, 15–31. [Google Scholar] [CrossRef] [Green Version]
- Samuel, S.; Nguyen, T.; Choi, H.A. Pharmacologic characteristics of corticosteroids. J. Neurocrit. Care 2017, 10, 53–59. [Google Scholar] [CrossRef] [Green Version]
- Dellinger, R.P.; Levy, M.M.; Carlet, J.M.; Bion, J.; Parker, M.M.; Jaeschke, R.; Reinhart, K.; Angus, D.C.; Brun-Buisson, C.; Beale, R.; et al. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock: 2008. Crit. Care Med. 2008, 36, 296–327. [Google Scholar] [CrossRef] [Green Version]
- Son, J.Y.; Shin, S.; Choi, Y.J. New Evidence of potential benefits of dexamethasone and added on therapy of fludrocortisone on clinical outcomes of corticosteroid in sepsis patients: A systematic review and meta-analysis. J. Pers. Med. 2021, 11, 544. [Google Scholar] [CrossRef]
- Minneci, P.C.; Deans, K.J.; Eichacker, P.Q.; Natanson, C. The effects of steroids during sepsis depend on dose and severity of illness: An updated meta-analysis. Clin. Microbiol. Infect. 2009, 15, 308–318. [Google Scholar] [CrossRef] [Green Version]
- Marik, P.E.; Pastores, S.M.; Annane, D.; Meduri, G.U.; Sprung, C.L.; Arlt, W.; Keh, D.; Briegel, J.; Beishuizen, A.; Dimopoulou, I.; et al. Recommendations for the diagnosis and management of corticosteroid insufficiency in critically ill adult patients: Consensus statements from an international task force by the American College of Critical Care Medicine. Crit. Care Med. 2008, 36, 1937–1949. [Google Scholar] [CrossRef]
- Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; Elmahi, E.; et al. Dexamethasone in hospitalized patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [PubMed]
- COVID-19 Treatment Guidelines. Therapeutic Management of Hospitalized Adults with COVID-19. 2021. Available online: https://www.covid19treatmentguidelines.nih.gov/management/clinical-management/hospitalized-adults--therapeutic-management/ (accessed on 2 February 2022).
- Sacha, G.L.; Chen, A.Y.; Palm, N.M.; Duggal, A. Evaluation of the initiation timing of hydrocortisone in adult patients with septic shock. Shock 2021, 55, 488–494. [Google Scholar] [CrossRef] [PubMed]
- El-Nawawy, A.; Khater, D.; Omar, H.; Wali, Y. Evaluation of early corticosteroid therapy in management of pediatric septic shock in pediatric intensive care patients: A Randomized clinical study. Pediatr. Infect. Dis. J. 2017, 36, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Youssef, J.; Novosad, S.A.; Winthrop, K.L. Infection risk and safety of corticosteroid use. Rheum. Dis. Clin. N. Am. 2016, 42, 157–176. [Google Scholar] [CrossRef] [Green Version]
- UpToDate. Comparison of Systemic Glucocorticoid Preparations, 2021. Available online: https://www.uptodate.com/contents/image/print?imageKey=ENDO%2F64138&topicKey=ANEST%2F94256&source=outline (accessed on 6 June 2021).
- Seemann, S.; Zohles, F.; Lupp, A. Comprehensive comparison of three different animal models for systemic inflammation. J. Biomed. Sci. 2017, 24, 60. [Google Scholar] [CrossRef]
- Piirsalu, M.; Taalberg, E.; Lilleväli, K.; Tian, L.; Zilmer, M.; Vasar, E. Treatment with lipopolysaccharide induces distinct changes in metabolite profile and body weight in 129Sv and Bl6 mouse strains. Front. Pharmacol. 2020, 11, 371. [Google Scholar] [CrossRef]
- Granger, J.I.; Ratti, P.L.; Datta, S.C.; Raymond, R.M.; Opp, M.R. Sepsis-induced morbidity in mice: Effects on body temperature, body weight, cage activity, social behavior and cytokines in brain. Psychoneuroendocrinology 2013, 38, 1047–1057. [Google Scholar] [CrossRef] [Green Version]
- Osuchowski, M.F.; Welch, K.; Yang, H.; Siddiqui, J.; Remick, D.G. Chronic sepsis mortality characterized by an individualized inflammatory response. J. Immunol. 2007, 179, 623–630. [Google Scholar] [CrossRef]
- Acheampong, A.; Vincent, J.L. A positive fluid balance is an independent prognostic factor in patients with sepsis. Crit. Care 2015, 19, 251. [Google Scholar] [CrossRef] [Green Version]
- Arnau-Barrés, I.; Pascual-Dapena, A.; López-Montesinos, I.; Gómez-Zorrilla, S.; Sorlí, L.; Herrero, M.; Nogués, X.; Navarro-Valls, C.; Ibarra, B.; Canchucaja, L.; et al. Severe hypoalbuminemia at admission is strongly associated with worse prognosis in older adults with SARS-CoV-2 infection. J. Clin. Med. 2021, 10, 5134. [Google Scholar] [CrossRef]
- Cao, C.; Yu, M.; Chai, Y. Pathological alteration and therapeutic implications of sepsis-induced immune cell apoptosis. Cell Death Dis. 2019, 10, 782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rehman, A.; Baloch, N.U.; Morrow, J.P.; Pacher, P.; Haskó, G. Targeting of G-protein coupled receptors in sepsis. Pharmacol. Ther. 2020, 211, 107529. [Google Scholar] [CrossRef] [PubMed]
- Kiers, D.; Koch, R.M.; Hamers, L.; Gerretsen, J.; Thijs, E.J.; van Ede, L.; Riksen, N.P.; Kox, M.; Pickkers, P. Characterization of a model of systemic inflammation in humans in vivo elicited by continuous infusion of endotoxin. Sci. Rep. 2017, 7, 40149. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Siddiqui, J.; Remick, D.G. Mechanisms of mortality in early and late sepsis. Infect. Immun. 2006, 74, 5227–5235. [Google Scholar] [CrossRef] [Green Version]
- Oh, S.-Y.; Cho, S.; Lee, H.; Chang, E.J.; Min, S.H.; Ryu, H.G. Sepsis in patients receiving immunosuppressive drugs in Korea: Analysis of the National Insurance Database from 2009 to 2013. Korean J. Crit. Care Med. 2015, 30, 249–257. [Google Scholar] [CrossRef]
- Park, Y.J.; Lee, M.J.; Bae, J.; Lee, J.H.; Lee, H.A.R.; Mun, S.; Kim, Y.S.; Yune, C.J.; Chung, T.N.; Kim, K. Effects of glucocorticoid therapy on sepsis depend both on the dose of steroids and on the severity and phase of the animal sepsis model. Life 2022, 12, 421. [Google Scholar] [CrossRef]
- Vincent, J.L. The clinical challenge of sepsis identification and monitoring. PLoS Med. 2016, 13, e1002022. [Google Scholar] [CrossRef] [Green Version]
- Gibbison, B.; López-López, J.A.; Higgins, J.P.; Miller, T.; Angelini, G.D.; Lightman, S.L.; Annane, D. Corticosteroids in septic shock: A systematic review and network meta-analysis. Crit. Care 2017, 21, 78. [Google Scholar] [CrossRef] [Green Version]
- Sprung, C.L.; Caralis, P.V.; Marcial, E.H.; Pierce, M.; Gelbard, M.A.; Long, W.M.; Duncan, R.C.; Tendler, M.D.; Karpf, M. The effects of high-dose corticosteroids in patients with septic shock. A prospective, controlled study. N. Engl. J. Med. 1984, 311, 1137–1143. [Google Scholar] [CrossRef]
- Wang, S.; Tan, K.S.; Beng, H.; Liu, F.; Huang, J.; Kuai, Y.; Zhang, R.; Tan, W. Protective effect of isosteviol sodium against LPS-induced multiple organ injury by regulating of glycerophospholipid metabolism and reducing macrophage-driven inflammation. Pharmacol. Res. 2021, 172, 105781. [Google Scholar] [CrossRef]
- Ono, S.; Tsujimoto, H.; Hiraki, S.; Aosasa, S. Mechanisms of sepsis-induced immunosuppression and immunological modification therapies for sepsis. Ann. Gastroenterol. Surg. 2018, 2, 351–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otto, G.P.; Sossdorf, M.; Claus, R.A.; Rödel, J.; Menge, K.; Reinhart, K.; Bauer, M.; Riedemann, N.C. The late phase of sepsis is characterized by an increased microbiological burden and death rate. Crit. Care 2011, 15, R183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marik, P.E.; Farkas, J.D. The changing paradigm of sepsis: Early diagnosis, early antibiotics, early pressors, and early adjuvant treatment. Crit. Care Med. 2018, 46, 1690–1692. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Whiteman, M.; Moore, P.K. Dexamethasone inhibits lipopolysaccharide-induced hydrogen sulphide biosynthesis in intact cells and in an animal model of endotoxic shock. J. Cell. Mol. Med. 2009, 13, 2684–2692. [Google Scholar] [CrossRef]
- Mihailidou, I.; Pelekanou, A.; Pistiki, A.; Spyridaki, A.; Tzepi, I.M.; Damoraki, G.; Giamarellos-Bourboulis, E.J. Dexamethasone down-regulates expression of triggering receptor expressed on myeloid cells-1: Evidence for a TNFα-related effect. Front. Public Health 2013, 1, 50. [Google Scholar] [CrossRef] [Green Version]
- Chuang, T.Y.; Cheng, A.J.; Chen, I.T.; Lan, T.Y.; Huang, I.H.; Shiau, C.W.; Hsu, C.L.; Liu, Y.W.; Chang, Z.F.; Tseng, P.H.; et al. Suppression of LPS-induced inflammatory responses by the hydroxyl groups of dexamethasone. Oncotarget 2017, 8, 49735–49748. [Google Scholar] [CrossRef]
- Al Shoyaib, A.; Archie, S.R.; Karamyan, V.T. Intraperitoneal route of drug administration: Should it be used in experimental animal studies? Pharm. Res. 2019, 37, 12. [Google Scholar] [CrossRef]
- Loew, D.; Schuster, O.; Graul, E.H. Dose-dependent pharmacokinetics of dexamethasone. Eur. J. Clin. Pharmacol. 1986, 30, 225–230. [Google Scholar] [CrossRef]
- Williams, D.M. Clinical pharmacology of corticosteroids. Respir. Care 2018, 63, 655–670. [Google Scholar] [CrossRef] [Green Version]
- Husabø, G.; Nilsen, R.M.; Flaatten, H.; Solligård, E.; Frich, J.C.; Bondevik, G.T.; Braut, G.S.; Walshe, K.; Harthug, S.; Hovlid, E. Early diagnosis of sepsis in emergency departments, time to treatment, and association with mortality: An observational study. PLoS ONE 2020, 15, e0227652. [Google Scholar] [CrossRef]
- Kim, H.I.; Park, S. Sepsis: Early recognition and optimized treatment. Tuberc. Respir. Dis. 2019, 82, 6–14. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Son, J.-y.; Kwack, W.G.; Chung, E.K.; Shin, S.; Choi, Y.J. Effects of Early Initiation of High-Dose Dexamethasone Therapy on Pro-Inflammatory Cytokines and Mortality in LPS-Challenged Mice. Healthcare 2022, 10, 1247. https://doi.org/10.3390/healthcare10071247
Son J-y, Kwack WG, Chung EK, Shin S, Choi YJ. Effects of Early Initiation of High-Dose Dexamethasone Therapy on Pro-Inflammatory Cytokines and Mortality in LPS-Challenged Mice. Healthcare. 2022; 10(7):1247. https://doi.org/10.3390/healthcare10071247
Chicago/Turabian StyleSon, Ji-young, Won Gun Kwack, Eun Kyoung Chung, Sooyoung Shin, and Yeo Jin Choi. 2022. "Effects of Early Initiation of High-Dose Dexamethasone Therapy on Pro-Inflammatory Cytokines and Mortality in LPS-Challenged Mice" Healthcare 10, no. 7: 1247. https://doi.org/10.3390/healthcare10071247
APA StyleSon, J.-y., Kwack, W. G., Chung, E. K., Shin, S., & Choi, Y. J. (2022). Effects of Early Initiation of High-Dose Dexamethasone Therapy on Pro-Inflammatory Cytokines and Mortality in LPS-Challenged Mice. Healthcare, 10(7), 1247. https://doi.org/10.3390/healthcare10071247





