Low-Volume Squat Jump Training Improves Functional Performance Independent of Myofibre Changes in Inactive Young Male Individuals
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Subjects
2.3. Plyometric Control and Training Groups
2.4. Biochemical Parameters
2.5. Functional Test
2.6. Muscle Biopsy and Sample Preparation
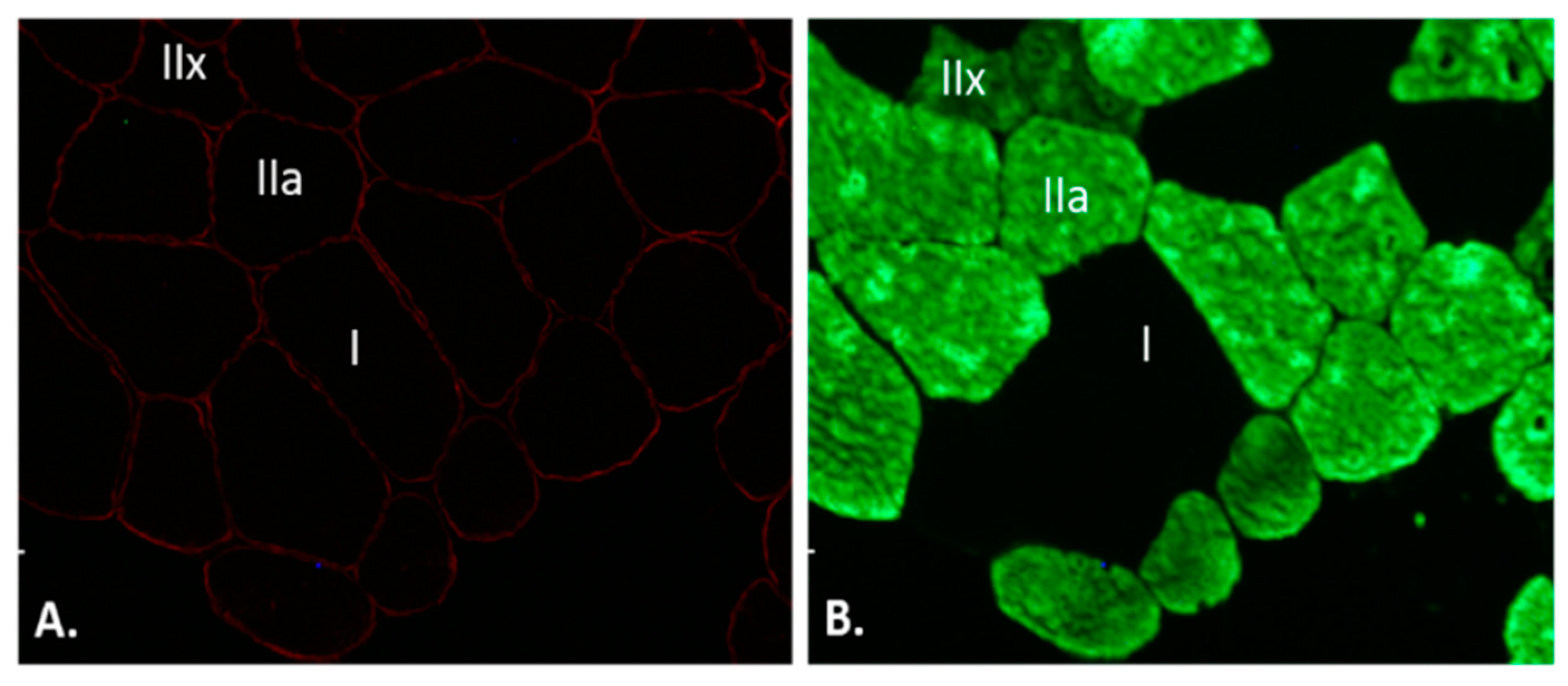
2.7. Immunofluorescence Staining
2.8. Quantitative Image Analysis
2.9. Statistical Analysis
3. Results
3.1. Blood Markers of Muscle Damage
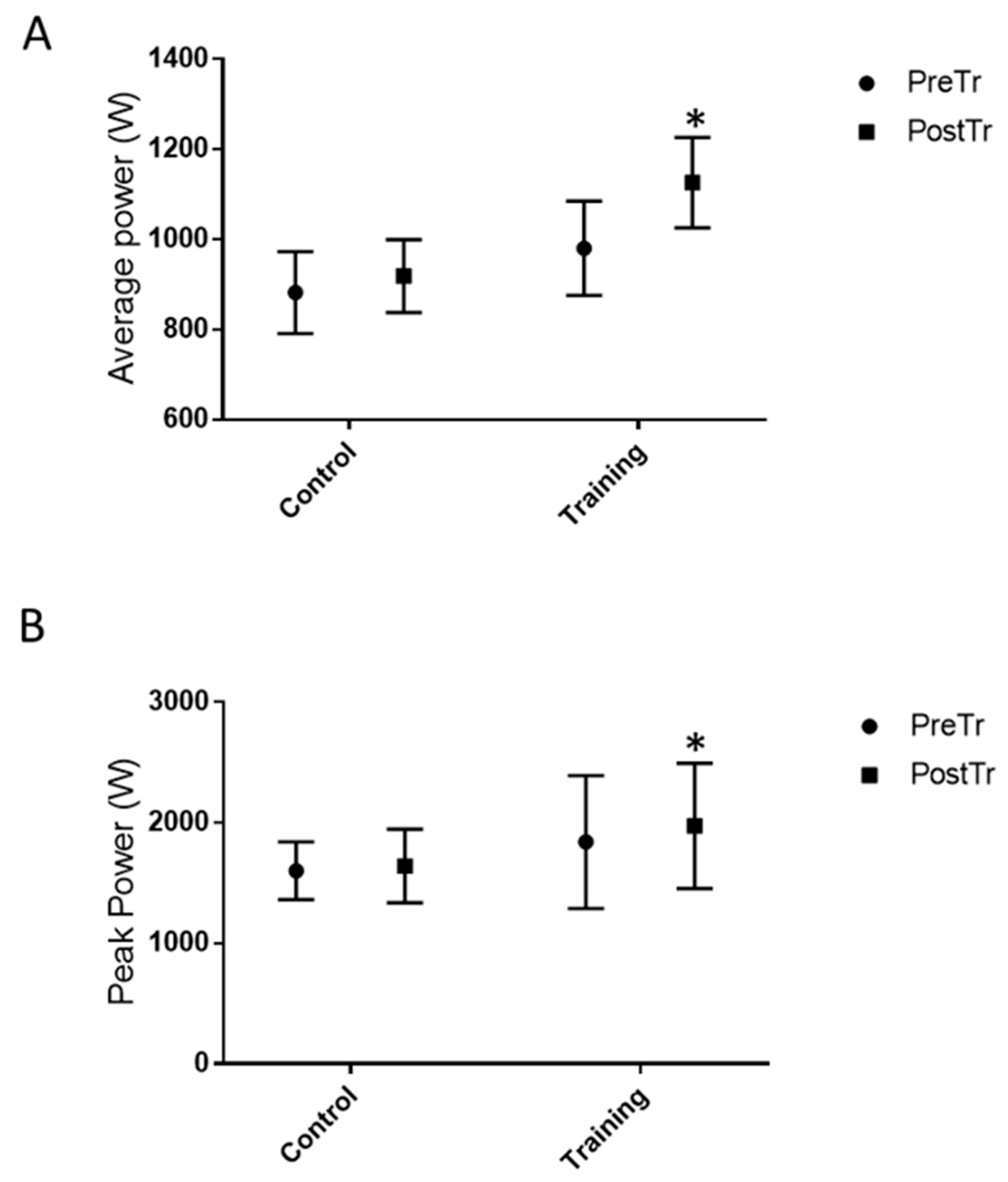
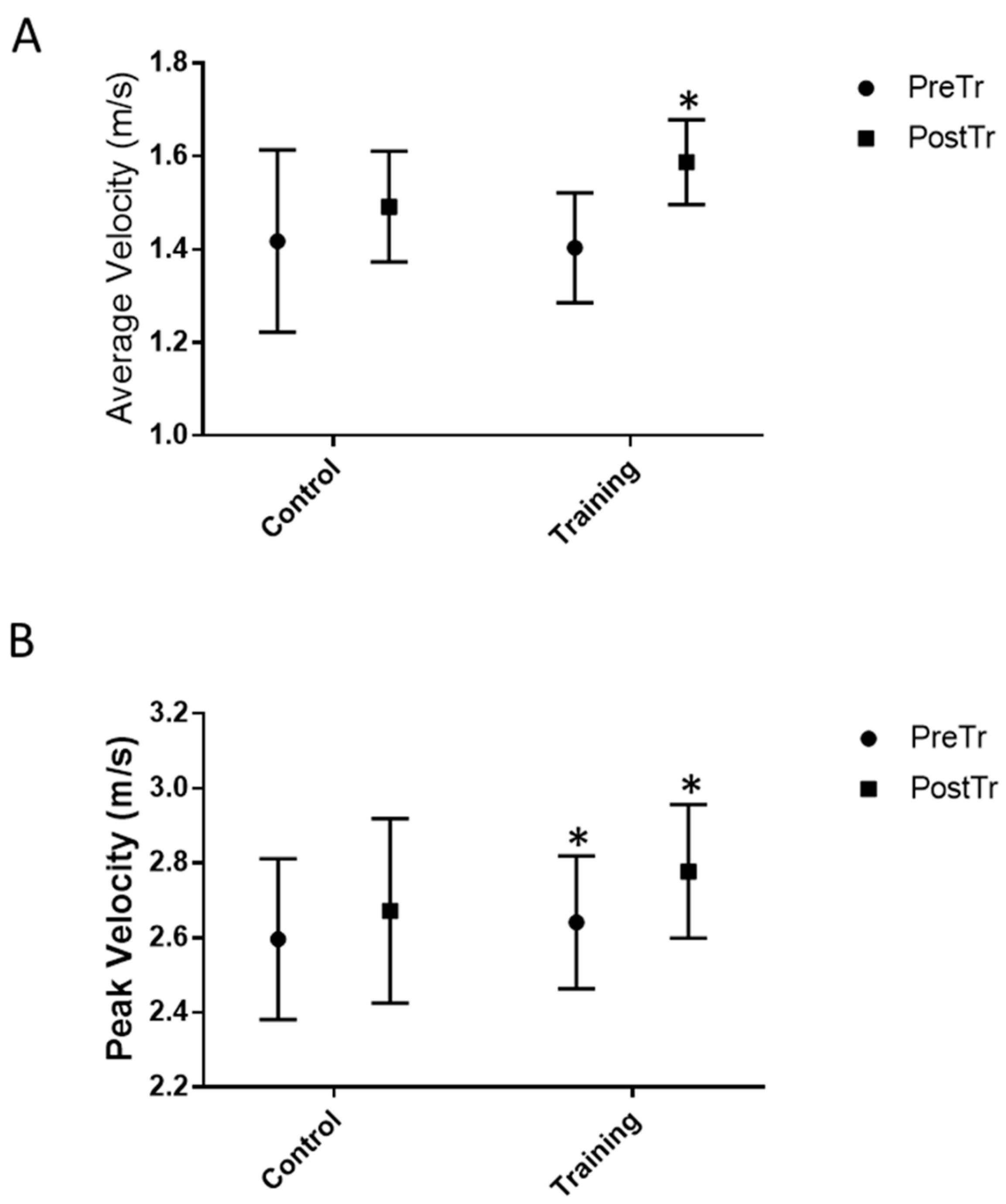
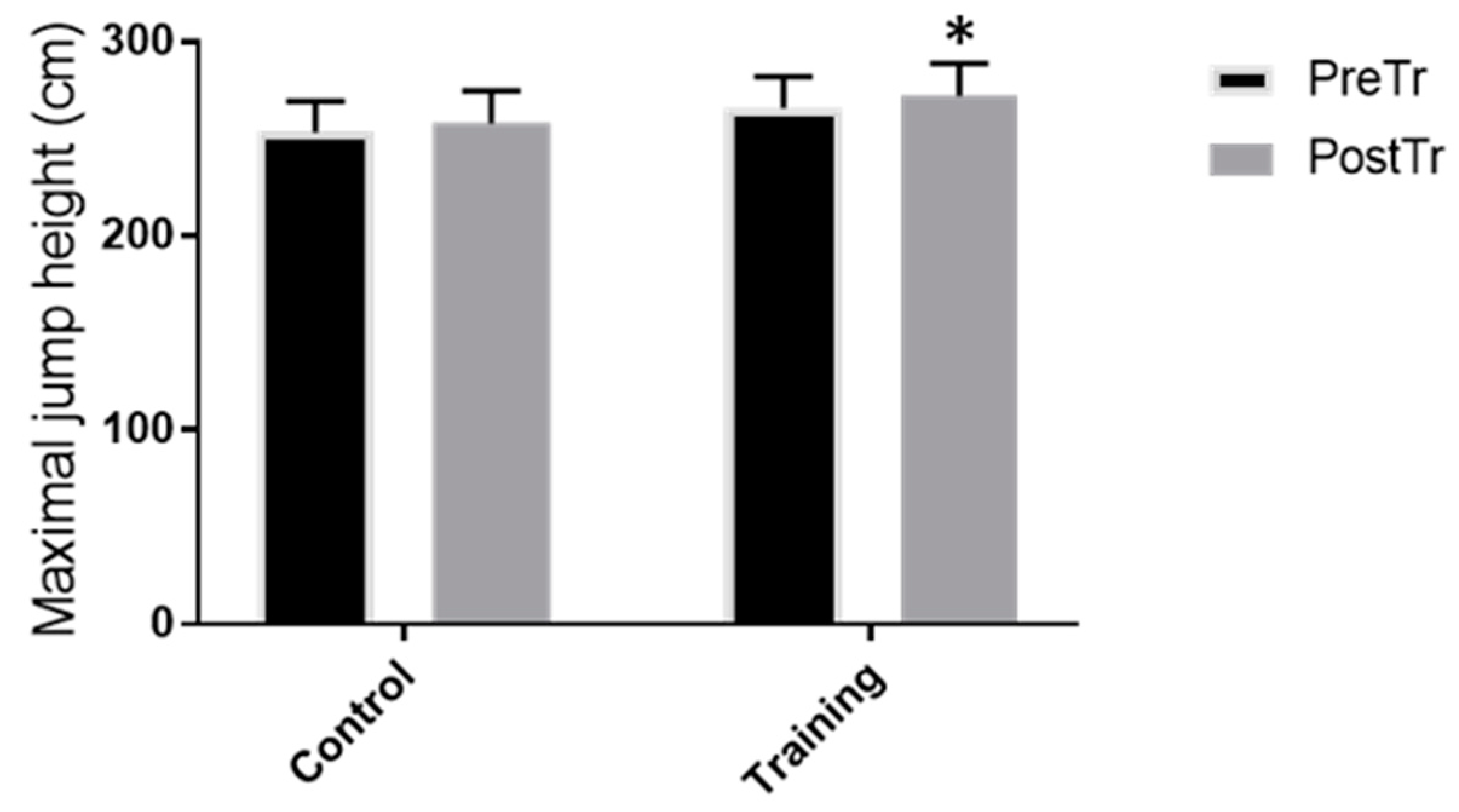
3.2. Functional Test Measures and Jump Height Performance
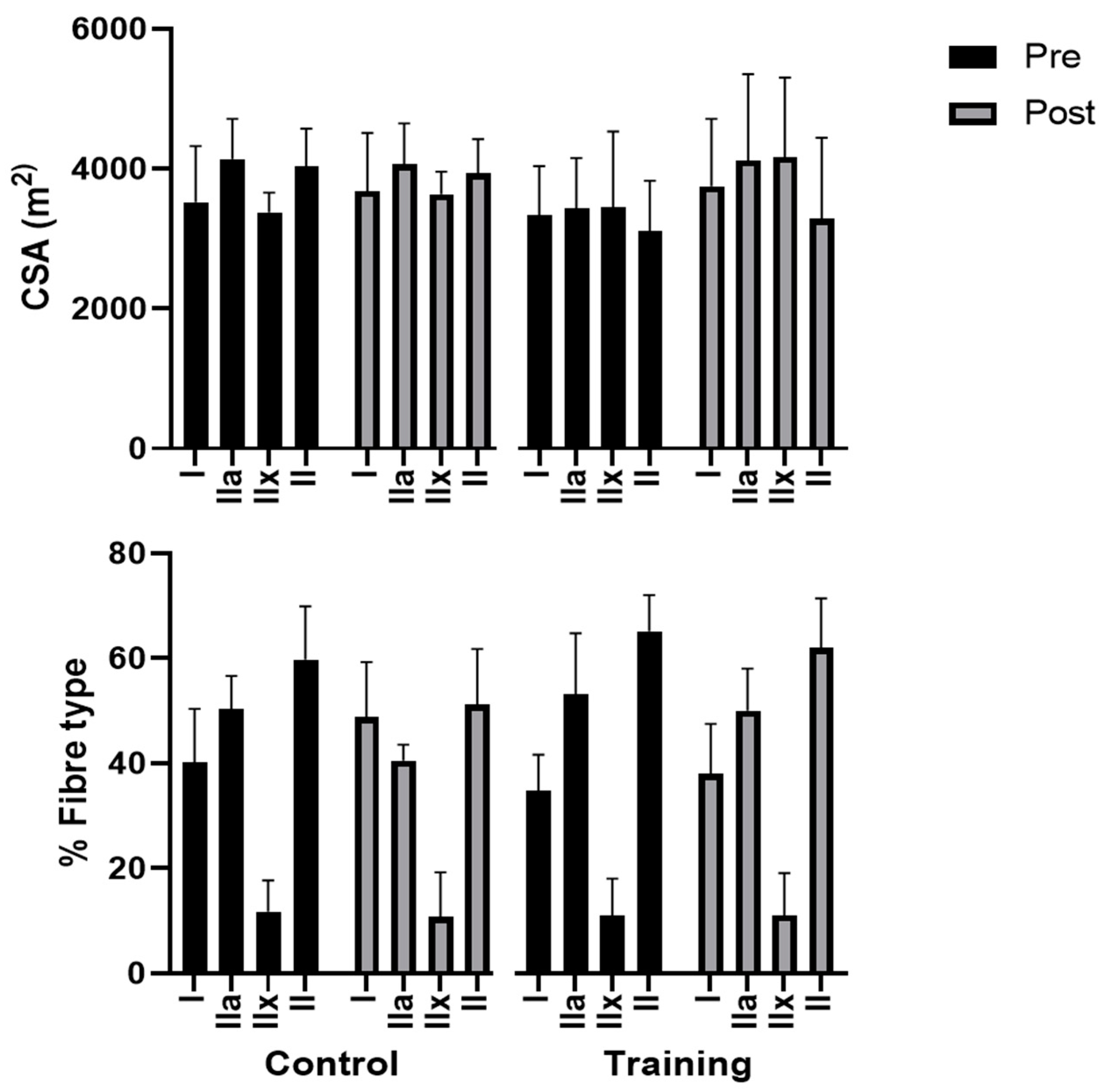
3.3. Skeletal Muscle Fibres Adaptations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Castaneda-Babarro, A.; Arbillaga-Etxarri, A.; Gutierrez-Santamaria, B.; Coca, A. Physical Activity Change during COVID-19 Confinement. Int. J. Environ. Res. Public Health 2020, 17, 6878. [Google Scholar] [CrossRef] [PubMed]
- Narici, M.; Vito, G.; Franchi, M.; Paoli, A.; Moro, T.; Marcolin, G.; Grassi, B.; Baldassarre, G.; Zuccarelli, L.; Biolo, G.; et al. Impact of sedentarism due to the COVID-19 home confinement on neuromuscular, cardiovascular and metabolic health: Physiological and pathophysiological implications and recommendations for physical and nutritional countermeasures. Eur. J. Sport Sci. 2021, 21, 614–635. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Larrad, A.; Manas, A.; Labayen, I.; Gonzalez-Gross, M.; Espin, A.; Aznar, S.; Serrano-Sanchez, J.A.; Vera-Garcia, F.J.; Gonzalez-Lamuno, D.; Ara, I.; et al. Impact of COVID-19 Confinement on Physical Activity and Sedentary Behaviour in Spanish University Students: Role of Gender. Int. J. Environ. Res. Public Health 2021, 18, 369. [Google Scholar] [CrossRef]
- Ruffieux, J.; Walchli, M.; Kim, K.M.; Taube, W. Countermovement Jump Training Is More Effective Than Drop Jump Training in Enhancing Jump Height in Non-professional Female Volleyball Players. Front. Physiol. 2020, 11, 231. [Google Scholar] [CrossRef] [PubMed]
- Kyrolainen, H.; Avela, J.; McBride, J.M.; Koskinen, S.; Andersen, J.L.; Sipila, S.; Takala, T.E.; Komi, P.V. Effects of power training on muscle structure and neuromuscular performance. Scand. J. Med. Sci. Sports 2005, 15, 58–64. [Google Scholar] [CrossRef]
- Vissing, K.; Brink, M.; Lonbro, S.; Sorensen, H.; Overgaard, K.; Danborg, K.; Mortensen, J.; Elstrom, O.; Rosenhoj, N.; Ringgaard, S.; et al. Muscle adaptations to plyometric vs. resistance training in untrained young men. J. Strength Cond. Res. 2008, 22, 1799–1810. [Google Scholar] [CrossRef]
- Wilk, K.E.; Voight, M.L.; Keirns, M.A.; Gambetta, V.; Andrews, J.R.; Dillman, C.J. Stretch-shortening drills for the upper extremities: Theory and clinical application. J. Orthop. Sports Phys. Ther. 1993, 17, 225–239. [Google Scholar] [CrossRef]
- Chimera, N.J.; Swanik, K.A.; Swanik, C.B.; Straub, S.J. Effects of Plyometric Training on Muscle-Activation Strategies and Performance in Female Athletes. J. Athl. Train. 2004, 39, 24–31. [Google Scholar]
- Potteiger, J.A.; Lockwood, R.H.; Haub, M.D.; Dolezal, B.A.; Almuzaini, K.S.; Schroeder, J.M.; Zebas, C.J. Muscle power and fiber characteristics following 8 weeks of plyometric training. J. Strength Cond. Res. 1999, 13, 275–279. [Google Scholar] [CrossRef]
- Van Cutsem, M.; Duchateau, J.; Hainaut, K. Changes in single motor unit behaviour contribute to the increase in contraction speed after dynamic training in humans. J. Physiol. 1998, 513 Pt 1, 295–305. [Google Scholar] [CrossRef]
- Almeida-Silveira, M.I.; Perot, C.; Pousson, M.; Goubel, F. Effects of stretch-shortening cycle training on mechanical properties and fibre type transition in the rat soleus muscle. Pflugers Arch. 1994, 427, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Pousson, M.; Perot, C.; Goubel, F. Stiffness changes and fibre type transitions in rat soleus muscle produced by jumping training. Pflugers Arch. 1991, 419, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Malisoux, L.; Francaux, M.; Nielens, H.; Theisen, D. Stretch-shortening cycle exercises: An effective training paradigm to enhance power output of human single muscle fibers. J. Appl. Physiol. 2006, 100, 771–779. [Google Scholar] [CrossRef]
- Perez-Gomez, J.; Olmedillas, H.; Delgado-Guerra, S.; Ara, I.; Vicente-Rodriguez, G.; Ortiz, R.A.; Chavarren, J.; Calbet, J.A. Effects of weight lifting training combined with plyometric exercises on physical fitness, body composition, and knee extension velocity during kicking in football. Appl. Physiol. Nutr. Metab. 2008, 33, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Herrero, J.A.; Izquierdo, M.; Maffiuletti, N.A.; Garcia-Lopez, J. Electromyostimulation and plyometric training effects on jumping and sprint time. Int. J. Sports Med. 2006, 27, 533–539. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kubo, K.; Morimoto, M.; Komuro, T.; Yata, H.; Tsunoda, N.; Kanehisa, H.; Fukunaga, T. Effects of plyometric and weight training on muscle-tendon complex and jump performance. Med. Sci. Sports Exerc. 2007, 39, 1801–1810. [Google Scholar] [CrossRef]
- Macaluso, F.; Isaacs, A.W.; Myburgh, K.H. Preferential type II muscle fiber damage from plyometric exercise. J. Athl. Train. 2012, 47, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Manojlovic, V.; Erculj, F. Using blood lactate concentration to predict muscle damage and jump performance response to maximal stretch-shortening cycle exercise. J. Sports Med. Phys. Fit. 2019, 59, 581–586. [Google Scholar] [CrossRef]
- Isaacs, A.W.; Macaluso, F.; Smith, C.; Myburgh, K.H. C-Reactive Protein Is Elevated Only in High Creatine Kinase Responders to Muscle Damaging Exercise. Front. Physiol. 2019, 10, 86. [Google Scholar] [CrossRef]
- Cleary, M.A.; Sadowski, K.A.; Lee, S.Y.; Miller, G.L.; Nichols, A.W. Exertional rhabdomyolysis in an adolescent athlete during preseason conditioning: A perfect storm. J. Strength Cond. Res. 2011, 25, 3506–3513. [Google Scholar] [CrossRef]
- Machado, A.F.; Baker, J.S.; Figueira, A.J., Jr.; Bocalini, D.S. High-intensity interval training using whole-body exercises: Training recommendations and methodological overview. Clin. Physiol. Funct. Imaging 2019, 39, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Davies, G.; Riemann, B.L.; Manske, R. Current Concepts of Plyometric Exercise. Int. J. Sports Phys. Ther. 2015, 10, 760–786. [Google Scholar] [PubMed]
- Macaluso, F.; Isaacs, A.W.; Di Felice, V.; Myburgh, K.H. Acute change of titin at mid-sarcomere remains despite 8 wk of plyometric training. J. Appl. Physiol. (1985) 2014, 116, 1512–1519. [Google Scholar] [CrossRef]
- Thivel, D.; Tremblay, A.; Genin, P.M.; Panahi, S.; Riviere, D.; Duclos, M. Physical Activity, Inactivity, and Sedentary Behaviors: Definitions and Implications in Occupational Health. Front. Public Health 2018, 6, 288. [Google Scholar] [CrossRef]
- Jennings, C.L.; Viljoen, W.; Durandt, J.; Lambert, M.I. The reliability of the FitroDyne as a measure of muscle power. J. Strength Cond. Res. 2005, 19, 859–863. [Google Scholar] [CrossRef]
- Kohn, T.A.; Essen-Gustavsson, B.; Myburgh, K.H. Do skeletal muscle phenotypic characteristics of Xhosa and Caucasian endurance runners differ when matched for training and racing distances? J. Appl. Physiol. 2007, 103, 932–940. [Google Scholar] [CrossRef]
- Barone, R.; Macaluso, F.; Sangiorgi, C.; Campanella, C.; Marino Gammazza, A.; Moresi, V.; Coletti, D.; Conway de Macario, E.; Macario, A.J.; Cappello, F.; et al. Skeletal muscle Heat shock protein 60 increases after endurance training and induces peroxisome proliferator-activated receptor gamma coactivator 1 alpha1 expression. Sci. Rep. 2016, 6, 19781. [Google Scholar] [CrossRef] [PubMed]
- Barone, R.; Sangiorgi, C.; Marino Gammazza, A.; D’Amico, D.; Salerno, M.; Cappello, F.; Pomara, C.; Zummo, G.; Farina, F.; Di Felice, V.; et al. Effects of Conjugated Linoleic Acid Associated With Endurance Exercise on Muscle Fibres and Peroxisome Proliferator-Activated Receptor gamma Coactivator 1 alpha Isoforms. J. Cell. Physiol. 2017, 232, 1086–1094. [Google Scholar] [CrossRef]
- Thoenes, M. Rhabdomyolysis: When exercising becomes a risk. J. Pediatr. Health Care 2010, 24, 189–193. [Google Scholar] [CrossRef]
- Silva, A.F.; Clemente, F.M.; Lima, R.; Nikolaidis, P.T.; Rosemann, T.; Knechtle, B. The Effect of Plyometric Training in Volleyball Players: A Systematic Review. Int. J. Environ. Res. Public Health 2019, 16, 2960. [Google Scholar] [CrossRef]
- Peterson, M.D.; Alvar, B.A.; Rhea, M.R. The contribution of maximal force production to explosive movement among young collegiate athletes. J. Strength Cond. Res. 2006, 20, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Stone, M.H.; O’Bryant, H.S.; McCoy, L.; Coglianese, R.; Lehmkuhl, M.; Schilling, B. Power and maximum strength relationships during performance of dynamic and static weighted jumps. J. Strength Cond. Res. 2003, 17, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Wisloff, U.; Castagna, C.; Helgerud, J.; Jones, R.; Hoff, J. Strong correlation of maximal squat strength with sprint performance and vertical jump height in elite soccer players. Br. J. Sports Med. 2004, 38, 285–288. [Google Scholar] [CrossRef]
- Sheppard, J.M.; Cronin, J.B.; Gabbett, T.J.; McGuigan, M.R.; Etxebarria, N.; Newton, R.U. Relative importance of strength, power, and anthropometric measures to jump performance of elite volleyball players. J. Strength Cond. Res. 2008, 22, 758–765. [Google Scholar] [CrossRef]
- Markovic, G. Does plyometric training improve vertical jump height? A meta-analytical review. Br. J. Sports Med. 2007, 41, 349–355; discussion 355. [Google Scholar] [CrossRef] [PubMed]
- Bogdanis, G.C.; Tsoukos, A.; Brown, L.E.; Selima, E.; Veligekas, P.; Spengos, K.; Terzis, G. Muscle Fiber and Performance Changes after Fast Eccentric Complex Training. Med. Sci. Sports Exerc. 2018, 50, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Winchester, J.B.; McBride, J.M.; Maher, M.A.; Mikat, R.P.; Allen, B.K.; Kline, D.E.; McGuigan, M.R. Eight weeks of ballistic exercise improves power independently of changes in strength and muscle fiber type expression. J. Strength Cond. Res. 2008, 22, 1728–1734. [Google Scholar] [CrossRef]
- Behrens, M.; Mau-Moeller, A.; Bruhn, S. Effect of plyometric training on neural and mechanical properties of the knee extensor muscles. Int. J. Sports Med. 2014, 35, 101–119. [Google Scholar] [CrossRef]
- Monti, E.; Franchi, M.V.; Badiali, F.; Quinlan, J.I.; Longo, S.; Narici, M.V. The Time-Course of Changes in Muscle Mass, Architecture and Power During 6 Weeks of Plyometric Training. Front. Physiol. 2020, 11, 946. [Google Scholar] [CrossRef]
- Ramirez-Campillo, R.; Vergara-Pedreros, M.; Henriquez-Olguin, C.; Martinez-Salazar, C.; Alvarez, C.; Nakamura, F.Y.; De La Fuente, C.I.; Caniuqueo, A.; Alonso-Martinez, A.M.; Izquierdo, M. Effects of plyometric training on maximal-intensity exercise and endurance in male and female soccer players. J. Sports Sci. 2016, 34, 687–693. [Google Scholar] [CrossRef]
- D’Amico, D.; Marino Gammazza, A.; Macaluso, F.; Paladino, L.; Scalia, F.; Spinoso, G.; Dimauro, I.; Caporossi, D.; Cappello, F.; Di Felice, V.; et al. Sex-based differences after a single bout of exercise on PGC1alpha isoforms in skeletal muscle: A pilot study. FASEB J. 2021, 35, e21328. [Google Scholar] [CrossRef] [PubMed]
- Miljkovic, N.; Lim, J.Y.; Miljkovic, I.; Frontera, W.R. Aging of skeletal muscle fibers. Ann. Rehabil. Med. 2015, 39, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Van Roie, E.; Walker, S.; Van Driessche, S.; Delabastita, T.; Vanwanseele, B.; Delecluse, C. An age-adapted plyometric exercise program improves dynamic strength, jump performance and functional capacity in older men either similarly or more than traditional resistance training. PLoS ONE 2020, 15, e0237921. [Google Scholar] [CrossRef] [PubMed]
- Zubac, D.; Paravlic, A.; Koren, K.; Felicita, U.; Simunic, B. Plyometric exercise improves jumping performance and skeletal muscle contractile properties in seniors. J. Musculoskelet Neuronal Interact. 2019, 19, 38–49. [Google Scholar]
- Franchi, M.V.; Monti, E.; Carter, A.; Quinlan, J.I.; Herrod, P.J.J.; Reeves, N.D.; Narici, M.V. Bouncing Back! Counteracting Muscle Aging With Plyometric Muscle Loading. Front. Physiol. 2019, 10, 178. [Google Scholar] [CrossRef]

| Control Group (n = 5) | Training Group (n = 8) | Total (n = 13) | |
|---|---|---|---|
| Age (year) | 21.4 ± 1.7 | 21.5 ± 1.7 | 21.5 ± 1.7 |
| Height (cm) | 169.3 ± 10.2 | 176.2 ± 10 | 173.6 ± 10.3 |
| Weight (kg) | 63.0 ± 6.8 | 71.6 ± 21.8 | 68.3 ± 17.7 |
| Ave max squat jump height (cm) | 215 ± 13.6 | 224 ± 12.6 | 220.2 ± 13.7 |
| 95% squat jump height (cm) | 204 ± 12.9 | 212 ± 12.0 | 209.2 ± 13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isaacs, A.W.; Myburgh, K.H.; Macaluso, F. Low-Volume Squat Jump Training Improves Functional Performance Independent of Myofibre Changes in Inactive Young Male Individuals. Healthcare 2022, 10, 1217. https://doi.org/10.3390/healthcare10071217
Isaacs AW, Myburgh KH, Macaluso F. Low-Volume Squat Jump Training Improves Functional Performance Independent of Myofibre Changes in Inactive Young Male Individuals. Healthcare. 2022; 10(7):1217. https://doi.org/10.3390/healthcare10071217
Chicago/Turabian StyleIsaacs, Ashwin Wayne, Kathryn Helen Myburgh, and Filippo Macaluso. 2022. "Low-Volume Squat Jump Training Improves Functional Performance Independent of Myofibre Changes in Inactive Young Male Individuals" Healthcare 10, no. 7: 1217. https://doi.org/10.3390/healthcare10071217
APA StyleIsaacs, A. W., Myburgh, K. H., & Macaluso, F. (2022). Low-Volume Squat Jump Training Improves Functional Performance Independent of Myofibre Changes in Inactive Young Male Individuals. Healthcare, 10(7), 1217. https://doi.org/10.3390/healthcare10071217






