Addressing Mild Cognitive Impairment and Boosting Wellness for the Elderly through Personalized Remote Monitoring
Abstract
:1. Introduction
- The RO-SmartAgeing system, due to its personalized smart environment, provides safe, minimally intrusive, low-cost, and privacy-protected remote monitoring with a strong accent on preventative and proactive functionalities.
- The results obtained during the development of the system demonstrate that IoT-based devices selected so as to provide full and free post-purchase access to data collected during monitoring increase the potential of developing comprehensive applications targeted to better sustain the health care management of the elderly with MCI.
- In Romania, there are currently no information systems that provide personalized remote health monitoring and interactive care of the elderly with MCI in an intelligent environment, customized according to the evolving needs, requirements, and dysfunctionalities of aged people, and that forecast the health status and MCI evolution by using modelling and AI-based prediction.
2. Related Work
2.1. Enhanced Cognitive Wellness through Emerging Technologies
2.2. Smart Environments Augmented by AI
2.2.1. Smart Environment
2.2.2. Continuum of Care Sustained by AI and Predictive Models
2.3. Patient-Centric Remote Monitoring
3. System Description and Methods
3.1. Main Objective
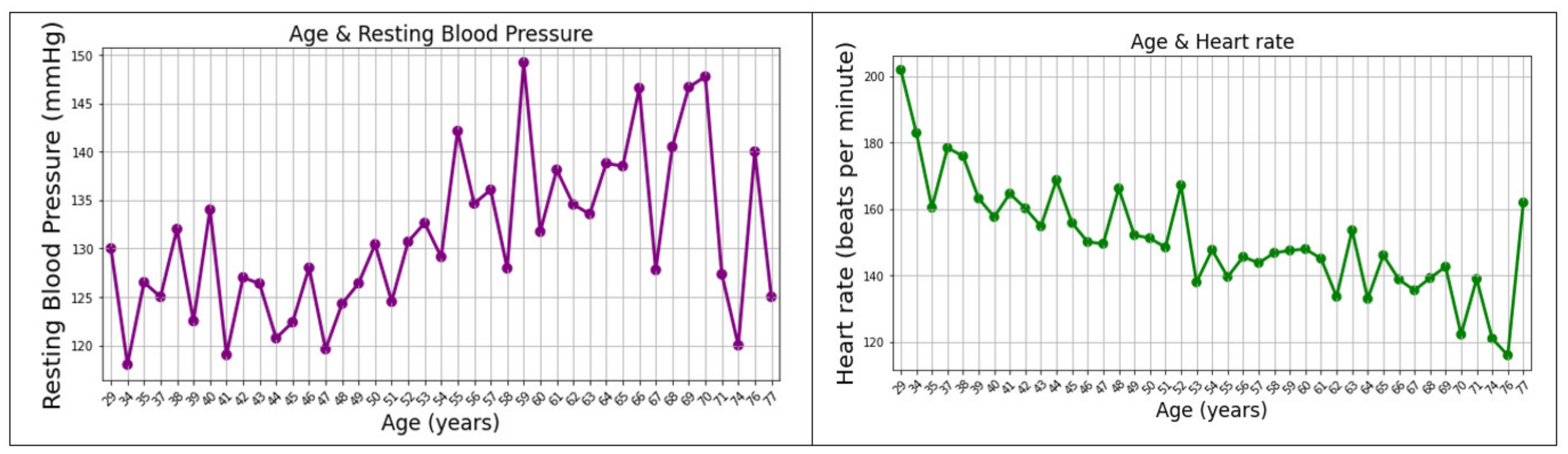
- Monitoring health and ambient parameters, daily movement, activities behavior, and routine tasks through accurate gathering of a quite comprehensive range of data collected by IoT devices (Withings devices, depth cameras, and devices built inside a project (the so-called “Blackboxes” and the “Leg Band”) for health parameter monitoring—such as heart rate, electrocardiogram (ECG), blood pressure, sleep-associated parameters, body position, bioelectric impedance analysis, body fat, total body water, muscle mass, bone mass—and motion and environmental parameters);
- Using open data sources for MCI.
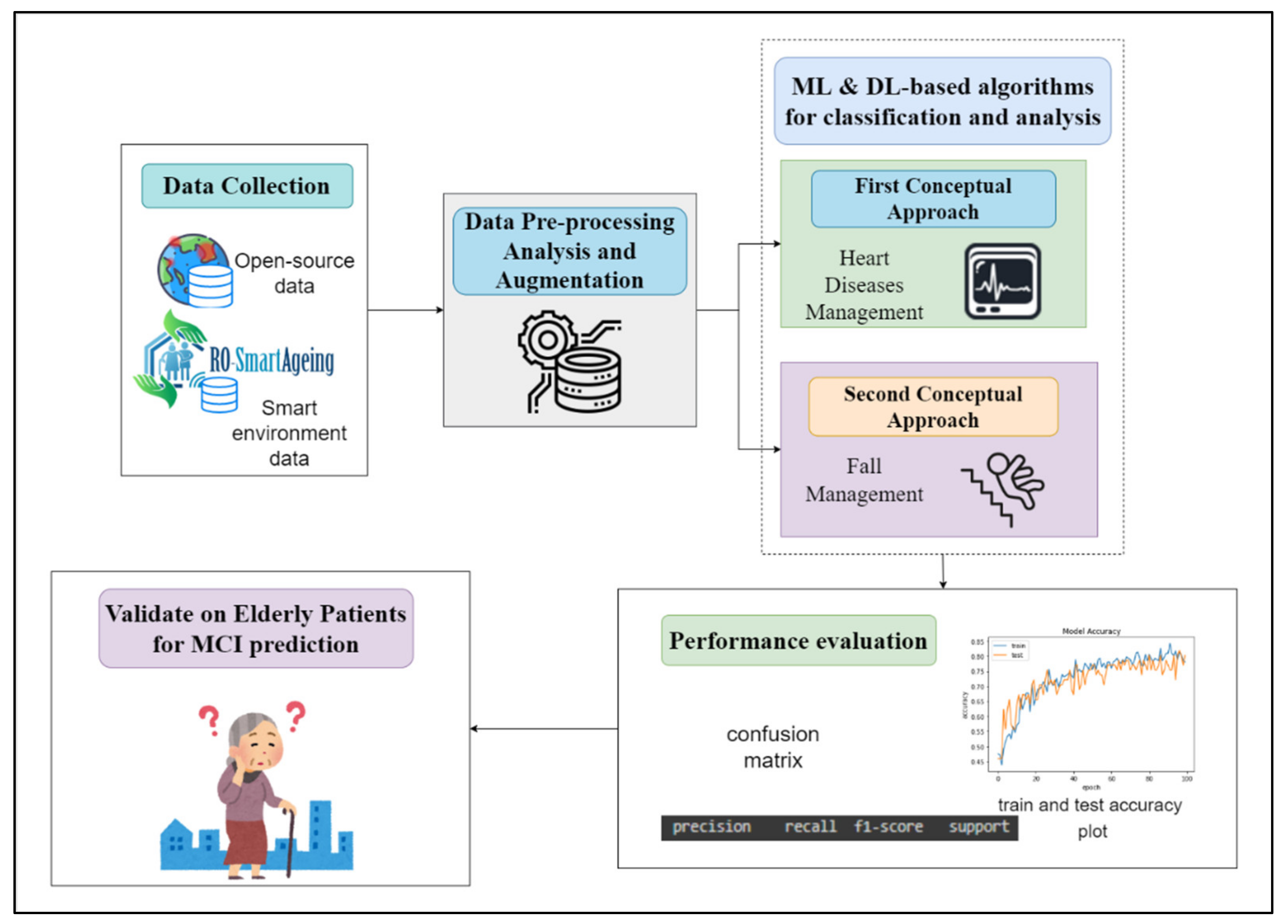
3.2. Method
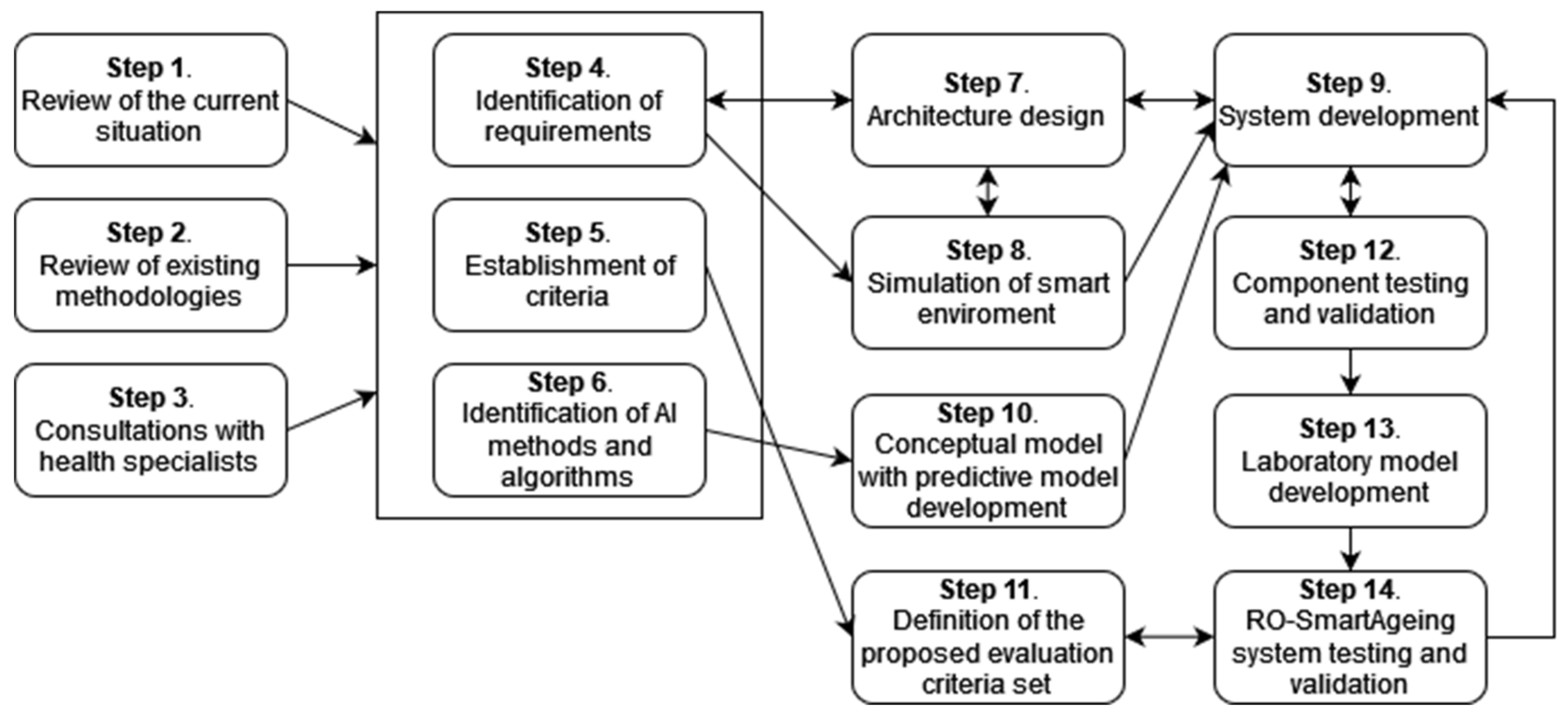
- Step 1. Authorized scientific sources. Performing a systematic review of a large number of authorized scientific sources in order to clearly identify the most relevant information about the current situation (with an emphasis on the Romanian one) in the domains of population ageing; healthcare services for elderly patients, including those with MCI; current and emerging digital technologies; and healthcare solutions;
- Step 2. Review of existing methodologies. Performing a literature review to identify currently used methodologies for the assessment of digital systems and technology, as well as the current legal framework and guidance;
- Step 3. Consultations with health specialists. Organizing several rounds of consultations with health specialists; taking into consideration the specificities in the Romanian health, social, and IT domains, and the most significant drawbacks, limitations, deficiencies, and facilities;
- Step 4. Identification of requirements. Identifying the functional and non-technical requirements for the digital system, based on the above-mentioned research;
- Step 5. Establishment of criteria. Setting up criteria for selecting the most appropriate devices and technologies according to the special needs and demands of elderly people with MCI and their physicians, for the development of an efficient, tailored smart environment;
- Step 6. Identification of AI methods and algorithms. Performing research for identifying high-performing AI (DL and ML) methods and algorithms, as well as MCI-related open data, used in the development of predictive models;
- Step 7. Architecture designing. Designing RO-SmartAgeing system based on an IoT- and microservice-based, multilevel architecture in order to provide a high degree of flexibility and scalability;
- Step 8. Simulation of the smart environment. Creating a smart environment in a laboratory setting that reproduces as closely as possible the real living conditions of an elderly person;
- Step 9. System development. Developing the system using an iterative approach;
- Step 10. Conceptual model with predictive model development. Including the predictive models in a conceptual model conceived to concatenate the health parameters collected via RO-SmartAgeing smart environment and the MCI-related data from the open data sources, in order to obtain a more accurate and trained result;
- Step 11. Definition of the proposed evaluation criteria set. Defining a proposed evaluation criteria set by taking into consideration the high-rate dynamics of the advances in the field of remote health-monitoring solutions;
- Step 12. Component testing and validation. Testing each component of the system and validating it in the laboratory environment before its integration into a comprehensive system;
- Step 13. Laboratory model development. Developing a laboratory model of the RO-SmartAgeing system;
- Step 14. Testing and validating the RO-SmartAgeing system. Testing and validating the RO-SmartAgeing system in laboratory and real conditions (Figure 1).
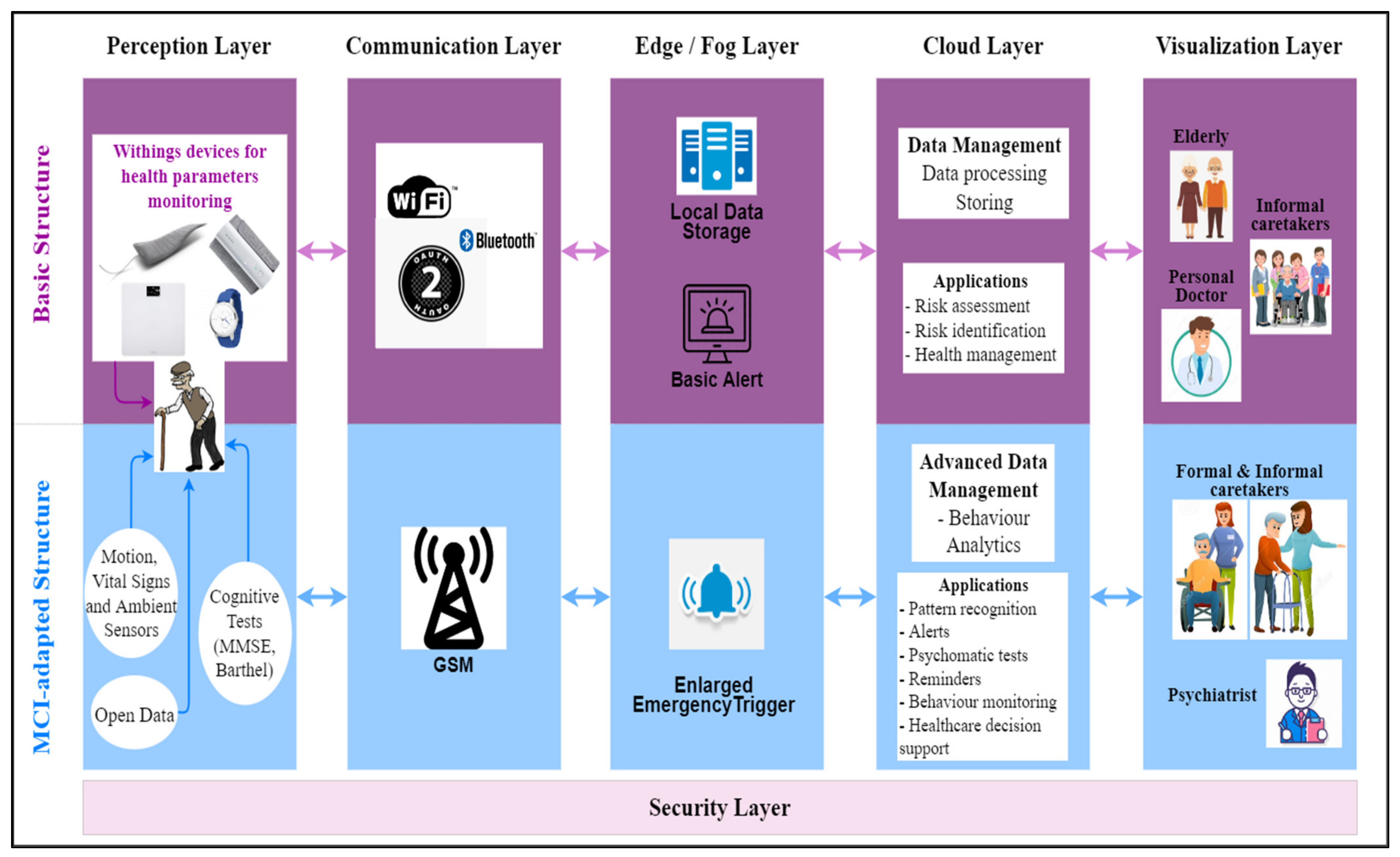
3.3. System Architecture
- The Perception Layer consists of a variety of devices and other type of sources for collecting health-related data;
- The Communication Layer allows the raw data to be transferred to the next layers;
- The Edge/Fog Layer performs preliminary analytics in order to analyse the data and trigger alarms, in real time (basic alerts). An enlarged emergency trigger functionality, built around some specificities of MCI, is provided for a fast response. Data is temporary stored in a local data storage;
- The Cloud Layer performs long-term analytics and data management in order to generate specific health-supporting applications, including the ones directly related to MCI, such as personalized predictive models;
- The Vizualization Layer gives access to the applications that provide the system users with different visualizations (in different formats or reports) of the stored data or new insights obtained from advanced processing;
- The Security Layer—the privacy, confidentiality and security issues associated to health data and a digital system are addressed across all layers, according to the local specificities and threats.
4. Implementation of RO-SmartAgeing System
4.1. Tailored Smart Environment for MCI Patients
- The Medical Blackbox is responsible for monitoring the healthcare parameters. It has been designed to offer the possibility to manage personalized monitoring according to the patient’s needs, as it is based on accessible, independent, and Arduino-compatible sensors.
- The Ambient Blackbox targets the measurement of the home-based environmental parameters while the patient is normally practicing their daily activities.
- The Leg Band includes an accelerometer and gyroscope-based sensor for patient’s movements, positioning, and fall detection, these measurements being essential for an MCI-related condition.
- Medical Blackbox—for pulse and oxygen in blood; body temperature; volatile organic compounds from breath; urine biochemistry;
- Leg Band—for fall detection and number of steps;
- Ambient Blackbox—for ambient temperature; environmental temperature and humidity; ambient light; flammable gases and smoke; infrared signal caused by motion; pressure and altitude; air quality;
- Smart depth camera (Orbbec Persee)—human activity recognition [35];
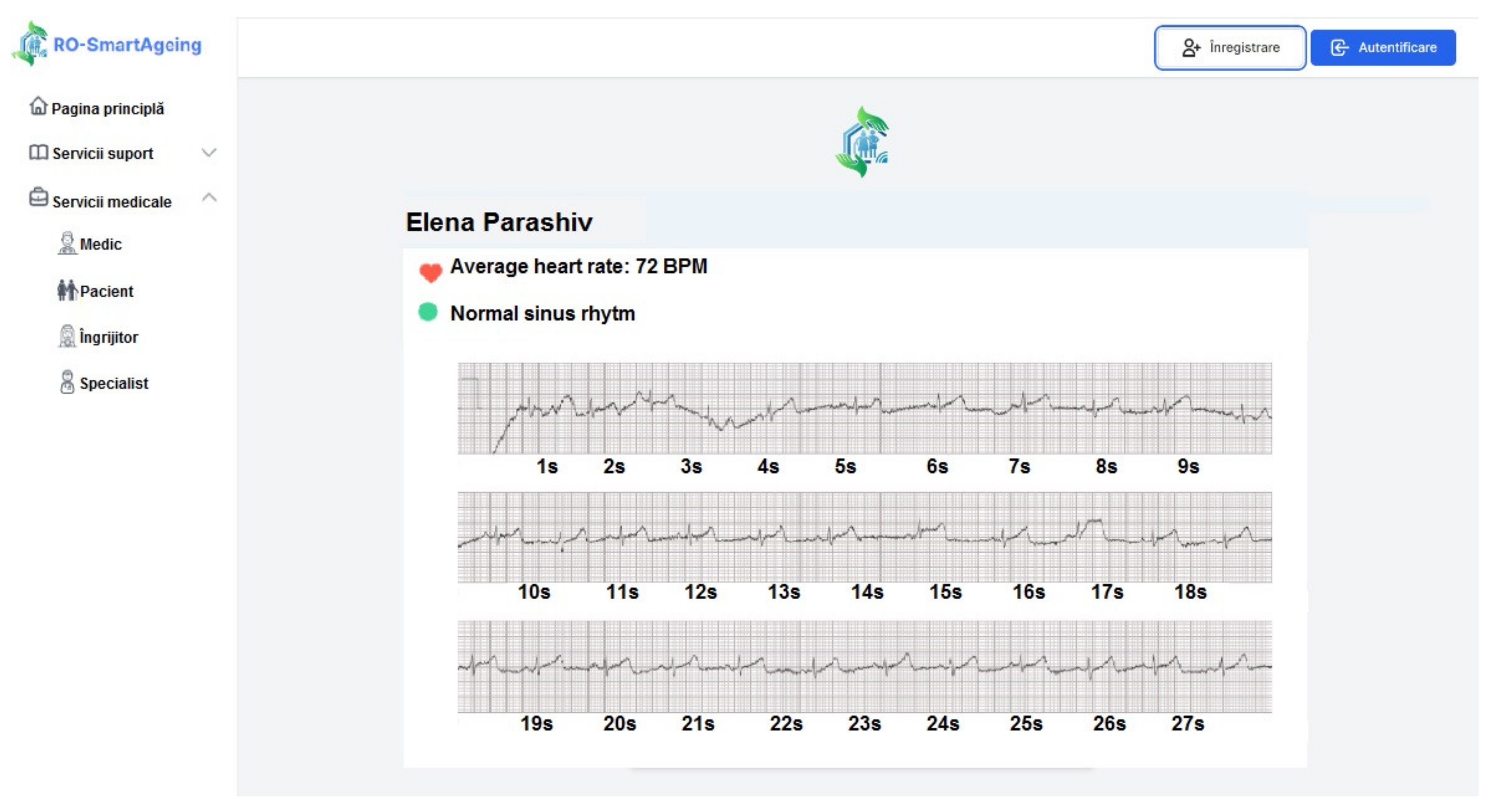
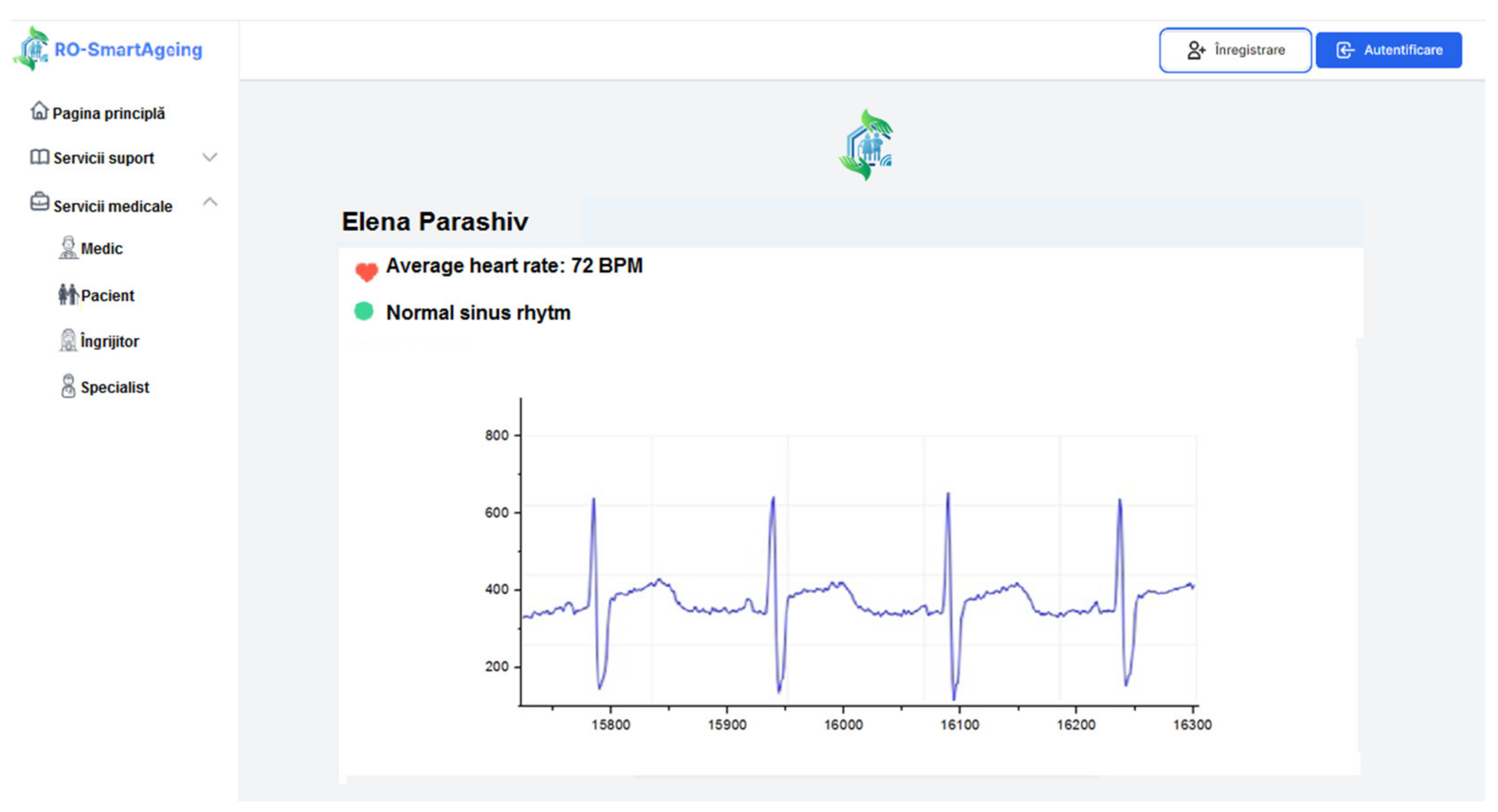
- Withings Smartwatch—ECG; pulse; GPS; activity monitoring; score for sleep quality assessment; sleep duration; sleep cycles (deep, light, REM sleep) [36];
- Withings Blood Pressure Monitor (BPM)—blood pressure; ECG; pulse [37];
- Withings Sleep monitor—score for sleep quality assessment; breathing disorders; sleep duration; sleep onset and waking time; sleep cycles: deep, light, REM; pulse during sleep; average pulse; snoring duration [38];
- Withings Smart Scale—body position detection; bioelectric impedance analysis; body fat (%); total body water (%); muscle mass (kg or lb.); bone mass (kg or lb) [39].
4.2. Predictive Models for MCI—Conceptual Approaches
5. Results
5.1. Functionalities for Monitoring MCI Provided by RO-SmartAgeing System
- The Medical Component
- ◦
- Scope: It allows personalized monitoring and dynamic management of medical data and information of an elderly patient with MCI. It allows the collection (in real time, at changeable established intervals) of data and information related to the evolution of the health status, the environment in which he/she carries out the daily activities, or other information essential to support the provision of a diagnosis or treatment.
- ◦
- Examples of provided functionalities: Management of the trigger alerts; monitoring planning; management of collected data; health report generation; evaluation of collected data and information in the context of MCI; planning daily activities; personalized remote configuration of the smart environment; administration of the interactions among patient, caretaker/family, and healthcare specialists.
- The Monitoring Component
- ◦
- Scope: It provides functionalities for the collection of data from the IoT-based devices that are integrated in the smart environment, and the transmission of this data to the Edge/Fog and Cloud Computing, where it is filtered, aggregated, analysed, and stored in the RO-SmartAgeing database. In the case of detecting an abnormal value of a measured parameter or an accidental fall, specific functionalities for alerting the caretaker and personal physician are triggered.
- ◦
- Examples of provided functionalities: Management of monitoring biomedical parameters; management of monitoring ambient parameters; management of smart environments components; management of monitoring current activities; management of monitoring fall detection and movements; generating trigger alerts.
- The Service Support Component
- ◦
- Scope: It provides specific information on a number of issues associated with supporting active, independent, and healthy ageing.
- ◦
- Examples of provided functionalities: Recommendations for healthy and independent living for the elderly; elderly self-assessment questionnaires on the quality of life and well-being of the elderly; support information for caretakers regarding information on specifics of care for the elderly; services for supporting/facilitating social relationships and cognitive abilities of the elderly; (self-)assessment of cognitive ability; personal fall risk awareness assessment; fall prevention information management; management of digital technologies for the elderly; information on legislation and regulations for the elderly; management of COVID-19 disease.
5.2. Proposed Evaluation Criteria for Health Remote Monitoring Systems for Elderly Patients, Including Those with MCI
- A validated good practice can be more quickly replicated, adapted, and implemented;
- The stakeholders have the necessary information to be able to estimate if the functionalities and features of a good practice correspond to their need and objectives;
- The developers have clear targets for improving health monitoring according to the evaluation results to refer to;
- The evaluation criteria represent a solid base for an objective validation process of a digital solution in order to see if it may be undertaken as a good practice.
- Concept—system under development;
- Emerging—system during testing phase;
- Pilot—an experimental version of the system is tried out in a real environment;
- Implemented—a functional version is ready-to-market and used in healthcare units;
- Upgraded—optimized versions are available;
- Cross-border—the system is integrated and functional in medical practices from different countries.
5.3. Organizing the Testing of RO-SmartAgeing System
- Step 1. Planning the testing: The elaboration of the test strategy, main objectives, schedule, the level from which the test begins, testing environments, and human resources.
- Step 2. Requirements testing: All the specifications used in the design phase are tested by the development team from different points of view: completeness, clarity, consistency, traceability, testability. In case an error is identified, the dysfunctional requirement and associated part of the system architecture are redesigned and integrated within the others.
- Step 3. Testing performed by the development team: It is structured according to the functional and non-functional features of the RO-SmartAgeing system. It is organized so as to begin with the testing of all the components of the system and their associated functionalities. The next phase is to test the entire system from the point of view of privacy, confidentiality, security, usability, performance, adaptability to a specific environment, and user. The bugs are reported, evaluated, and addressed. The software is updated, and potential dysfunctionalities of the smart environment are solved. Based on the data collected through the smart environment during this step, the development team will test some of the functionalities of the conceptual models of the predictive models.
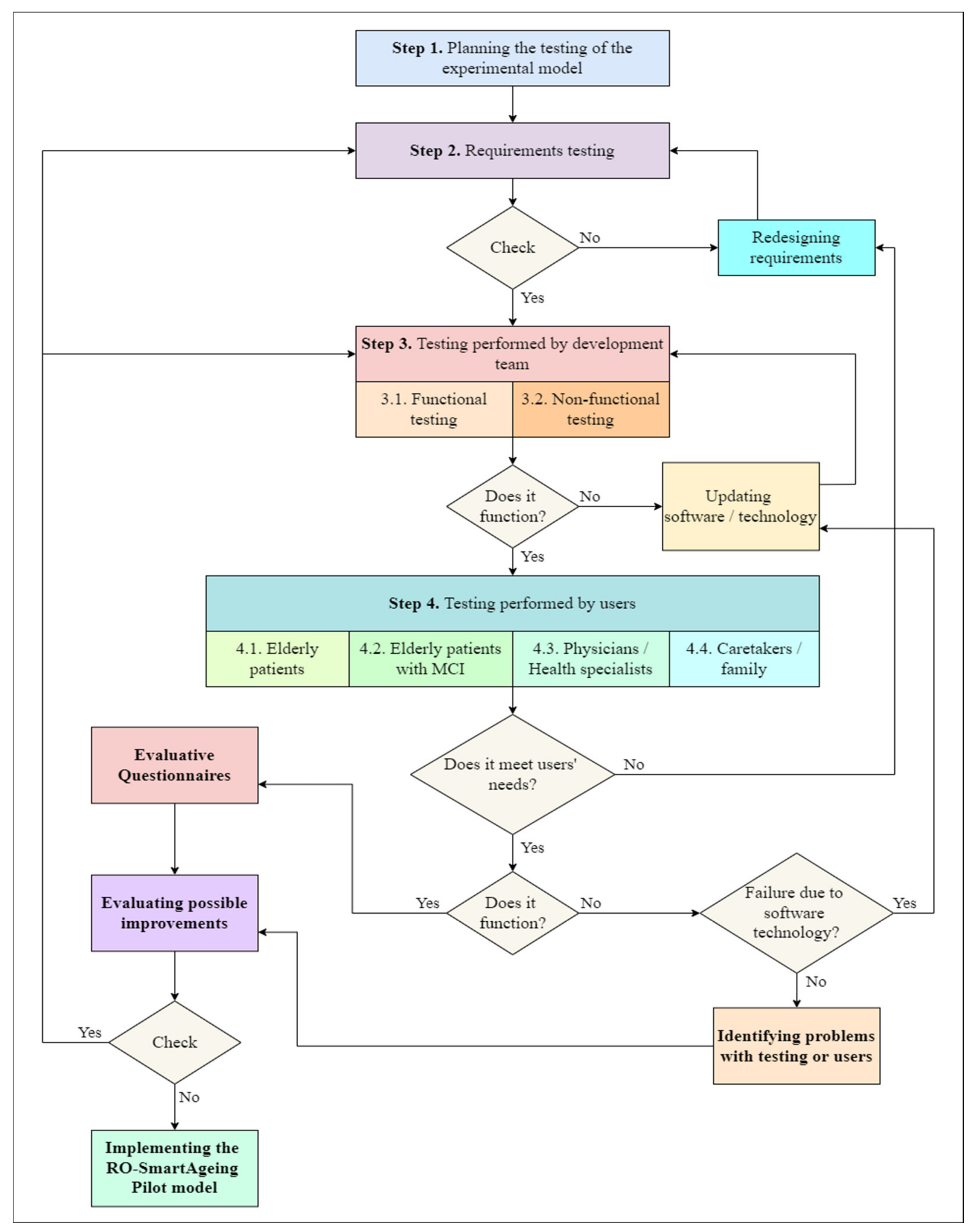
- Step 4. Testing performed by the users: All the categories of final users/beneficiaries of the RO-SmartAgeing system are involved in this phase. They have total access to the complete functionalities of the system and the possibility to test it in different environments. The objectives of step 4 are similar to the ones of step 3. Eventual errors or dysfunctionalities are reported, the development team takes them over, and the process is restarted from step 2 or 3.
- Human resources: The development team involved in testing is divided into three group of testers that simulate the range of users of the RO-SmartAgeing system. Several agreements with public and private healthcare units and associations—“Bucharest Center for Seniors”, ”Nicolae Cajal Association for the Elderly”, “Ana Aslan National Institute of Gerontology and Geriatrics”, “Carol Davila University of Medicine and Pharmacy”, and ”Center for Medical Innovation, InoMed—non-governmental organization”—have been concluded in order to involve representative lots of elderly patients, physicians, and caretakers. Fifty patients (25 women, 25 men) will participate. They have an average age of 78 years, and they are grouped in two lots, according to their Mini-Mental State Examination score: 24 and higher for normal cognition, and 19–23 for MCI. Ten physicians have already agreed to participate, and at least twenty caretakers are going to be involved in testing. The patients and caretakers are recommended by their physicians. They will receive written information, and after their consent, they will sign a consent document in accordance with the legal and ethical provisions in force.
- Locations for testing the RO-SmartAgeing system: Most of the locations in which the RO-SmartAgeing smart environment will be installed are going to be at the elderly patients’ homes. If the local conditions can be assimilated to the ones from a home, the smart environment is scheduled to also be installed inside a nursing home.
6. Discussion
- Cross-patient-centered approaches, which support an efficient management of multimorbidity associated with ageing and MCI, an improved empowerment and responsibility of the patients towards their own health, and an active and healthy ageing, which provides a sustaining framework for health specialists in developing new preventative approaches and therapeutic protocols for people with MCI;
- Implementation of new methods based on scalable ITC services to assess problems related to health, lifestyle, and degenerative diseases related to ageing, and provision of new flexible ways of collecting and processing information and data of a medical nature;
- Development of new AI-based models that integrate high-dimensional data for identification of alarming changes in the cognitive state, behavior, and other vital sign parameters of elderly people with MCI;
- Personalization of services induced by the heterogeneity of the typologies of elderly people with various degenerative dysfunctions and related co-morbidities;
- Substantiation of a new type of long-distance relationship between doctor and elderly person that centralizes several directions of action, such as personalized health monitoring, health status assessment, supporting the independence of the elderly person in a familiar environment, and so on.
- IoT-based devices provide the potential to provide a continuous stream of real-time health and daily-activities-related data;
- The Withings devices were selected due to their performances, and, no less importantly, to the lack of necessity of having a separate future subscription at the Withings company for total access to the data collected through them (see Supplementary Section S2);
- The smart environment can be personalized not only according to the specific needs of an elderly patient with MCI, but also based on the changes of their health status that are likely to appear in time during the monitoring;
- The trigger alarm functionalities are built in the fog computing in order to get a faster response in case of an abnormal value of a vital sign measurement or behavior being recorded;
- Microservices were chosen due to the fact that they are loosely coupled, organized around a single business capability, and able to be developed with different technologies. These characteristics confer the system fault tolerance, agility, flexibility, scalability, and faster deployment and upgrading;
- As connecting a large number of smart devices increases the risk of vulnerabilities, strict security procedures for applications as well as for the protection of the entire network were developed;
- Current EU standards were used in working with sensitive health data in order to ensure privacy, security, and confidentiality, which will increase confidence in using the system for different types of health specialists, patients, and medical environments;
- The possibility to analyse and process large amounts of data on MCI patient monitoring that were acquired over long periods of time makes it possible to conduct longitudinal studies, and observe meaningful changes in behavior and health for the elderly;
- Recorded data offers the system the opportunity to monitor any problems the MCI patient might encounter in terms of mobility, orientation, self-assessment, memory, or sleep-related or any other cognitive-based activities;
- Mobile networks are the optimal solution to allow real-time access to data collected through the RO-SmartAgeing smart environment. Even if the platform, sensors, and network work well, the accuracy of the diagnosis transmitted to the patient relies on the accuracy of the data provided to the doctor.
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ageing and Health. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 29 March 2022).
- 2020 Alzheimer’s Disease Facts and Figures. Available online: https://www.ncbi.nlm.nih.gov/pubmed/32157811 (accessed on 29 March 2022).
- Brandão, P.R.P.; Munhoz, R.P.; Grippe, T.C.; Cardoso, F.E.C.; e Castro, B.M.D.A.; Titze-de-Almeida, R.; Tomaz, C.; Tavares, M.C.H. Cognitive impairment in Parkinson’s disease: A clinical and pathophysiological overview. J. Neurol. Sci. 2020, 419, 117177. [Google Scholar] [CrossRef] [PubMed]
- Thapliyal, H.; Rajdeep, K.N.; Mohanty, P.S. Smart Home Environment for Mild Cognitive Impairment Population: Solutions to Improve Care and Quality of Life. IEEE Consum. Electron. Mag. 2017, 7, 68–76. [Google Scholar] [CrossRef]
- Lovett, R.M.; Curtis, L.M.; Persell, S.D.; Griffith, J.W.; Cobia, D.; Federman, A.; Wolf, M.S. Cognitive impairment no dementia and associations with health literacy, self-management skills, and functional health status. Patient Educ. Couns. 2020, 103, 1805–1811. [Google Scholar] [CrossRef] [PubMed]
- Mc Ardle, R.; Del Din, S.; Galna, B.; Thomas, A.; Rochester, L. Differentiating dementia disease subtypes with gait analysis: Feasibility of wearable sensors? Gait Posture 2020, 76, 372–376. [Google Scholar] [CrossRef]
- Lee-Cheong, S.; Amanullah, S.; Jardine, M. New Assistive Technologies in Dementia and Mild Cognitive Impairment Care: A PubMed Review. Asian J. Psychiatry 2022, 73, 103135. [Google Scholar] [CrossRef]
- Javed, A.R.; Fahad, L.G.; Farhan, A.A.; Abbas, S.; Srivastava, G.; Parizi, R.M.; Khan, M.S. Automated cognitive health assessment in smart homes using machine learning. Sustain. Cities Soc. 2021, 65, 102572. [Google Scholar] [CrossRef]
- Khaire, P.; Kumar, P. A semi-supervised deep learning based video anomaly detection framework using RGB-D for surveillance of real-world critical environments. Forensic Sci. Int. Digit. Investig. 2022, 40, 301346. [Google Scholar] [CrossRef]
- Ianculescu, M.; Alexandru, A.; Tudora, E.; Neagu, G. Mapping the Behavioral Change Evaluation of Elderly with Mild Cognitive Impairment in a Smart Environment. In Proceedings of the IEEE International Conference on e-Health and Bioengineering—EHB 2020, Iasi, Romania, 29–30 October 2020; pp. 1–4. [Google Scholar]
- He, S.; Li, S.; Nagc, A.; Feng, S.; Hane, T.; Mukhopadhyay, A.C.; Powel, W. A comprehensive review of the use of sensors for food intake detection. Sens. Actuators A Phys. 2020, 315, 112318. [Google Scholar] [CrossRef]
- Fahad, L.G.; Tahir, S.F. Activity recognition and anomaly detection in smart homes. Neurocomputing 2021, 423, 362–372. [Google Scholar] [CrossRef]
- Javed, A.R.; Faheem, R.; Asim, M.; Baker, T.; Beg, M.O. A smartphone sensors-based personalized human activity recognition system for sustainable smart cities. Sustain. Cities Soc. 2021, 71, 102970. [Google Scholar] [CrossRef]
- Triboan, D.; Chen, L.; Chen, F.; Wang, Z. A semantics-based approach to sensor data segmentation in real-time Activity Recognition. Future Gener. Comput. Syst. 2019, 93, 224–236. [Google Scholar] [CrossRef]
- Riboni, D.; Bettini, C.; Civitarese, G.; Janjua, Z.H.; Helaoui, R. SmartFABER: Recognizing fine-grained abnormal behaviors for early detection of mild cognitive impairment. Artif. Intell. Med. 2016, 67, 57–74. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Lu, Y.; Pei, Z. Intelligent diagnosis of Alzheimer’s disease based on internet of things monitoring system and deep learning classification method. Microprocess. Microsyst. 2021, 83, 104007. [Google Scholar] [CrossRef]
- Katayama, O.; Lee, S.; Bae, S.; Makino, K.; Shinkai, Y.; Chiba, I.; Harada, K.; Shimada, H. Lifestyle changes and outcomes of older adults with mild cognitive impairment: A 4-year longitudinal study. Arch. Gerontol. Geriatr. 2021, 94, 104376. [Google Scholar] [CrossRef]
- Lee, J.M.; Hauskrecht, M. Modeling multivariate clinical event time-series with recurrent temporal mechanisms. Artif. Intell. Med. 2021, 112, 102021. [Google Scholar] [CrossRef]
- Bhardwaj, V.; Joshi, R.; Gaur, A.M. IoT-Based Smart Health Monitoring System for COVID-19. SN Comput. Sci. 2022, 3, 137. [Google Scholar] [CrossRef]
- Yacchirema, D.; Chura, A. SafeMobility: An IoT- based System for safer mobility using machine learning in the age of COVID-19. Procedia Comput. Sci. 2021, 184, 524–531. [Google Scholar] [CrossRef]
- Vanus, J.; Belesova, J.; Martinek, R.; Nedoma, J.; Fajkus, M.; Bilik, P.; Zidek, J. Monitoring of the daily living activities in smart home care. Hum.-Cent. Comput. Inf. Sci. 2017, 7, 30. [Google Scholar] [CrossRef] [Green Version]
- Enshaeifar, S.; Zoha, A.; Skillman, S.; Markides, A.; Acton, S.T.; Elsaleh, T.; Kenny, M.; Rostill, H.; Nilforooshan, R.; Barnaghi, P. Machine learning methods for detecting urinary tract infection and analysing daily living activities in people with dementia. PLoS ONE 2019, 14, e0209909. [Google Scholar] [CrossRef] [Green Version]
- Yacchirema, D.; de Puga, J.; Palau, C.; Esteve, M. Fall detection system for elderly people using IoT and Big Data. Procedia Comput. Sci. 2018, 130, 603–610. [Google Scholar] [CrossRef]
- Alberdi, A.; Weakley, A.; Schmitter-Edgecombe, M.; Cook, D.J.; Aztiria, A.; Basarab, A.; Barrenechea, M. Smart Home-Based Prediction of Multidomain Symptoms Related to Alzheimer’s Disease. IEEE J. Biomed. Health Inform. 2018, 22, 1720–1731. [Google Scholar] [CrossRef] [Green Version]
- Schinle, M.; Papantonis, I.; Stork, W. Personalization of monitoring system parameters to support ambulatory care for dementia patients. In Proceedings of the 2018 IEEE Sensors Applications Symposium (SAS), Seoul, Korea, 12–14 March 2018; pp. 1–6. [Google Scholar]
- Lu, P.; Hu, L.; Zhang, N.; Liang, H.; Tian, T.; Lu, L. A Two-Stage Model for Predicting Mild Cognitive Impairment to Alzheimer’s Disease Conversion. Front. Aging Neurosci. 2022, 14, 826622. [Google Scholar] [CrossRef]
- Yang, S.; Bornot, J.M.S.; Wong-Lin, K.; Prasad, G. M/EEG-based bio-markers to predict the MCI and Alzheimer’s disease: A review from the ML perspective. IEEE Trans. Biomed. Eng. 2019, 66, 2924–2935. [Google Scholar] [CrossRef]
- Akl, A.; Chikhaoui, B.; Mattek, N.; Kaye, J.; Austin, D.; Mihailidis, A. Clustering home activity distributions for automatic detection of mild cognitive impairment in older adults. J. Ambient. Intell. Smart Environ. 2016, 8, 437–451. [Google Scholar] [CrossRef] [Green Version]
- Ahamed, F.; Shahrestani, S.; Cheung, H. Internet of Things and Machine Learning for Healthy Ageing: Identifying the Early Signs of Dementia. Sensors 2020, 20, 6031. [Google Scholar] [CrossRef]
- Arifoglu, D.; Bouchachia, A. Detection of abnormal behaviour for dementia sufferers using Convolutional Neural Networks. Artif. Intell. Med. 2019, 94, 88–95. [Google Scholar] [CrossRef]
- Narasimhan, R.; Muthukumaran, G.; McGlade, C.; Ramakrishnan, A. Early Detection of Mild Cognitive Impairment Progression Using Non-Wearable Sensor Data–a Deep Learning Approach. In Proceedings of the 2020 IEEE Bangalore Humanitarian Technology Conference (B-HTC), Virtual Conference, 8–10 October 2020; pp. 1–6. [Google Scholar]
- Grand View Research. Digital Health Market Size, Share & Trends Analysis Report by Solution, By Technology (Deep Learning, Machine Learning, Natural Language Processing, Machine Vision), By End Use, By Region, And Segment Forecasts, 2021–2028. 2021. Available online: https://www.grandviewresearch.com/industry-analysis/digital-health-market/toc (accessed on 9 April 2022).
- Tabaa, M.; Monteiro, F.; Bensag, H.; Dandache, A. Green Industrial Internet of Things from a smart industry perspectives. Energy Rep. 2020, 6, 430–446. [Google Scholar] [CrossRef]
- ICT Services for Life Improvement for the Elderly. Available online: https://cordis.europa.eu/project/id/690090 (accessed on 20 February 2022).
- Persee. Available online: https://orbbec3d.com/index/Product/info.html?cate=38&id=37 (accessed on 2 May 2022).
- Record an ECH Anytime, Anywhere. Available online: https://www.withings.com/de/en/move-ecg (accessed on 7 February 2022).
- Manage Your Blood Pressure & Detect Silent Heart Conditions. Available online: https://www.withings.com/de/en/bpm-core (accessed on 7 February 2022).
- Sleep-Lab Results, at Home. Available online: https://www.withings.com/de/en/sleep-analyzer (accessed on 8 February 2022).
- Knowlegde Is Power. Available online: https://www.withings.com/it/en/scales (accessed on 8 February 2022).
- Ianculescu, M.; Alexandru, A.; Dragoș, N. Ensuring the Completeness and Accuracy of Data in a Customizable Remote Health Monitoring System. In Proceedings of the 14th International Conference on Electronics, Computers and Artificial Intelligence (ECAI 2022), Ploiești, Romania, 30 June–1 July 2022. [Google Scholar]
- Zuo, W.; Wu, J. The interaction and pathogenesis between cognitive impairment and common cardiovascular diseases in the elderly. Ther. Adv. Chronic Dis. 2022, 13, 20406223211063020. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.Y.; Zhao, X.D.; Zhu, B.G.; Hu, C.P. Demographic factors and cognitive function assessments associated with mild cognitive impairment progression for the elderly. BioMed Res. Int. 2020, 2020, 3054373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makino, K.; Lee, S.; Bae, S.; Shinkai, Y.; Chiba, I.; Shimada, H. Relationship between instrumental activities of daily living performance and incidence of mild cognitive impairment among older adults: A 48-month follow-up study. Arch. Gerontol. Geriatr. 2020, 88, 104034. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Ali, N.; Gao, Y.; Wu, H.; Wu, X.; Sun, C.; Ma, J.; Thabane, L.; Xiao, M.; Zhou, Q.; et al. Gait kinematic and kinetic characteristics of older adults with mild cognitive impairment and subjective cognitive decline: A cross-sectional study. Front. Aging Neurosci. 2021, 13, 664558. [Google Scholar] [CrossRef]
- Paraschiv, E.A.; Ianculescu, M.; Bica, O.; Sipică, A. Underpinning Improved Outcomes through Preventative Patient Care Models Based on Remote Monitoring and AI. In Proceedings of the 2021 International Conference on e-Health and Bioengineering (EHB), Iasi, Romania, 18–19 November 2021. [Google Scholar]
- Blake, C.; Merz, C.J. UCI Repository of Machine Learning Databases; Department of Information and Computer Science, University of California: Irvine, CA, USA, 1998; Available online: http://www.archive.ics.uci.edu/ml (accessed on 9 April 2022).
- Ianculescu, M.; Gavrilă, V.; Petrache, C.; Țirlea, C. Improving the Elderly’s Fall Management through Innovative Personalized Remote Monitoring Solution. In Proceedings of the 2021 International Conference on e-Health and Bioengineering (EHB), Iasi, Romania, 18–19 November 2021. [Google Scholar]
- COVID-19 Disrupting Mental Health Services in Most Countries, WHO Survey. Available online: https://www.who.int/news/item/05-10-2020-covid-19-disrupting-mental-health-services-in-most-countries-who-survey (accessed on 13 April 2022).
- O’Rourke, B.; Oortwijn, W.; Schuller, T.; International Joint Task Group. The new definition of health technology assessment: A milestone in international collaboration. Int. J. Technol. Assess. Health Care 2020, 36, 187–190. [Google Scholar] [CrossRef]
- Good Machine Learning Practice for Medical Device Development: Guiding Principles. Available online: https://www.gov.uk/government/publications/good-machine-learning-practice-for-medical-device-development-guiding-principles (accessed on 9 April 2022).
- Unsworth, H.; Dillon, B.; Collinson, L.; Powell, H.; Salmon, M.; Oladapo, T.; Ayiku, L.; Shield, G.; Holden, J.; Patel, N.; et al. The NICE Evidence Standards Framework for digital health and care technologies–Developing and maintaining an innovative evidence framework with global impact. Digit. Health 2021, 7, 20552076211018617. [Google Scholar] [CrossRef]
- Evaluating Digital Health Products. Available online: https://www.gov.uk/government/collections/evaluating-digital-health-products (accessed on 9 April 2022).
- WHO Guideline: Recommendations on Digital Interventions for Health System Strengthening. Available online: https://apps.who.int/iris/bitstream/handle/10665/311941/9789241550505-eng.pdf?ua=1 (accessed on 9 April 2022).
- Health Information Systems Interoperability Maturity Toolkit. Available online: https://www.measureevaluation.org/resources/tools/health-information-systems-interoperability-toolkit (accessed on 9 April 2022).
- A Minimum HTA Inspired Framework to Assess the Value of National eHealth Projects. Available online: https://jasehn.eu/wordpress/wp-content/uploads/2018/05/D7.4_REPORT_Minimum_HTA_inspired_framework_assess_value_of_National_eHealth_projects-1-1.pdf (accessed on 9 April 2022).
- Ianculescu, M.; Alexandru, A.; Pop, F. Critical Analysis and Evaluation of Current Digital Healthcare Solutions. In Proceedings of the 23nd International Conference on Control Systems and Computer Science (23rd CSCS 2021)—MEDEA-2021: International Workshop on Monitoring and EviDEnce-Based Protocols, Applications and Platforms for Active Living and Aging, Bucharest, Romania, 26–28 May 2021; pp. 482–488. [Google Scholar]


| Type of Recognition | Advantages | Necessary Hardware and Software | Limitations |
|---|---|---|---|
| Recognition of simple activities | Collecting data from wearable sensors Applying supervised learning methods [11] | Wearable accelerometers (possibly coupled with biometrical sensors and integrated in clothes) | Contextual information (current location, environmental conditions, ambient objects, etc.) for improving the accuracy of recognition is not considered |
| Considering the user’s context | Several environmental sensors | Not suited for more complex activities | |
| Recognition of complex activities | Using sensors to recognize the elderly person’s movements and their interactions with objects and furniture (such as IADLs executed at home) | Time series data analysis method [12] to segment sequences of sensor events. Hidden Markov models inference [13] to recognize activities based on features extracted from recent sensor events. Ontologies to model complex human activities [14]. Machine learning for recognizing activity instances [15], and differentiating cognitively healthy seniors from Alzheimer’s patients based on activity execution and gait events [16]. | Detection of abnormal behaviors on a short-term basis Patient’s personal habits are not considered |
| Long-term analysis of activity data | Model the patient’s usual behavior from the activities performed in the past to detect anomalies as changes | Using an alert system to detect changes in the activity patterns and generate health alerts [17]. Detecting recurrent IADL patterns and their variations by using data mining of heterogeneous multivariate time-series from sensor data [18]. | Necessity of big amount of data |
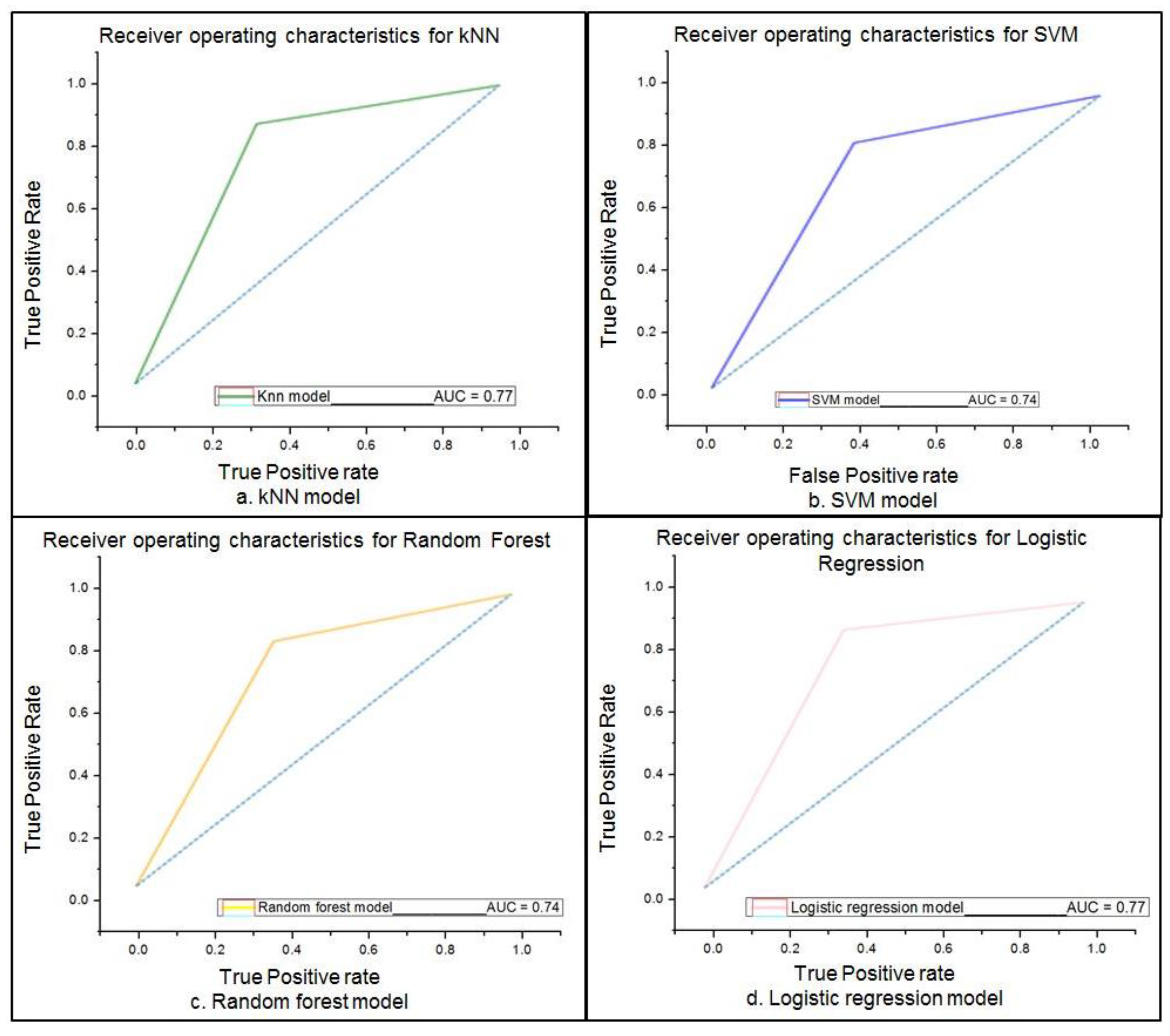
| Model | Sensitivity | Specificity | Accuracy | |
|---|---|---|---|---|
| ML | kNN | 87.09% | 66.67% | 76.01% |
| SVM | 83.87% | 63.4% | 74% | |
| Random Forest | 83.9% | 64% | 73.8% | |
| Logistic Regression | 91% | 65% | 77% | |
| DL | 94% | 68% | 82% | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ianculescu, M.; Paraschiv, E.-A.; Alexandru, A. Addressing Mild Cognitive Impairment and Boosting Wellness for the Elderly through Personalized Remote Monitoring. Healthcare 2022, 10, 1214. https://doi.org/10.3390/healthcare10071214
Ianculescu M, Paraschiv E-A, Alexandru A. Addressing Mild Cognitive Impairment and Boosting Wellness for the Elderly through Personalized Remote Monitoring. Healthcare. 2022; 10(7):1214. https://doi.org/10.3390/healthcare10071214
Chicago/Turabian StyleIanculescu, Marilena, Elena-Anca Paraschiv, and Adriana Alexandru. 2022. "Addressing Mild Cognitive Impairment and Boosting Wellness for the Elderly through Personalized Remote Monitoring" Healthcare 10, no. 7: 1214. https://doi.org/10.3390/healthcare10071214
APA StyleIanculescu, M., Paraschiv, E.-A., & Alexandru, A. (2022). Addressing Mild Cognitive Impairment and Boosting Wellness for the Elderly through Personalized Remote Monitoring. Healthcare, 10(7), 1214. https://doi.org/10.3390/healthcare10071214







