Intraocular Pressure Fluctuation during Aerobic Exercise at Different Exercise Intensities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
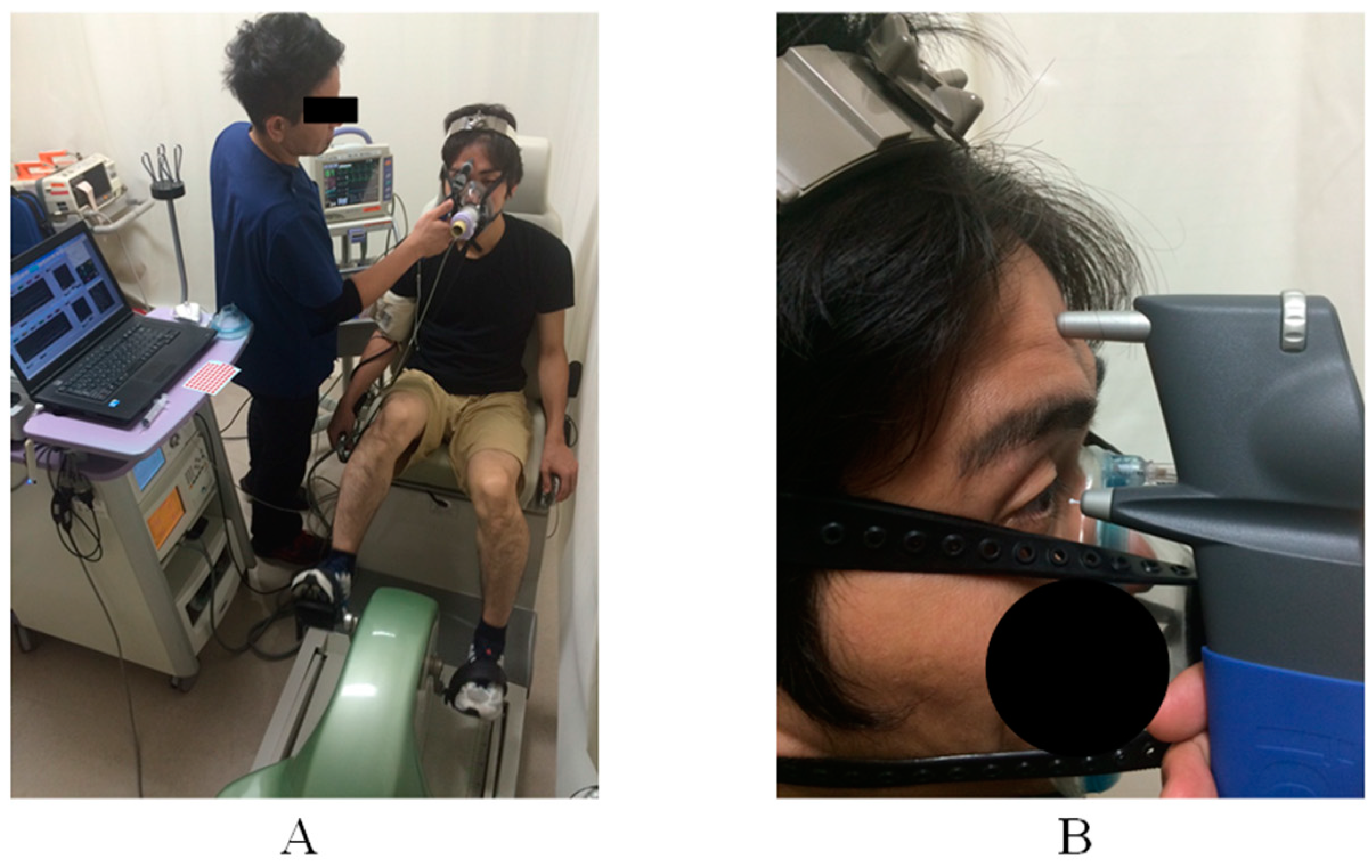
2.2. Measurement Protocol
2.3. Maximal Cardiopulmonary Exercise Test
2.4. Constant Aerobics Exercise Protocol
2.5. Oxygen Consumption
2.6. Intraocular Pressure
2.7. Statistical Analysis
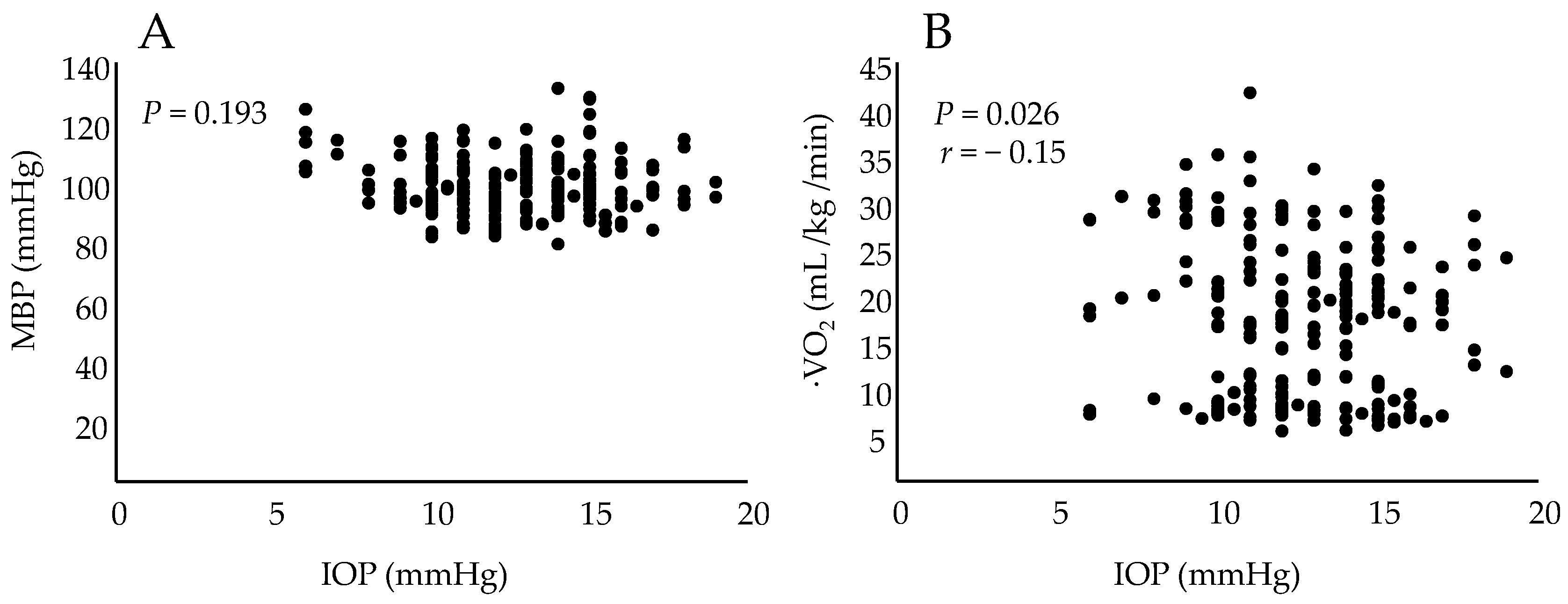
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas: Diabetes around the World in 2021. Available online: https://diabetesatlas.org/ (accessed on 28 September 2021).
- Boulé, N.G.; Haddad, E.; Kenny, G.P.; Wells, G.A.; Sigal, R.J. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: A meta-analysis of controlled clinical trials. JAMA 2001, 286, 1218–1227. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Cai, X.; Schumann, U.; Velders, M.; Sun, Z.; Steinacker, J.M. Impact of walking on glycemic control and other cardiovascular risk factors in type 2 diabetes: A meta-analysis. PLoS ONE 2014, 9, e109767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabag, A.; Way, K.L.; Keating, S.E.; Sultana, R.N.; O’Connor, H.T.; Baker, M.K.; Chuter, V.H.; George, J.; Johnson, N.A. Exercise and ectopic fat in type 2 diabetes: A systematic review and meta-analysis. Diabetes Metab. 2017, 43, 195–210. [Google Scholar] [CrossRef] [PubMed]
- Araki, E.; Goto, A.; Kondo, T.; Noda, M.; Noto, H.; Origasa, H.; Osawa, H.; Taguchi, A.; Tanizawa, Y.; Tobe, K.; et al. Japanese Clinical Practice Guideline for Diabetes 2019. J. Diabetes Investig. 2020, 11, 1020–1076. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Li, J.; Lian, Y.; Tang, Z.X.; Zen, Z.; Yu, P.; Li, Y. Long-term intensive lifestyle intervention promotes improvement of stage III diabetic nephropathy. Med. Sci. Monit. 2019, 25, 3061–3068. [Google Scholar] [CrossRef] [PubMed]
- Robinson-Cohen, C.; Littman, A.J.; Duncan, G.E.; Weiss, N.S.; Sachs, M.C.; Ruzinski, J.; Kundzins, J.; Rock, D.; de Boer, I.H.; Ikizler, T.A.; et al. Physical activity and change in estimated GFR among persons with CKD. J. Am. Soc. Nephrol. 2014, 25, 399–406. [Google Scholar] [CrossRef] [Green Version]
- Yoo, M.; D’Silva, L.J.; Martin, K.; Sharma, N.K.; Pasnoor, M.; LeMaster, J.W.; Kluding, P.M. Pilot study of exercise therapy on painful diabetic peripheral neuropathy. Pain Med. 2015, 16, 1482–1489. [Google Scholar] [CrossRef] [Green Version]
- Brown, R.; Sharafi, A.; Slade, J.M.; Convit, A.; Davis, N.; Baete, S.; Milton, H.; Mroczek, K.J.; Kluding, P.M.; Regatte, R.R.; et al. Lower extremity MRI following 10-week supervised exercise intervention in patients with diabetic peripheral neuropathy. BMJ Open Diabetes Res. Care 2021, 9, e002312. [Google Scholar] [CrossRef]
- Aro, A.; Kauppinen, A.; Kivinen, N.; Selander, T.; Kinnunen, K.; Tuomilehto, J.; Keinänen-Kiukaanniemi, S.; Lindström, J.; Uusitupa, M.; Kaarniranta, K. Life style intervention improves retinopathy status-the Finnish Diabetes Prevention Study. Nutrients 2019, 11, 1691. [Google Scholar] [CrossRef] [Green Version]
- Kuwata, H.; Okamura, S.; Hayashino, Y.; Tsujii, S.; Ishii, H.; Diabetes Distress and Care Registry at Tenri Study Group. Higher levels of physical activity are independently associated with a lower incidence of diabetic retinopathy in Japanese patients with type 2 diabetes: A prospective cohort study, Diabetes Distress and Care Registry at Tenri (DDCRT15). PLoS ONE 2017, 12, e0172890. [Google Scholar] [CrossRef]
- Woodcock, M.G.L.; Richards, J.C.; Murray, A.D.N. The last 11 years of Molteno implantation at the University of Cape Town. Refining our indications and surgical technique. Eye 2008, 22, 18–25. [Google Scholar] [CrossRef]
- Al-Shamsi, H.N.; Dueker, D.K.; Nowilaty, S.R.; Al-Shahwan, S.A. Neovascular glaucoma at King Khaled Eye Specialist Hospital—Etiologic consideration. Middle East Afr. J. Ophthalmol. 2009, 16, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Natsis, K.; Asouhidou, I.; Nousios, G.; Chatzibalis, T.; Vlasis, K.; Karabatakis, V. Aerobic exercise and intraocular pressure in normotensive and glaucoma patients. BMC Ophthalmol. 2009, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Vieira, G.M.; Oliveira, H.B.; de Andrade, D.T.; Bottaro, M.; Ritch, R. Intraocular pressure variation during weight lifting. Arch. Ophthalmol. 2006, 124, 1251–1254. [Google Scholar] [CrossRef] [Green Version]
- Shapiro, A.; Shoenfeld, Y.; Shapiro, Y. The effect of standardised submaximal work load on intraocular pressure. Br. J. Ophthalmol. 1978, 62, 679–681. [Google Scholar] [CrossRef] [Green Version]
- Karvonen, M.J.; Kentala, E.; Mustala, O. The effects of training on heart rate; a longitudinal study. Ann. Med. Exp. Biol. Fenn. 1957, 35, 307–315. [Google Scholar] [PubMed]
- Wu, F.; Zhao, Y.; Zhang, H. Ocular autonomic nervous system: An update from anatomy to physiological functions. Vision 2022, 6, 6. [Google Scholar] [CrossRef]
- Kiel, J.W.; Reitsamer, H.A.; Walker, J.S.; Kiel, F.W. Effects of nitric oxide synthase inhibition on ciliary blood flow, aqueous production and intraocular pressure. Exp. Eye Res. 2001, 73, 355–364. [Google Scholar] [CrossRef]
- Phillips, C.I.; Howitt, G.; Rowlands, D.J. Propranolol as ocular hypotensive agent. Br. J. Ophthalmol. 1967, 51, 222–226. [Google Scholar] [CrossRef] [Green Version]
- Harris, A.; Malinovsky, V.; Martin, B. Correlates of acute exercise-induced ocular hypotension. Investig. Ophthalmol. Vis. Sci. 1994, 35, 3852–3857. [Google Scholar]
- Alnawaiseh, M.; Lahme, L.; Treder, M.; Rosentreter, A.; Eter, N. Short-term effects of exercise on optic nerve and macular perfusion measured by optical coherence tomography angiography. Retina 2017, 37, 1642–1646. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, B.; Nelis, P.; Rolfes, F.; Alnawaiseh, M.; Klose, A.; Krüger, M.; Eter, N.; Brand, S.-M.; Alten, F. Effects of high-intensity interval training on optic nerve head and macular perfusion using optical coherence tomography angiography in healthy adults. Atherosclerosis 2018, 247, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Shiose, Y. The aging effect on intraocular pressure in an apparently normal population. Arch. Ophthalmol. 1984, 102, 883–887. [Google Scholar] [CrossRef] [PubMed]
| 30%-intensity aerobic exercise | ||||||
| Rest | 5 min | 10 min | 15 min | 20 min | Recovery | |
| IOP (mmHg) | 13.7 ± 2.2 | 13.0 ± 2.2 | 12.9 ± 2.2 | 12.4 ± 2.4 | 12.1 ± 2.3 | 12.5 ± 2.9 |
| MBP (mmHg) | 93.2 ± 9.5 | 97.6 ± 6.3 | 95.2 ± 8.7 | 95.5 ± 7.1 | 95.0 ± 8.5 | 93.6 ± 7.1 |
| ·VO2 (mL/kg/min) | 5.5 ± 1.5 | 8.7 ± 2.7 * | 8.4 ± 2.7 * | 8.6 ± 2.9 * | 8.6 ± 2.7 * | 5.7 ± 1.5 * |
| 50%-intensity aerobic exercise | ||||||
| Rest | 5 min | 10 min | 15 min | 20 min | Recovery | |
| IOP (mmHg) | 14.3 ± 2.7 | 13.9 ± 2.5 | 13.1 ± 2.7 | 13.4 ± 2.9 | 13.1 ± 3.3 | 13.0 ± 2.9 |
| MBP (mmHg) | 93.7 ± 11.2 | 97.5 ± 7.1 | 95.7 ± 7.9 | 97.3 ± 8.1 | 96.8 ± 9.9 | 93.6 ± 8.3 |
| ·VO2 (mL/kg/min) | 5.7 ± 1.6 | 17.7 ± 4.3 * | 17.6 ± 4.4 * | 16.5 ± 3.8 * | 15.8 ± 4.3 * | 7.8 ± 2.6 |
| 70%-intensity aerobic exercise | ||||||
| Rest | 5 min | 10 min | 15 min | 20 min | Recovery | |
| IOP (mmHg) | 14.2 ± 2.6 | 12.4 ± 2.8 * | 11.5 ± 2.6 * | 11.5 ± 2.5 * | 11.6 ± 2.8 * | 13.1 ± 2.3 |
| MAP (mmHg) | 94.3 ± 10.4 | 110.0 ± 12.4 * | 103.3 ± 9.9 * | 102.2 ± 7.5 * | 100.3 ± 7.1 * | 94.1 ± 11.2 |
| ·VO2 (mL/kg/min) | 5.8 ± 1.7 | 28.7 ± 5.2 * | 25.7 ± 4.7 * | 24.6 ± 3.9 * | 24.6 ± 4.3 * | 7.0 ± 2.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawae, T.; Nomura, T.; Iwaki, D.; Nakashima, Y.; Fudeyasu, K.; Kataoka, H.; Ishiguro, T.; Kimura, H. Intraocular Pressure Fluctuation during Aerobic Exercise at Different Exercise Intensities. Healthcare 2022, 10, 1196. https://doi.org/10.3390/healthcare10071196
Kawae T, Nomura T, Iwaki D, Nakashima Y, Fudeyasu K, Kataoka H, Ishiguro T, Kimura H. Intraocular Pressure Fluctuation during Aerobic Exercise at Different Exercise Intensities. Healthcare. 2022; 10(7):1196. https://doi.org/10.3390/healthcare10071196
Chicago/Turabian StyleKawae, Toshihiro, Takuo Nomura, Daisuke Iwaki, Yuki Nakashima, Kenichi Fudeyasu, Hiroaki Kataoka, Tomoyasu Ishiguro, and Hiroaki Kimura. 2022. "Intraocular Pressure Fluctuation during Aerobic Exercise at Different Exercise Intensities" Healthcare 10, no. 7: 1196. https://doi.org/10.3390/healthcare10071196
APA StyleKawae, T., Nomura, T., Iwaki, D., Nakashima, Y., Fudeyasu, K., Kataoka, H., Ishiguro, T., & Kimura, H. (2022). Intraocular Pressure Fluctuation during Aerobic Exercise at Different Exercise Intensities. Healthcare, 10(7), 1196. https://doi.org/10.3390/healthcare10071196






