Anterior Ocular Biometrics as Measured by Ultrasound Biomicroscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
2.2. Examination Technique
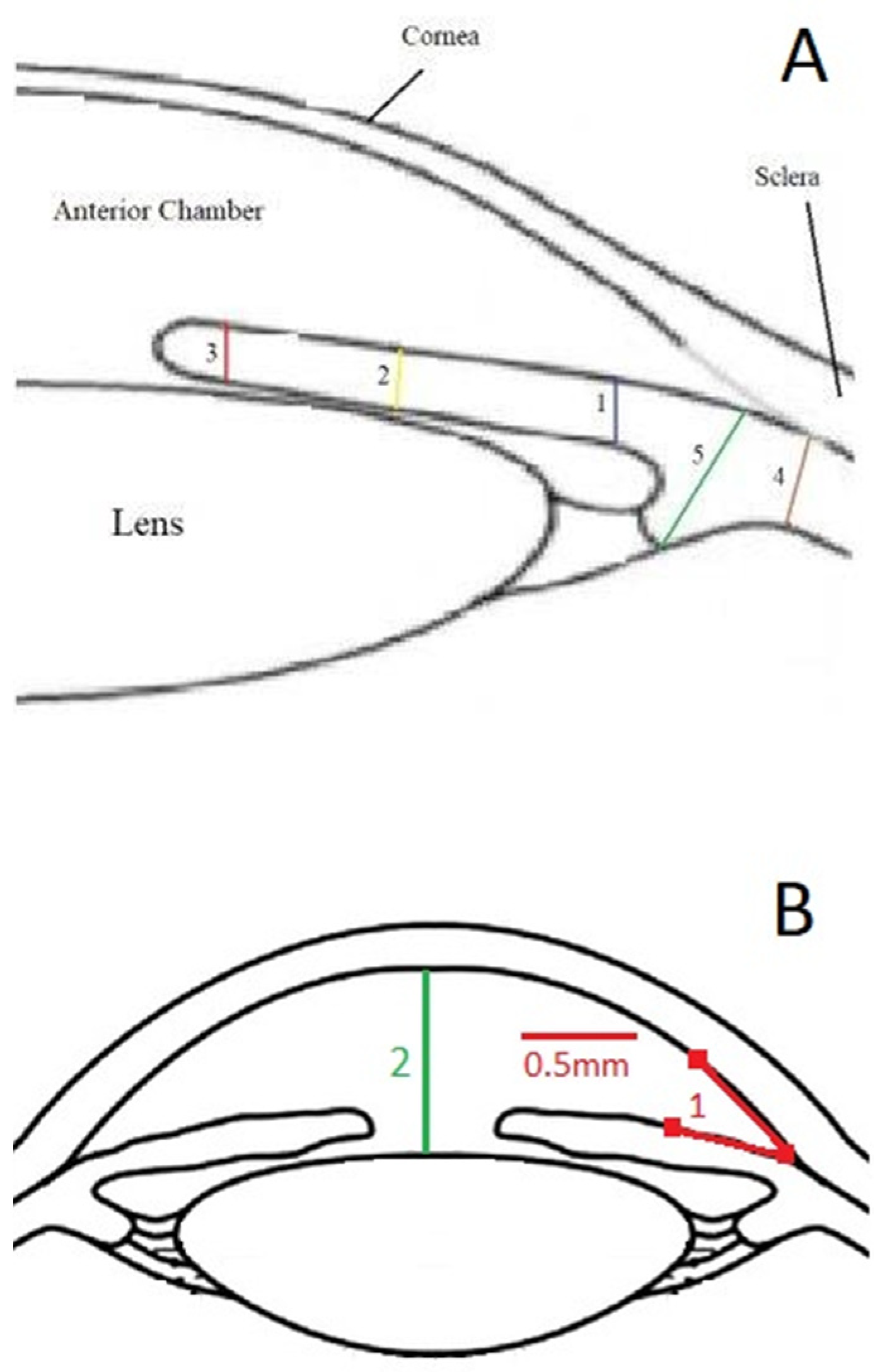
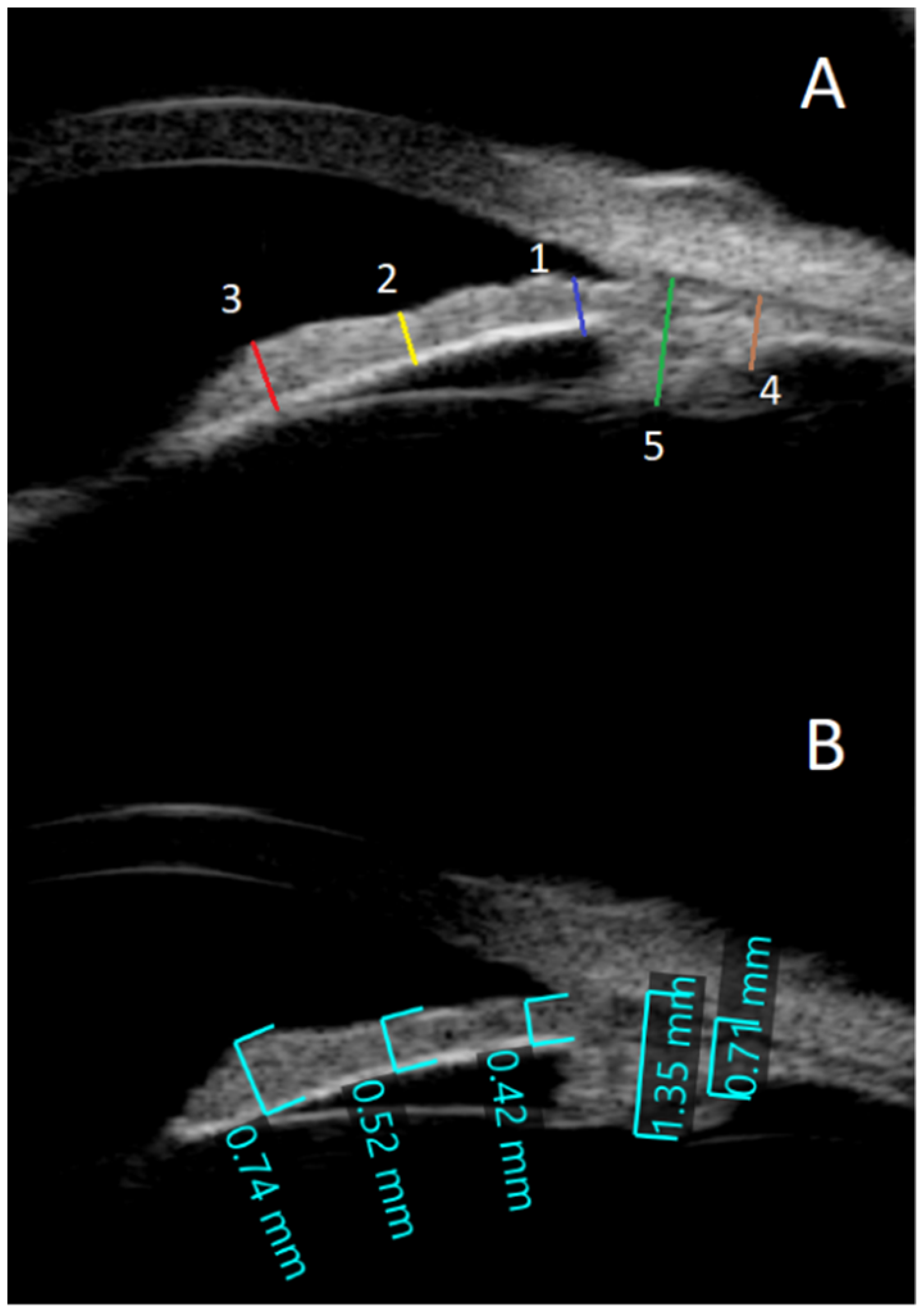
- The anterior chamber angle (in degrees) was calculated using two 0.5 mm lines that began at the iris root and traveled across the posterior cornea and the anterior surface of the iris (Figure 1B). Images were obtained from each quadrant (upper, lower, nasal, and temporal).
- To determine the anterior segment depth (ASD), the axial distance between the anterior corneal surface and the anterior surface of the lens was measured; views were as vertical as feasible, as indicated on the screen (See Figure 1B).
- The thicknesses of the iris were measured at 0.8 mm from the iris root, amid the iris, and at the juxtapupillary edge of the iris (thickest area of the iris at 1 mm distance from the pupil).
- The perpendicular distance between the apex of the ciliary body (inner tip) and the inner wall of the sclera was measured to assess CB thickness. The distance between the inner-most point of the ciliary process and the inner wall of the sclera was used to calculate the CB + ciliary process thickness (perpendicular to the scleral wall).
- The sulcus-to-sulcus diameters (STS) were measured as cross-sectional images and were obtained on the following two meridians: vertical (up-down, 90°) and horizontal (nasal-temporal 180°). The STS diameters were measured offline in the images with the widest pupil diameter.
2.3. Statistical Analysis
3. Results
3.1. Anterior Segment Depth and Anterior Chamber Angle Measurements
3.2. Iris Measurements
3.3. Ciliary Body Measurements
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pavlin, C.J.; Sherar, M.D.; Foster, F.S. Subsurface ultrasound microscopic imaging of the intact eye. Ophthalmology 1990, 97, 244–250. [Google Scholar] [CrossRef]
- Marigo, F.A.; Finger, P.T.; McCormick, S.A.; Iezzi, R.; Esaki, K.; Ishikawa, H.; Seedor, J.; Liebmann, J.M.; Ritch, R. Anterior segment implantation cysts. Ultrasound biomicroscopy with histopathologic correlation. Arch. Ophthalmol. 1998, 116, 1569–1575. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Marigo, F.A.; Esaki, K.; Finger, P.T.; Ishikawa, H.; Greenfield, D.S.; Liebmann, J.M.; Ritch, R. Differential diagnosis of anterior segment cysts by ultrasound biomicroscopy. Ophthalmology 1999, 106, 2131–2135. [Google Scholar] [CrossRef]
- Marigo, F.A.; Finger, P.T.; McCormick, S.A.; Iezzi, R.; Esaki, K.; Ishikawa, H.; Liebmann, J.M.; Ritch, R. Iris and ciliary body melanomas: Ultrasound biomicroscopy with histopathologic correlation. Arch. Ophthalmol. 2000, 118, 1515–1521. [Google Scholar] [CrossRef] [PubMed]
- Finger, P.T.; Reddy, S.; Chin, K. High-frequency ultrasound characteristics of 24 iris and iridociliary melanomas: Before and after plaque brachytherapy. Arch. Ophthalmol. 2007, 125, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- Finger, P.T.; Meskin, S.W.; Wisnicki, H.J.; Albekioni, Z.; Schneider, S. High-frequency ultrasound of anterior segment retinoblastoma. Am. J. Ophthalmol. 2004, 137, 944–946. [Google Scholar] [CrossRef]
- Wada, S.; Kohno, T.; Yanagihara, N.; Hirabayashi, M.; Tabuchi, H.; Shiraki, K.; Miki, T. Ultrasound biomicroscopic study of ciliary body changes in the post-treatment phase of Vogt-Koyanagi-Harada disease. Br. J. Ophthalmol. 2002, 86, 1374–1379. [Google Scholar] [CrossRef]
- Finger, P.T.; Narayana, K.; Iacob, C.E.; Samson, C.M.; Latkany, P. Giant sarcoid tumor of the iris and ciliary body. Ocul. Immunol. Inflamm. 2007, 15, 121–125. [Google Scholar] [CrossRef]
- Finger, P.T.; Tran, H.V.; Turbin, R.E.; Perry, H.D.; Abramson, D.H.; Chin, K.; Della Rocca, R.; Ritch, R. High-frequency ultrasonographic evaluation of conjunctival intraepithelial neoplasia and squamous cell carcinoma. Arch. Ophthalmol. 2003, 121, 168–172. [Google Scholar] [CrossRef]
- Garcia, J.P.; de la Cruz, J.; Rosen, R.B.; Buxton, D.F. Imaging implanted keratoprostheses with anterior-segment optical coherence tomography and ultrasound biomicroscopy. Cornea 2008, 27, 180–188. [Google Scholar] [CrossRef]
- Pavlin, C.J.; Harasiewicz, K.; Foster, F.S. Ultrasound biomicroscopy of anterior segment structures in normal and glaucomatous eyes. Am. J. Ophthalmol. 1992, 113, 381–389. [Google Scholar] [CrossRef]
- Garcia, J.P.; Spielberg, L.; Finger, P.T. High-frequency ultrasound measurements of the normal ciliary body and iris. Ophthalmic. Surg. Lasers Imaging 2011, 42, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Gohdo, T.; Tsumura, T.; Iijima, H.; Kashiwagi, K.; Tsukahara, S. Ultrasound biomicroscopic study of ciliary body thickness in eyes with narrow angles. Am. J. Ophthalmol. 2000, 129, 342–346. [Google Scholar] [CrossRef]
- El Shakankiri, N.M.; Bayoumi, N.H.; Abdallah, A.H.; El Sahn, M.M. Role of ultrasound and biomicroscopy in evaluation of anterior segment anatomy in congenital and developmental cataract cases. J. Cataract. Refract. Surg. 2009, 35, 1893–1905. [Google Scholar] [CrossRef]
- Aptel, F.; Denis, P. Optical coherence tomography quantitative analysis of iris volume changes after pharmacologic mydriasis. Ophthalmology 2010, 117, 3–10. [Google Scholar] [CrossRef]
- Invernizzi, A.; Cigada, M.; Savoldi, L.; Cavuto, S.; Fontana, L.; Cimino, L. In vivo analysis of the iris thickness by spectral domain optical coherence tomography. Br. J. Ophthalmol. 2014, 98, 1245–1249. [Google Scholar] [CrossRef]
- Ragab, I.T.; Abdelkader, A.M.E.; Kishk, H.M.; Elshal, A.A. Assessment of Post-Operative Pseudophakic Glaucoma by Ultrasound Biomicroscopy. Clin. Ophthalmol. 2020, 14, 1495–1501. [Google Scholar] [CrossRef] [PubMed]
- Dada, T.; Agarwal, A.; Vanathi, M.; Gadia, R.; Panda, A.; Gupta, V.; Sihota, R. Ultrasound biomicroscopy in opaque grafts with post-penetrating keratoplasty glaucoma. Cornea 2008, 27, 402–405. [Google Scholar] [CrossRef]
- Xu, L.; Cao, W.F.; Wang, Y.X.; Chen, C.X.; Jonas, J.B. Anterior chamber depth and chamber angle and their associations with ocular and general parameters: The Beijing Eye Study. Am. J. Ophthalmol. 2008, 145, 929–936. [Google Scholar] [CrossRef]
- Bell, N.P.; Nagi, K.S.; Cumba, R.J.; Chuang, A.Z.; Lee, D.A.; Prager, T.C.; Rao, K.; Feldman, R.M. Age and positional effect on the anterior chamber angle: Assessment by ultrasound biomicroscopy. ISRN Ophthalmol. 2013, 2013, 706201. [Google Scholar] [CrossRef]
- Friedman, D.S.; Gazzard, G.; Min, C.B.; Broman, A.T.; Quigley, H.; Tielsch, J.; Seah, S.; Foster, P.J. Age and sex variation in angle findings among normal Chinese subjects: A comparison of UBM, Scheimpflug, and gonioscopic assessment of the anterior chamber angle. J. Glaucoma 2008, 17, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Esaki, K.; Ishikawa, H.; Liebmann, J.M.; Greenfield, D.S.; Uji, Y.; Ritch, R. Angle recess area decreases with age in normal Japanese. Jpn. J. Ophthalmol. 2000, 44, 46–51. [Google Scholar] [CrossRef]
- Pan, Z.; Furuya, T.; Kashiwagi, K. Longitudinal changes in anterior chamber configuration in eyes with open-angle glaucoma and associated factors. J. Glaucoma 2012, 21, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Bao, Y.Z.; Pei, X.T. Morphologic changes in the anterior chamber in patients with cortical or nuclear age-related cataract. J. Cataract. Refract. Surg. 2011, 37, 77–82. [Google Scholar] [CrossRef]
- Chen, X.; Han, T.; Zhao, W.; Wang, X.; Xu, Y.; Cheng, M.; Wang, X.; Zhou, X. Effect of the Difference Between the White-to-White and Sulcus-to-Sulcus on Vault and the Related Factors After ICL Implantation. Ophthalmol. Ther. 2021, 10, 947–955. [Google Scholar] [CrossRef]
- Izatt, J.A.; Hee, M.R.; Swanson, E.A.; Lin, C.P.; Huang, D.; Schuman, J.S.; Puliafito, C.A.; Fujimoto, J.G. Micrometer-scale resolution imaging of the anterior eye in vivo with optical coherence tomography. Arch. Ophthalmol. 1994, 112, 1584–1589. [Google Scholar] [CrossRef]
- Henzan, I.M.; Tomidokoro, A.; Uejo, C.; Sakai, H.; Sawaguchi, S.; Iwase, A.; Araie, M. Ultrasound biomicroscopic configurations of the anterior ocular segment in a population-based study the Kumejima Study. Ophthalmology 2010, 117, 1720–1728. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Rollins, A.M.; Roth, J.E.; Yazdanfar, S.; Westphal, V.; Bardenstein, D.S.; Izatt, J.A. Real-time optical coherence tomography of the anterior segment at 1310 nm. Arch. Ophthalmol. 2001, 119, 1179–1185. [Google Scholar] [CrossRef]
- He, M.; Wang, D.; Console, J.W.; Zhang, J.; Zheng, Y.; Huang, W. Distribution and heritability of iris thickness and pupil size in Chinese: The Guangzhou Twin Eye Study. Invest. Ophthalmol. Vis. Sci. 2009, 50, 1593–1597. [Google Scholar] [CrossRef]
- Garcia, J.P.; Rosen, R.B. Anterior segment imaging: Optical coherence tomography versus ultrasound biomicroscopy. Ophthalmic. Surg. Lasers Imaging 2008, 39, 476–484. [Google Scholar] [CrossRef]
- Liu, B.; Kang, C.; Fang, F. Biometric Measurement of Anterior Segment: A Review. Sensors 2020, 20, 4285. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, D.; Zeng, Y.; Wang, Y.; Liang, X.; Liu, X. Comparison of anterior segment optical coherence tomography and ultrasound biomicroscopy for iris parameter measurements in patients with primary angle closure glaucoma. Eye Sci. 2013, 28, 1–6. [Google Scholar] [PubMed]
- Radhakrishnan, S.; Goldsmith, J.; Huang, D.; Westphal, V.; Dueker, D.K.; Rollins, A.M.; Izatt, J.A.; Smith, S.D. Comparison of optical coherence tomography and ultrasound biomicroscopy for detection of narrow anterior chamber angles. Arch. Ophthalmol. 2005, 123, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Ziaul, Y.H.; Mahale, A.; Varghese, S.; Khanam, F.; AlFutaise, M.; Ahad, M.A.; Edward, D.P.; Khandekar, R.B. The Iris Thickness in a Healthy Saudi Population. Cureus 2021, 13, e12521. [Google Scholar] [CrossRef]
- Huang, W.; Gao, X.; Li, X.; Wang, J.; Chen, S.; Wang, W.; Du, S.; He, M.; Zhang, X. Anterior and posterior ocular biometry in healthy Chinese subjects: Data based on AS-OCT and SS-OCT. PLoS ONE 2015, 10, 121740. [Google Scholar] [CrossRef]
- He, N.; Wu, L.; Qi, M.; He, M.; Lin, S.; Wang, X.; Yang, F.; Fan, X. Comparison of Ciliary Body Anatomy between American Caucasians and Ethnic Chinese Using Ultrasound Biomicroscopy. Curr. Eye Res. 2016, 41, 485–491. [Google Scholar] [CrossRef]
- Alibet, Y.; Levytska, G.; Umanets, N.; Pasyechnikova, N.; Henrich, P.B. Ciliary body thickness changes after preoperative anti-inflammatory treatment in rhegmatogenous retinal detachment complicated by choroidal detachment. Graefes Arch. Clin. Exp. Ophthalmol. 2017, 255, 1503–1508. [Google Scholar] [CrossRef]
- Oliveira, C.; Tello, C.; Liebmann, J.M.; Ritch, R. Ciliary body thickness increases with increasing axial myopia. Am. J. Ophthalmol. 2005, 140, 324–325. [Google Scholar] [CrossRef]
- Velazquez-Martin, J.P.; Krema, H.; Fulda, E.; Yücel, Y.H.; Simpson, E.R.; Pavlin, C.J. Ultrasound biomicroscopy of the ciliary body in ocular/oculodermal melanocytosis. Am. J. Ophthalmol. 2013, 155, 681–687.e1-2. [Google Scholar] [CrossRef]
- Galvis, V.; Villamil, J.F.; Acuña, M.F.; Camacho, P.A.; Merayo-Lloves, J.; Tello, A.; Zambrano, S.L.; Rey, J.J.; Espinoza, J.V.; Prada, A.M. Long-term endothelial cell loss with the iris-claw intraocular phakic lenses (Artisan®). Graefes Arch. Clin. Exp. Ophthalmol. 2019, 257, 2775–2787. [Google Scholar] [CrossRef]
| Parameter | Anterior Chamber Depth (mm) | Anterior Chamber Angle (Degree) |
|---|---|---|
| Overall (95 eyes) | 2.92,2.91 ± 0.41 | 34.3, 34.1 ± 12.1 |
| Sex | ||
| Female (n = 37 eyes, 21 patient) | 2.90,2.90 ± 0.40 | 34.1, 34.0 ± 12.1 |
| Male (n = 58 eyes, 31 patient) | 2.96,2.93 ± 0.41 | 34.4, 34.2 ± 12.1 |
| p-value | 0.58 | 0.52 |
| Age (year) | ||
| 20–40 (50 eyes) | 3.01,2.92 ± 0.41 | 34.8, 34.6 ± 12.1 |
| 40–60 (29 eyes) | 2.91,2.91 ± 0.40 | 34.3, 34.2 ± 12.1 |
| 60–80 (10 eyes) | 2.88,2.90 ± 0.39 | 33.9, 33.8 ± 12.1 |
| p-value | 0.24 | 0.18 |
| Height | ||
| Less than 160 cm (55 eyes) | 2.91,2.90 ± 0.38 | 32.2, 32.1 ± 12.2 |
| More than 160 cm (40 eyes) | 2.94,2.92 ± 0.41 | 35.3, 34.1 ± 12.1 |
| p-value | 0.78 | 0.42 |
| Eye | ||
| OD (46 eyes) | 2.93,2.92 ± 0.41 | 34.4, 34.2 ± 12.1 |
| OS (49 eyes) | 2.91,2.90 ± 0.41 | 34.3, 34.1 ± 12.1 |
| p-value | 0.89 | 0.91 |
| Quadrant | ||
| Upper quadrant (95 eyes) | NA | 35.2, 34.2 ± 12.1 |
| Lower quadrant (95 eyes) | NA | 33.8, 34.1 ± 12.1 |
| p-value | 0.04 | |
| Nasal quadrant (95 eyes) | NA | 34.5, 34.3 ± 12.1 |
| Temporal quadrant (95 eyes) | NA | 34.2, 34.0 ± 12.1 |
| p-value | 0.86 | |
| Parameter | Iris Root Thickness * [Mean, Median ± SD (range)] | Mid of the Iris [Mean, Median ± SD (Range)] | Tip of the Iris [Mean, Median ± SD (Range)] |
|---|---|---|---|
| Measured thickness of the iris (95 eyes) | 0.41, 0.40 ± 0.1 (0.28–0.65) | 0.51, 0.50 ± 0.1 (0.30–0.73) | 0.71, 0.70 ± 0.1 (0.41–0.93) |
| Sex (Male/Female) | |||
| Female (37 eyes, 21 patients) | 0.40, 0.40 ± 0.1 (0.28–0.62) | 0.50, 0.50 ± 0.1 (0.30–0.70) | 0.70, 0.69 ± 0.1 (0.41–0.88) |
| Male (58 eyes, 31 patients) | 0.41, 0.40 ± 0.1 (0.29–0.65) | 0.51, 0.50 ± 0.1 (0.31–0.73) | 0.72, 0.71 ± 0.1 (0.43–0.93) |
| p-value | 0.89 | 0.89 | 0.82 |
| Age (in years) | |||
| Less than 40 (44 eyes) | 0.41, 0.40 ± 0.1 (0.29–0.65) | 0.51, 0.50 ± 0.1 (0.32–0.73) | 0.71, 0.70 ± 0.1 (0.42–0.93) |
| More than 40 (41 eyes) | 0.41, 0.40 ± 0.1 (0.28–0.62) | 0.50, 0.50 ± 0.1 (0.30–0.70) | 0.70, 0.70 ± 0.1 (0.41–0.90) |
| p-value | 1.00 | 0.89 | 0.93 |
| Height (in cm) | |||
| Less than 160 cm (55 eyes) | 0.40, 0.40 ± 0. 0.1 (0.29–0.61) | 0.51, 0.50 ± 0.1 (0.31-0.70) | 0.71, 0.70 ± 0.1 (0.41–0.91) |
| More than 160 cm (40 eyes) | 0.41, 0.40 ± 0.1 (0.28–0.65) | 0.51, 0.50 ± 0.1 (0.32–0.73) | 0.72, 0.70 ± 0.1 (0.41–0.93) |
| p-value | 0.84 | 0.84 | 0.93 |
| Side | |||
| OD (46 eyes) | 0.40, 0.39 ± 0.1 (0.32–0.65) | 0.50, 0.49 ± 0.1 (0.30–0.73) | 0.70, 0.69 ± 0.1 (0.41–0.93) |
| OS (49 eyes) | 0.39, 0.39 ± 0.1 (0.28–0.64) | 0.50, 0.50 ± 0.1 (0.30–0.72) | 0.70, 0.69 ± 0.1 (0.41–0.91) |
| p-value | 0.93 | 0.94 | 0.89 |
| Measured Quadrant of the Eye | |||
| Upper quadrant (95 eyes) | 0.43, 0.41 ± 0.1 (0.30–0.63) | 0.53, 0.51 ± 0.1 (0.30–0.70) | 0.74, 0.73 ± 0.1 (0.44–0.91) |
| Lower quadrant (95 eyes) | 0.37, 0.36 ± 0.1 (0.28–0.62) | 0.47, 0.46 ± 0.1 (0.31–0.71) | 0.67, 0.66 ± 0.1 (0.41–0.89) |
| p-value | 0.045 | 0.043 | 0.018 |
| Nasal quadrant (95 eyes) | 0.43, 0.41 ± 0.1 (0.30–0.65) | 0.53, 0.51 ± 0.1 (0.32–0.73) | 0.73, 0.71 ± 0.1 (0.47–0.93) |
| Temporal quadrant (95 eyes) | 0.40, 0.39 ± 0.1 (0.28–0.62) | 0.48, 0.47 ± 0.1 (0.30–0.70) | 0.69, 0.69 ± 0.1 (0.41–0.90) |
| p-value | 0.046 | 0.040 | 0.016 |
| Parameter | The Thickness of the Ciliary Body [Mean, Median ± SD (Range)] | The Thickness of the Ciliary Body + Ciliary Processes [Mean, Median ± SD (Range)] |
|---|---|---|
| Measured thickness (83 eyes) | 0.71, 0.70 ± 0.15 (0.42–1.14) | 1.41, 1.36 ± 0.15 (1.19–1.75) |
| Sex | ||
| Female (n = 37 eyes, 21 patients) | 0.69, 0.68 ± 0.15 (0.42–1.14) | 1.39, 1.34 ± 0.15 (1.19–1.70) |
| Male (n = 58 eyes, 31 patients) | 0.71, 0.69 ± 0.15 (0.44–1.12) | 1.43, 1.37 ± 0.15 (1.31–1.75) |
| p-value | 0.34 | 0.44 |
| Age (year) | ||
| Less than 40 (44 eyes) | 0.74, 0.73 ± 0.15 (0.46–1.14) | 1.43, 1.39 ± 0.15 (1.24–1.75) |
| More than 40 (41 eyes) | 0.69, 0.68 ± 0.15 (0.42–1.08) | 1.37, 1.35 ± 0.15 (1.19–1.60) |
| p-value | 0.56 | 0.68 |
| Height | ||
| Less than 160 cm (55 eyes) | 0.70, 0.69 ± 0.1 (0.42–1.1) | 1.39, 1.33 ± 0.1 (1.19–1.60) |
| More than 160 cm (40 eyes) | 0.73, 0.71 ± 0.2 (0.45–1.14) | 1.44, 1.37 ± 0.2 (1.29–1.75) |
| p-value | 0.92 | 0.76 |
| Eye | ||
| OD (46 eyes) | 0.71, 0.70 ± 0.15 (0.43–1.12) | 1.42, 1.37 ± 0.15 (1.29–1.75) |
| OS (49 eyes) | 0.71, 0.69 ± 0.15 (0.42–1.14) | 1.40, 1.35 ± 0.15 (1.19–1.68) |
| p-value | 0.68 | 0.48 |
| Measured site (Quadrant) | ||
| Upper quadrant (95 eyes) | 0.73, 0.71 ± 0.15 (0.45–1.12) | 1.44, 1.36 ± 0.15 (1.33–1.75) |
| Lower quadrant (95 eyes) | 0.69, 0.68 ± 0.15 (0.42–1.10) | 1.39, 1.34 ± 0.15 (1.19–1.67) |
| p-value | 0.044 | 0.031 |
| Nasal quadrant (95 eyes) | 0.72, 0.71 ± 0.15 (0.45–1.12) | 1.43, 1.37 ± 0.15 (1.33–1.75) |
| Temporal quadrant (95 eyes) | 0.69, 0.688 ± 0.15 (0.42–1.10) | 1.39, 1.34 ± 0.15 (1.19–1.67) |
| p-value | 0.27 | 0.88 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elfalah, M.; Mohammad, M.; Toro, M.D.; Abu-Yaghi, N.; Rejdak, R.; Yousef, Y.A. Anterior Ocular Biometrics as Measured by Ultrasound Biomicroscopy. Healthcare 2022, 10, 1188. https://doi.org/10.3390/healthcare10071188
Elfalah M, Mohammad M, Toro MD, Abu-Yaghi N, Rejdak R, Yousef YA. Anterior Ocular Biometrics as Measured by Ultrasound Biomicroscopy. Healthcare. 2022; 10(7):1188. https://doi.org/10.3390/healthcare10071188
Chicago/Turabian StyleElfalah, Mutasem, Mona Mohammad, Mario Damiano Toro, Nakhleh Abu-Yaghi, Robert Rejdak, and Yacoub A. Yousef. 2022. "Anterior Ocular Biometrics as Measured by Ultrasound Biomicroscopy" Healthcare 10, no. 7: 1188. https://doi.org/10.3390/healthcare10071188
APA StyleElfalah, M., Mohammad, M., Toro, M. D., Abu-Yaghi, N., Rejdak, R., & Yousef, Y. A. (2022). Anterior Ocular Biometrics as Measured by Ultrasound Biomicroscopy. Healthcare, 10(7), 1188. https://doi.org/10.3390/healthcare10071188







