Abstract
Parkinson Disease (PD) primarily affects older adults. It is the second-most common neurodegenerative disease after Alzheimer’s disease. Currently, more than 10 million people suffer from PD, and this number is expected to grow, considering the increasing global longevity. Freezing of Gait (FoG) is a symptom present in approximately 80% of advanced-stage PD’s patients. FoG episodes alter the continuity of gait, and may be the cause of falls that can lead to injuries and even death. The recent advances in the development of hardware and software systems for the monitoring, stimulus, or rehabilitation of patients with FoG has been of great interest to researchers because detection and minimization of the duration of FoG events is an important factor in improving the quality of life. This article presents a review of the research on non-invasive medical devices for FoG, focusing on the acquisition, processing, and stimulation approaches used.
1. Introduction
Parkinson Disease (PD) is the second-most common neurodegenerative disease, after Alzheimer’s disease, among the elderly. At present, there are 10 million people patients with PD worldwide [1,2]. Statistics show that the prevalence of PD is higher in Europe, North America, and South America in comparison with Africa and Asia, with an incidence of 13.4 per 100,000 people per year [2,3,4]. The causes of PD remain unknown, but some studies have attributed it to environmental exposure factors and genetic factors [5]. Moreover, sex and ethnicity have been shown to be influencing factors, with the male:female ratio of patients with PD being approximately 3:2 [3]. Age remains the main risk factor for the development of PD, and the prevalence and incidence of the disease increases exponentially after 60 years of age [3,6]. This trend has important implications for public health, since greater longevity, which is the trend in most countries, is expected to increase the number of people with PD by 50% in 2030 [3,7].
Parkinson disease is associated with non-motor and motor symptoms. Non-motor symptoms include dementia, depression, psychotic characteristics, autonomic dysfunction, oculomotor abnormalities, and olfactory and visual impairments. The motor symptoms include tremor, stiffness, bradykinesia, postural instability, festination, decreased blink frequency, blepharospasm, and Freezing of Gait (FoG) [8]. Medical evaluation of non-motor symptoms is usually performed by a neuropsychologist, while motor symptoms are diagnosed by a neurologist on the basis of medical history, a review of signs and symptoms, and physical and neurological examinations.
The FoG symptom is related on bradykinesia, rigidity, tremor, and postural instability, together with perceptive malfunction and frontal executive dysfunction [8,9]. It presents as a reduction in the forward progression of the feet, despite the person’s intention to walk [10,11,12]. This symptom occurs in 21–27% of patients in the early stages of PD [13,14], and this percentage continues to increase during PD evolution, with the symptom appearing in 80% of patients more than 17 years from the initial diagnosis. FoG may be the cause of falls that can lead to injuries and even death [15].
FoG has its origin in the brain, specifically in the mesencephalic locomotive region (MLR), where the performance of the pedunculopontinal nucleus (PPN) diminishes their connections with basal ganglia. Similarly, the region of the brain stem is related to the condition of freezing, which has been validated with Functional Magnetic Resonance Imaging (FMRI) and ElectroEncephaloGraphy (EEG) data. FoG is no longer considered a strictly motor symptom but is a part of cognitive impairments that originate from areas of the brain that allow the body to be able to walk without hindrance [16,17].
FoG does not respond to existing drugs, and neurorehabilitation exercises tend to be repetitive and tiring. Several invasive methods, such as deep brain stimulation (DBS) [18] or vagus nerve invasive stimulation (VNS) [19], have been developed for the treatment of FoG, but they are expensive, do not guarantee elimination of freezing, and may increase other symptoms. Patients with an episode of FoG can resume walking after receiving external stimulation, and non-invasive methods such as visual, vibratory, and tactile stimulation devices can provide such stimulation at a relatively low cost and without health risks.
Thus, the objective of this study was to analyze non-invasive monitoring systems focused on detection, minimization, and even prediction of FoG, and also evaluate active extracorporeal medical devices that can break FoG episodes. Most devices integrate both functions: monitoring plus stimulation. We aim to compare their underlying processes, methodologies, and outcomes.
2. Brain Activity during a FoG Episode
To understand the parameters to be measured by those systems, it was necessary to analyze the brain motor activity involved in PD during episodes FoG. Brain activity occurs when the brain generates electrical impulses known as action potentials, which travel through neurons. Electrical impulses contain information that travels from neuron to neuron making use of hundreds of thousands of them to get transported and perform a specific function, any alteration provokes a change in their contiguous connections [20]. When the brain generates an impulse to move a muscle, the impulse passes through the basal ganglia that help to smooth muscle movements and coordinate changes in posture, such as the gait.
A statistical parametric mapping analysis applied to healthy subjects during the gait revealed that the following areas were activated in their brain activity: supplementary motor, medial primary sensorimotor, striatum, cerebellar vermis, and visual cortex. These results indicate that the cerebral cortexes that control: motor functions, visual cortex, basal ganglia, and cerebellum, may be involved in the bipedal locomotor activities in humans [21]. When a person has PD, there is a degeneration in the cells of the basal ganglia that causes a decrease production of dopamine and reduces connectivity between nerve cells and muscles [22].
Encephalography was used to understand the bioelectrical connections of those who suffer from PD and present FoG. The Figure 1 shows the electrode arranged system in a 10–20 scheme. This scheme was used to analyze brain activity utilizing electrodes on the hair scalp in FOG patients. The presence of FOG episodes generates different levels of energy in the brain waves of the parietal zone (P4), suggesting that this zone has been deeply affected by the disease. Measurements of P4 and the central zone (Cz) are the features that most contributed to the analysis for detecting FoG transition in PD patients [23,24,25].
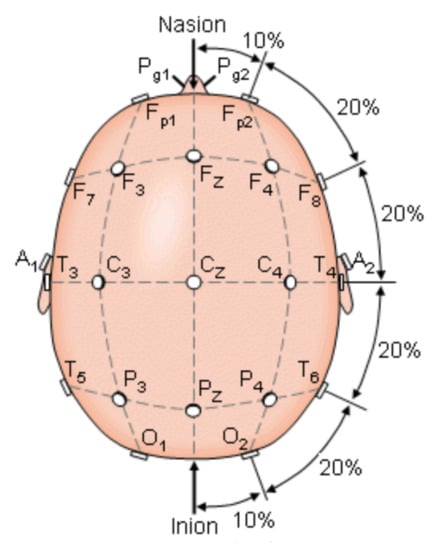
Figure 1.
The international 10–20 system seen above the head. A = Ear lobe, C = central, Pg = nasopharyngeal, P = parietal, F = frontal, Fp = frontal polar, O = occipital [23].
When the fronto-parietal zone is affected, there is a decrease in executive functions (including cognitive skills), aggravating the problems in people with FoG, who also have failures in their visuospatial network. Some authors such as Amboni et al. compared the progression of cognitive impairment in 26 Parkinsonian patients with FoG (FoG+) and without FoG (FoG-) over a follow-up period of 2 years, finding that FoG+ patients had a faster progression of cognitive impairment, while in FoG- patients, cognitive alteration remained unchanged during this period [26]. Another technique used is magnetic resonance imaging, where it was concluded that FoG+ patients present predominantly frontal-executive dysfunction compared to FoG- patients [27]. At the same time, the right hemisphere of the brain looked more affected in FoG+ patients, which would make sense due to the great influence of this hemisphere on visuospatial abilities [28].
At a neuronal level, the important role of the pedunculopontine nucleus (PPN) located in the MLR, likely play a crucial role in the appearance of axial symptoms in PD. The aim was to activate the remaining PPN cholinergic neurons to improve axial symptoms including FoG and balance deficits in PD patients. Its importance resides in that it is the main center of the mesencephalic locomotor region and controls the initiation, maintenance, and modulation of posture and gait [29]. The presence of FoG was associated with altered functional connectivity between the PPN and the corticopontine-cerebellar pathways (in the bilateral cerebellum and in the pons) and visual temporal areas compared to healthy subjects, additionally marked abnormalities in white matter extending to motor, sensory and cognitive regions [30].
A significant number of PD patients increasingly rely on visual and auditory signals to control the locomotion [31], which creates a problem because visual impairments have been correlated with gait disturbance, deficits in visual attention, memory, and visuospatial abilities [32,33]. Lenka et al. managed to establish that a reduction in inter-hemispheric connectivity between bilateral parietal operculum, somatosensory cortex, and primary auditory areas are correlated with FoG [34]. The hearing deficiency was tested with the Rey Auditory-Verbal Learning Test (RAVLT) [35]. This shows a decline in vision and hearing in those who have episodes of FoG.
3. Motor Characteristics during FoG
Although FoG originates in the brain, it manifests as an irregularity in gait. As PD progresses, this irregularity becomes more frequent and disabling for the patient, which results in longer FoG episodes. These episodes can present as complete akinesia when the patient is not medicated (off state, FoG-). When the patient is medicated (on state, FoG+), the FoG episodes are shorter and rarely become akinetic. FoG+ patients present deficiencies during motor initiation that are not present in FoG- patients or healthy people. Additionally, FoG+ patients are slower to initiate motor activity, and in particular, to respond to the signal from the brain to initiate walking. Moreover, these patients require more time to react to stop walking in comparison with FoG- patients and healthy people [36,37].
In muscular-system analyses, FoG is characterized by co-contraction of agonist and antagonist muscles [10,38]. The patients show changes in the pressure of the foot and the behavior of the distance between the steps, as these become shorter compared to those in a slow walk. The frequency of this type of walk is between 4 and 5 Hz [39,40]. Patients with FoG respond to classic auditory, visual, and tactile stimulations [41]. However, these stimulations cannot effectively induce the muscular system to react to bradykinesia [42], which results in alterations in symmetry, rhythm, and bilateral coordination in the patients’ walk with FoG [43,44].
On the other hand, dysfunction in cognitive networks and interictal gait changes may contribute to Parkinson’s disease patients presenting with episodes of FoG. An analysis of patients performing two activities at the same time is presented in one study [45], and the FoG+ patients showed a shorter stride length and slower speed, except during postural balance, in that study. In contrast, during the turn, both groups (FoG+ and FoG-) showed a slower turning speed in the tests involving double activities compared to the findings for the single-task condition.
Analyses of the FoG to date have focused on the lower extremities. However, some studies have revealed determining characteristics in the upper limbs, specifically in the wrists, which show FoG earlier than the legs and feet [36,46]. These studies revealed that FoG can be detected using wrist motion and machine learning models with an FoG hit rate of 0.9 and specificity between 0.66–0.8. Additionally, the standard deviation, acceleration, and rotation were analyzed, in addition to the power between the frequency ranges of 0 to 1 Hz, 5 to 6 Hz, and in the intervals of 0 to 4 Hz, 5 to 8 Hz, and 9 to 12 Hz [46,47].
Some studies have focused on analyzing single-leg posture using portable inertial sensors and performing statistical calculations. The results of these studies indicate that the acceleration peak of the medial-lateral trunk in FoG+ and FoG- patients was significantly lower than that of healthy patients (p < 0.05), and the equilibrium was longer in FoG- patients in comparison with that in FoG+ patients [48].
Various studies have revealed significant differences in the duration of the balanced phase of the feet during walking in FoG+ and FoG- patients and healthy subjects. This reduction in the duration of equilibrium is generally associated with an increased risk of falls [49,50,51,52,53].
In one study [54], the two main reasons for falls in PD patients were identified as FoG and impaired balance. This argument is supported by other investigations [55,56,57], which validated the relationship between FoG and falls. These falls are consequences of FoG and appear from the early stages of the disease [58,59,60]. Additionally, falls could be disabling and can deteriorate the quality of life of patients [61,62,63,64]. These falls can occur in multiple directions and are associated with motor symptoms but also with non-motor symptoms, such as mood and cognitive disorders [65]. When the FoG occurs, the center of gravity continues to advance due to the effect of inertia, but the feet stop moving body while the body continues forward, which causes the majority of falls. Therefore, to initiate the walk, FoG+ patients require wider movements than FoG- patients [17].
4. Devices Developed for Detection and Stimulation of FoG Episodes
In recent years, various invasive and non-invasive devices have been developed to monitor and thereby minimize FoG. Invasive systems are effective in improving and minimizing some of the symptoms of Parkinson’s disease, but they involve expensive surgical procedures and may be risky for the patient’s health. At present, invasive devices are used when the patients do not respond to drug treatment. The most frequently used invasive systems are those involving deep brain stimulation, stimulation of the vagus nerve, and electrical stimulation of the spinal cord.
- Brain stimulation involves three components: an implantable pulse generator (IPG) that houses the battery and electronic components, an implanted electrode, and a lead extender that connects each electrode to the IPG [66]. The electrode is inserted through a small opening in the skull and implanted in the brain, and its tip is positioned within the target area of the brain, which is usually the thalamus, subthalamic nucleus, or globus pallidus. After completion of the procedure, impulses are sent from the neurostimulator to the extension cord and the electrode within the brain; these impulses interfere with and block the electrical signals that cause the symptoms of PD. Deep brain stimulation has been proven to be effective in the management of the symptoms of PD patients but is limited by its complexity and has been associated with mortality rates ranging from 1% to 2% [18].
- Stimulation of the vagus nerve synapse monoamine optimizes the addition activates the cholinergic neural network circuit that is affected in patients with PD and unlocks up gait. This method activates neurons through stimulation of afferent fibers of the left vagus nerve by a small electrical pulse generator implanted in the upper thorax. The adverse effects, in addition to the risks of implant surgery, are vocal changes and hoarseness [19].
- Researchers at Duke University have also proposed a new invasive technique based on electrical stimulation of the spinal cord. This procedure involves placing a plate with 16 electrodes on the spinal cord, which stimulates electrical impulses to the neurons to improve the information carried from the legs to the brain so that the patient can regain control of the lower limbs [67].
To minimize the adverse effects of invasive techniques, non-invasive devices that aim to reduce the symptoms of PD, especially FoG, have been developed. Different methodologies have been used for the construction of devices, such as those focused on acquiring EEG signals [24,68,69,70]. Moreover, using accelerometers located at different parts of the lower extremities, including the calf, hip, legs, and ankle [68,71,72,73,74,75], these devices can stimulate the muscle or nerve externally and help patients out of the episode of freezing. All of these devices are aimed at improving the patients’ lifestyles.
Some studies have used video recordings of the progress of PD patients [71,72,76,77]. In one study [78], the authors used a genetic algorithm and an SVM classifier. In another study [79], researchers developed a real-time FoG detection algorithm based on a generalized spectrogram with asymmetric windows, while the simulations in this study show that the proposed algorithm has greater precision than other methods for the detection of the FoG. In one study, an algorithm based on time characteristics and frequency with only a 1-s delay for the identification of FoG was proposed [80]. On the other hand, in another study, a previously trained artificial neural network (ANN) was used [76]. Other researchers have focused on data processing via a discrete wavelet transform-based algorithm that detects FoG episodes in real-time [81]. These systems use an acceleration sensor for data capture.
Some systems used more than one sensor to capture more gait characteristics [80,82,83,84]. The use of inertial sensors (accelerometers, gyroscopes, etc.) provides versatility and good range results in gait analysis. Moreover, the use of pressure sensors contributed to determining the gait phases; however, such sensors cannot accurately determine the distance [85].
Another approach involves calculation of the energy threshold, as described in one previous study [83]; this approach requires 2 s to detect the presence of the FoG and in another study [80], it takes only 1 s. Both approaches compute the power spectral density (PSD).
The increasing penetration of smartphones in the daily life of PD patients has allowed the use of smartphone components, such as the accelerometer, and the data-processing capacity of smartphones for the detection of FoG [86,87]. The use of smartphones to minimize the number of connected external devices for FoG patient monitoring has been presented in a previous study [75,81,87,88].
The existing research on this topic reflects the need for identification of devices for acquiring and processing data as well as for providing FoG-unlocking stimuli [70,88]. The stimuli used can be visual, vibrational, or electrical. In visual stimulation, for example, a projection of parallel lines is used on the floor, which significantly reduces FoG episodes if it is used as continuous stimulation in approximately 51% of the patients and as noncontinuous stimulation in approximately 69% of the patients [81,84,89]. Vibratory stimulation can reduce the time required for resumption of gait and improve the postural relationship of oscillation after only one week of treatment [85]. This form of stimulation is widely accepted by patients because it is comfortable, and women respond faster to a vibratory stimulus when it is placed near the posterior tibial nerve, with a sensitivity of 89% and effectiveness of 96% [74]. On the other hand, electrical stimulation reduced walking time by 19% and FoG episodes by up to 58% [90,91]. In one study [75], the effectiveness of the stimulus for restarting gait was 82.35%, consolidating it as an appropriate method for unlocking the FoG.
A previous study [88] presented research describing the perception of the patient to auditory and vibratory stimuli in an environment with external disturbances. The authors also used glasses and earplugs during testing, simulating the conditions experienced by elderly PD patients. The patients determined that earmuffs are easier and more pleasant to use than glasses. When both were used simultaneously, 66% of the patients perceived glasses as more restrictive than earmuffs. Moreover, in the presence of high levels of external disturbance, the audible system is considered easier to use and more pleasant than the visual system.
Virtual Reality (VR) is another technology explored by different researchers, usually more focused on physical rehabilitation. VR allows you to create personalized rehabilitation programs based on the characteristics of the patient and the progress of the disease, these programs are a highly immersive experience. VR typically uses a lens-type display placed on the patient’s head, sometimes sound, and controls that can be placed on the hand or leg. These devices can be used at home or in physical therapy laboratories, due to their ease of use, relatively low cost, and portability. Specifically, in FoG patients, these devices were used to provide visual stimulus to resume gait [89,92,93].
Table 1 summarizes acquisition approaches used in the studies analyzed in this article, with favorable results in patients with FoG, in Table 2 we did the same based on the used classifier, and in Table 3 based on the used stimulus.

Table 1.
Description of devices developed until today to help patients with episodes of FoG based in the acquisition type.

Table 2.
Description of devices developed until today to help patients with episodes of FoG based in the used classifier.

Table 3.
Description of devices developed until today to help patients with episodes of FoG based in the used stimulus.
In addition, Figure 2 lists the research on acquisition technologies, processing methods, the transmission means, in relation to the display module and stimulation mechanisms underlying devices aimed at treating the FoG, along with their location relative to the patient.
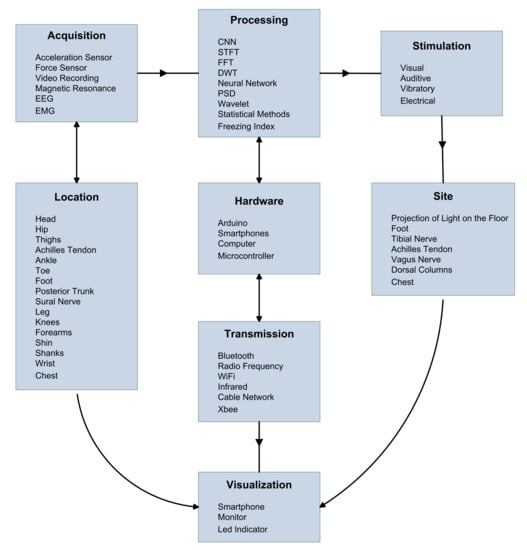
Figure 2.
Analysis of non-invasive techniques most used in patients with FoG: acquisition systems, devices, transmission, visualization, data processing, and stimulation. ElectroEncephaloGraphy (EEG), ElectroMyoGraphy (EMG), Convolutional Neural Network (CNN), Short-Time Fourier Transform (STFT), Fast Fourier Transform (FFT), Discrete Wavelet Transform (DTW), and Power Spectral Density (PSD).
Figure 2 related references:
- Acquisition
Acceleration Sensor [36,68,71,72,73,74,75,79,80,81,83,85,86,87,88,89,92,93,94,95]
Force Sensor [76,85,88,96]
Video Recording [37,71,72,76,77,92,96]
Magnetic Resonance [17,27,30,34]
ElectroEncephaloGraphy (EEG) [24,68,69,70]
ElectroMyoGraphy (EMG) [80]
- Location
Head [17,70,73,89,92]
Hip [71,72]
Thighs [80,83]
Achilles Tendon [88]
Ankle [46,82,87,94]
Toe [68,72,76,95]
Foot [73,76,90,92,93,95,96]
Posterior Trunk [86]
Sural Nerve [74,75,81]
Leg [36,37,71,73,74,86,89]
Knees [73,90,97]
Forearms [80]
Shin [73,80]
Shanks [83]
Wrist [36,46]
Chest [36]
- Processing
Convolutional Neural Network (CNN) [76]
Short-Time Fourier Transform (STFT) [79,95]
Fast Fourier Transform (FFT) [71,72,94,96]
Discrete Wavelet Transform (DTW) [70,75,87]
Neural Network [80]
Power Spectral Density (PSD) [70,71,80,83,86]
Wavelet [24,68,81]
Statistical methods [36,46,64,65,69,73,75,82,88,89,90,91,92,93,95]
Freezing Index [68,73,79,97]
- Hardware
Arduino [74,75,81,85,87,94,97]
Smartphones [46,74,75,76,81,87]
Computer [64,71,79]
Microcontroller [71,76,81,83,88]
- Transmission
Bluetooth [36,46,74,75,81,87,94,97]
Radio Frequency [74,76,83,86,87]
WiFi [71,82]
Infrared [73]
Cable Network [83,86,88]
Xbee [85]
- Stimulation
Visual [82,84,88,89,92,93,98]
Auditive [70,84,85]
Vibratory [74,75,81,85,88]
Electrical [90,91]
- Site
Projection of light on the floor [82,84,89,92,98]
Foot [90]
Tibial Nerve [74,75,81]
Achilles Tendon [88]
Vagus nerve [19]
Dorsal Columns [67]
Chest [82]
- Visualization
Smartphone [74,75,81,87,88]
Monitor [65,73,79,82,83,86,90,95,96]
Led Indicator [71,84]
5. Discussion
Since the 1970s, proposals and prototypes have been developed to help understand, detect, avoid, and predict episodes of FoG in patients with PD. Currently, signal-acquisition and processing techniques are still being improved, including analyses of brain activity to understand the origin of the pathology and its connections with motor function. These studies focused on achieving greater sensitivity and specificity in their results aimed at user autonomy, wherein the use of acceleration sensors yielded up to 85% effectiveness in detecting FoG. Similarly, the application of stimuli, such as vibration, addressed FoG episodes by up to 90%, with these results being dependent on the studied population. Research related to FoG episodes has primarily focused on two methods: invasive and non-invasive.
The application of invasive devices has been shown to be effective in minimizing some of the symptoms of PD; however, these devices are associated with numerous risks because the patient must undergo surgery, which requires many hours in the operating room, and is costly. Both factors limited the use of these devices for many patients. Invasive methods are currently being applied when patients do not respond to drug treatment or when the patient wishes to minimize the use of medications. On the basis of the scientific literature, the most frequently applied invasive methods are as follows: deep brain stimulation, stimulation of the vagus nerve, and electrical stimulation of the spinal cord.
With respect to non-invasive devices, the designs primarily involve the use of Inertial Measurement Units (IMU), using accelerometers, for data acquisition in the lower body. Although, the evidence indicates that the use of EEG sensors provide greater signal accuracy and allow faster detection of the presence of FoG in comparison with the inertial sensor response, it is less used because of its higher complexity.
The data is normally transmitted by wireless devices because this technique allows reduction of cost, better ergonomics, and easy implementation. From analyzed papers, Bluetooth technology was the most common choice due to its speed, convenience, and low power consumption. However, since the Bluetooth protocol uses point-to-point connections, the use of this protocol limits the number of concurrent sensors in the system. Other authors have used WiFi networks that allow them to access a server (usually with an Internet link) where record their measurements, and use multiple sensors concurrently.
With advancements in technologies, new hardware prototypes and software algorithms have been appearing, yielding systems with smaller physical sizes and greater processing speeds. This includes the use of greater sampling frequencies ranging from 10 Hz to 1 KHz for processing, allowing the application of frequency analysis techniques (such as FFT and DWT) or the use of neural networks. However, a higher sample rate analysis does not guarantee better results. It must be in accordance with the activity to be measured, since motor responses would hardly reach high frequencies, contrary to electroencephalographic signals. The processing system also needs to be at the level of data generation and complexity, greater demand for systems that use electroencephalographic signals and less in the case of IMU.
Although most studies and systems are focused on the characterization of FoG, some of them also made the next step and implement solutions to unblocking detected FoG episodes. Since the symptoms precede the cognitive function decline that underlies the pedunculopontine nucleus, methods have been proposed to reactivate this neuronal activity through visual, auditory, haptic, or electrical stimulation. Their functions are based on the patient concentrating on an external signal so that they can redirect their attention from freezing and the neuronal link can be reactivated to allow them to resume their walk.
Some authors recommend in future studies, that patterns in PD patients’ gait will be determined to allow prediction of FoG episodes. In addition, exists a consensus about the importance of database analysis of walking data is carried out using artificial intelligence to determine the possible causes of FoG.
We detected that few studies have focused on seeking standardization of algorithms to determine FoG and that these do not depend on posture or location of sensors, which would mean giving patients independence.
6. Conclusions
FoG is one of the most disabling consequences of Parkinson’s disease. Many studies have explored different approaches for its detection and overcoming. In this review, we have made a classification and compendium of all of them. Synthesized in Table 1, Table 2 and Table 3 and Figure 2.
As has been indicated in the discussion, the advancement of technology is allowing the improvement of the effectiveness of the techniques already proven, at the same time that the usability of the devices is improved. In any case, the study of devices for detecting and overcoming FoG is still a field open to new techniques.
Author Contributions
Conceptualization, M.H., B.B., C.P., A.G.-C. and R.C.; methodology, M.H, B.B., C.P. and R.C.; validation, M.H., B.B., C.P., A.G.-C. and R.C.; formal analysis, M.H., B.B., C.P., A.G.-C. and R.C.; investigation, M.H., B.B., C.P., A.G.-C. and R.C.; resources, M.H. and R.C.; writing—original draft preparation, M.H., B.B., C.P. and R.C.; writing—review and editing, M.H. and R.C.; visualization, M.H., A.G.-C. and R.C.; supervision, M.H. and R.C.; All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to express their gratitude to the Ibero-American Program of Science and Technology for Development, project code 320RT0006.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
The authors gratefully acknowledge the support of the NEURO-SISMO project, Universidad Politécnica Salesiana from Ecuador and CYTED Network 320RT0006, a network with the goal of accelerating the transition of SMEs to industry 4.0 with low-cost technology, Carlos Llumiguano and Diego Chimbo for his professional contributions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dauer, W.; Przedborski, S. Parkinson’s Disease. Neuron 2003, 39, 889–909. [Google Scholar] [CrossRef]
- Kalia, L.; Lang, A. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Pringsheim, T.; Jette, N.; Frolkis, A.; Steeves, T. The prevalence of Parkinson’s disease: A systematic review and meta-analysis. Mov. Disord. 2014, 29, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Van Den Eeden, S. Incidence of Parkinson’s Disease: Variation by Age, Gender, and Race/Ethnicity. Am. J. Epidemiol. 2003, 157, 1015–1022. [Google Scholar] [CrossRef]
- Lee, A.; Gilbert, R. Epidemiology of Parkinson Disease. Neurol. Clin. 2016, 34, 955–965. [Google Scholar] [CrossRef]
- Driver, J.; Logroscino, G.; Gaziano, J.; Kurth, T. Incidence and remaining lifetime risk of Parkinson disease in advanced age. Neurology 2009, 72, 432–438. [Google Scholar] [CrossRef]
- Calabrese, V.; Dorsey, E.; Constantinescu, R.; Thompson, J.; Biglan, K.; Holloway, R.; Kieburtz, K.; Marshall, F.; Ravina, B.; Schifitto, G.; et al. Projected Number of People With Parkinson Disease in the Most Populous Nations, 2005 Through 2030. Neurology 2007, 69, 223–224. [Google Scholar] [CrossRef]
- Gelb, D.; Oliver, E.; Gilman, S. Diagnostic Criteria for Parkinson Disease. Arch. Neurol. 1999, 56, 33. [Google Scholar] [CrossRef]
- Heremans, E.; Nieuwboer, A.; Vercruysse, S. Freezing of Gait in Parkinson’s Disease: Where Are We Now? Curr. Neurol. Neurosci. Rep. 2013, 13, 350. [Google Scholar] [CrossRef]
- Nutt, J.; Bloem, B.; Giladi, N.; Hallett, M.; Horak, F.; Nieuwboer, A. Freezing of gait: Moving forward on a mysterious clinical phenomenon. Lancet Neurol. 2011, 10, 734–744. [Google Scholar] [CrossRef]
- Rahman, S.; Griffin, H.; Quinn, N.; Jahanshahi, M. Quality of life in Parkinson’s disease: The relative importance of the symptoms. Mov. Disord. 2008, 23, 1428–1434. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; Danoudis, M.; McGinley, J.; Morris, M. Relationships between motor aspects of gait impairments and activity limitations in people with Parkinson’s disease: A systematic review. Park. Relat. Disord. 2012, 18, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Giladi, N.; McDermott, M.; Fahn, S.; Przedborski, S.; Jankovic, J.; Stern, M.; Tanner, C. Freezing of gait in PD: Prospective assessment in the DATATOP cohort. Neurology 2001, 56, 1712–1721. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; McGinley, J.; Danoudis, M.; Iansek, R.; Morris, M. Freezing of Gait and Activity Limitations in People With Parkinson’s Disease. Arch. Phys. Med. Rehabil. 2011, 92, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Hely, M.; Morris, J.; Reid, W.; Trafficante, R. Sydney multicenter study of Parkinson’s disease: Non-L-dopa-responsive problems dominate at 15 years. Mov. Disord. 2005, 20, 190–199. [Google Scholar] [CrossRef]
- Peterson, D.; Pickett, K.; Duncan, R.; Perlmutter, J.; Earhart, G. Gait-Related Brain Activity in People with Parkinson Disease with Freezing of Gait. PLoS ONE 2014, 9, e90634. [Google Scholar] [CrossRef]
- Peterson, D.; Fling, B.; Mancini, M.; Cohen, R.; Nutt, J.; Horak, F. Dual-task interference and brain structural connectivity in people with Parkinson’s disease who freeze. J. Neurol. Neurosurg. Psychiatry 2014, 86, 786–792. [Google Scholar] [CrossRef]
- Perlmutter, J.S.; Mink, J.W. Deep brain stimulation. Annu. Rev. Neurosci. 2006, 29, 229–257. [Google Scholar] [CrossRef]
- George, M.S.; Sackeim, H.A.; Rush, A.J.; Marangell, L.B.; Nahas, Z.; Husain, M.M.; Lisanby, S.; Burt, T.; Goldman, J.; Ballenger, J.C. Vagus nerve stimulation: A new tool for brain research and therapy. Biol. Psychiatry 2000, 47, 287–295. [Google Scholar] [CrossRef]
- Rattay, F. The basic mechanism for the electrical stimulation of the nervous system. Neuroscience 1999, 89, 335–346. [Google Scholar] [CrossRef]
- Fukuyama, H.; Ouchi, Y.; Matsuzaki, S.; Nagahama, Y.; Yamauchi, H.; Ogawa, M.; Kimura, J.; Shibasaki, H. Brain functional activity during gait in normal subjects: A SPECT study. Neurosci. Lett. 1997, 228, 183–186. [Google Scholar] [CrossRef]
- Rivlin-Etzion, M.; Marmor, O.; Saban, G.; Rosin, B.; Haber, S.N.; Vaadia, E.; Prut, Y.; Bergman, H. Low-Pass filter properties of basal ganglia–cortical–muscle loops in the normal and MPTP primate model of Parkinsonism. J. Neurosci. 2008, 28, 633–649. [Google Scholar] [CrossRef] [PubMed]
- Bhavsar, R.; Sun, Y.; Helian, N.; Davey, N.; Mayor, D.; Steffert, T. The correlation between EEG signals as measured in different positions on scalp varying with distance. Procedia Comput. Sci. 2018, 123, 92–97. [Google Scholar] [CrossRef]
- Handojoseno, M.A.; Shine, J.M.; Nguyen, T.N.; Tran, Y.; Lewis, S.J.G.; Nguyen, H.T. The detection of Freezing of Gait in Parkinson’s disease patients using EEG signals based on Wavelet decomposition. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; pp. 69–72. [Google Scholar]
- Handojoseno, A.M.A.; Shine, J.M.; Nguyen, T.N.; Tran, Y.; Lewis, S.J.G.; Nguyen, H.T. Using EEG spatial correlation, cross frequency energy, and wavelet coefficients for the prediction of Freezing of Gait in Parkinson’s Disease patients. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; pp. 4263–4266. [Google Scholar]
- Amboni, M.; Barone, P.; Picillo, M.; Cozzolino, A.; Longo, K.; Erro, R.; Iavarone, A. A two-year follow-up study of executive dysfunctions in Parkinsonian patients with freezing of gait at on-state. Mov. Disord. 2010, 25, 800–802. [Google Scholar] [CrossRef] [PubMed]
- Tessitore, A.; Amboni, M.; Esposito, F.; Russo, A.; Picillo, M.; Marcuccio, L.; Pellecchia, M.; Vitale, C.; Cirillo, M.; Tedeschi, G.; et al. Resting-state brain connectivity in patients with Parkinson’s disease and freezing of gait. Park. Relat. Disord. 2012, 18, 781–787. [Google Scholar] [CrossRef]
- Almeida, Q.; Lebold, C. Freezing of gait in Parkinson’s disease: A perceptual cause for a motor impairment? J. Neurol. Neurosurg. Psychiatry 2009, 81, 513–518. [Google Scholar] [CrossRef]
- Grabli, D.; Karachi, C.; Welter, M.; Lau, B.; Hirsch, E.; Vidailhet, M.; François, C. Normal and pathological gait: What we learn from Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2012, 83, 979–985. [Google Scholar] [CrossRef]
- Wang, M.; Jiang, S.; Yuan, Y.; Zhang, L.; Ding, J.; Wang, J.; Zhang, J.; Zhang, K.; Wang, J. Alterations of functional and structural connectivity of freezing of gait in Parkinson’s disease. J. Neurol. 2016, 263, 1583–1592. [Google Scholar] [CrossRef]
- Sage, M.; Almeida, Q. A positive influence of vision on motor symptoms during sensory attention focused exercise for Parkinson’s disease. Mov. Disord. 2010, 25, 64–69. [Google Scholar] [CrossRef]
- Uc, E.; Rizzo, M.; Anderson, S.; Qian, S.; Rodnitzky, R.; Dawson, J. Visual dysfunction in Parkinson disease without dementia. Neurology 2005, 65, 1907–1913. [Google Scholar] [CrossRef]
- Yogev-Seligmann, G.; Hausdorff, J.; Giladi, N. The role of executive function and attention in gait. Mov. Disord. 2008, 23, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Lenka, A.; Naduthota, R.; Jha, M.; Panda, R.; Prajapati, A.; Jhunjhunwala, K.; Saini, J.; Yadav, R.; Bharath, R.; Pal, P. Freezing of gait in Parkinson’s disease is associated with altered functional brain connectivity. Park. Relat. Disord. 2016, 24, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, L.; Bloem, B.R.; Snijders, A.H.; Daniele, A.; Quaranta, D.; Bentivoglio, A.R.; Fasano, A. Freezing of gait in Parkinson’s disease: The paradoxical interplay between gait and cognition. Park. Relat. Disord. 2014, 20, 824–829. [Google Scholar] [CrossRef] [PubMed]
- Tripoliti, E.; Tzallas, A.; Tsipouras, M.; Rigas, G.; Bougia, P.; Leontiou, M.; Konitsiotis, S.; Chondrogiorgi, M.; Tsouli, S.; Fotiadis, D. Automatic detection of freezing of gait events in patients with Parkinson’s disease. Comput. Methods Programs Biomed. 2013, 110, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Schaafsma, J.; Balash, Y.; Gurevich, T.; Bartels, A.; Hausdorff, J.; Giladi, N. Characterization of freezing of gait subtypes and the response of each to levodopa in Parkinson’s disease. Eur. J. Neurol. 2003, 10, 391–398. [Google Scholar] [CrossRef]
- Dietz, M.; Goetz, C.; Stebbins, G. Evaluation of a modified inverted walking stick as a treatment for Parkinsonian freezing episodes. Mov. Disord. 1990, 5, 243–247. [Google Scholar] [CrossRef]
- Okuma, Y. Freezing of gait in Parkinson’s disease. J. Neurol. 2006, 253, vii27–vii32. [Google Scholar] [CrossRef]
- Freeman, J.S.; Cody, F.W.; Schady, W. The influence of external timing cues upon the rhythm of voluntary movements in Parkinsonś disease. J. Neurol. Neurosurg. Psychiatry 1993, 56, 1078–1084. [Google Scholar] [CrossRef]
- Rutz, D.G.; Benninger, D.H. Physical therapy for freezing of gait and gait impairments in Parkinson disease: A systematic review. PM&R 2020, 12, 1140–1156. [Google Scholar]
- Bartels, A.; Balash, Y.; Gurevich, T.; Schaafsma, J.; Hausdorff, J.; Giladi, N. Relationship between freezing of gait (FOG) and other features of Parkinson’s: FOG is not correlated with bradykinesia. J. Clin. Neurosci. 2003, 10, 584–588. [Google Scholar] [CrossRef]
- Hausdorff, J.; Schaafsma, J.; Balash, Y.; Bartels, A.; Gurevich, T.; Giladi, N. Impaired regulation of stride variability in Parkinson’s disease subjects with freezing of gait. Exp. Brain Res. 2003, 149, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Plotnik, M.; Giladi, N.; Hausdorff, J. Bilateral coordination of walking and freezing of gait in Parkinson’s disease. Eur. J. Neurosci. 2008, 27, 1999–2006. [Google Scholar] [CrossRef]
- Fortaleza, A.d.; Mancini, M.; Carlson-Kuhta, P.; King, L.; Nutt, J.; Chagas, E.; Freitas, I.; Horak, F. Dual task interference on postural sway, postural transitions and gait in people with Parkinson’s disease and freezing of gait. Gait Posture 2017, 56, 76–81. [Google Scholar] [CrossRef]
- Mazilu, S.; Blanke, U.; Calatroni, A.; Gazit, E.; Hausdorff, J.; Tröster, G. The role of wrist-mounted inertial sensors in detecting gait freeze episodes in Parkinson’s disease. Pervasive Mob. Comput. 2016, 33, 1–16. [Google Scholar] [CrossRef]
- Daneault, J.F.; Vergara-Diaz, G.; Parisi, F.; Admati, C.; Alfonso, C.; Bertoli, M.; Bonizzoni, E.; Carvalho, G.F.; Costante, G.; Fabara, E.E.; et al. Accelerometer data collected with a minimum set of wearable sensors from subjects with Parkinson’s disease. Sci. Data 2021, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Bonora, G.; Mancini, M.; Carpinella, I.; Chiari, L.; Ferrarin, M.; Nutt, J.; Horak, F. Investigation of Anticipatory Postural Adjustments during One-Leg Stance Using Inertial Sensors: Evidence from Subjects with Parkinsonism. Front. Neurol. 2017, 8, 361. [Google Scholar] [CrossRef]
- Jacobs, J. Multiple balance tests improve the assessment of postural stability in subjects with Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2005, 77, 322–326. [Google Scholar] [CrossRef]
- Smithson, F.; Morris, M.; Iansek, R. Performance on Clinical Tests of Balance in Parkinson’s Disease. Phys. Ther. 1998, 78, 577–592. [Google Scholar] [CrossRef][Green Version]
- Morris, M.; Iansek, R.; Smithson, F.; Huxham, F. Postural instability in Parkinson’s disease: A comparison with and without a concurrent task. Gait Posture 2000, 12, 205–216. [Google Scholar] [CrossRef]
- Adkin, A.; Frank, J.; Jog, M. Fear of falling and postural control in Parkinson’s disease. Mov. Disord. 2003, 18, 496–502. [Google Scholar] [CrossRef]
- Vellas, B.; Wayne, S.; Romero, L.; Baumgartner, R.; Rubenstein, L.; Garry, P. One-Leg Balance Is an Important Predictor of Injurious Falls in Older Persons. J. Am. Geriatr. Soc. 1997, 45, 735–738. [Google Scholar] [CrossRef] [PubMed]
- Youn, J.; Okuma, Y.; Hwang, M.; Kim, D.; Cho, J. Falling Direction can Predict the Mechanism of Recurrent Falls in Advanced Parkinson’s Disease. Sci. Rep. 2017, 7, 3921. [Google Scholar] [CrossRef] [PubMed]
- Kerr, G.; Worringham, C.; Cole, M.; Lacherez, P.; Wood, J.; Silburn, P. Predictors of future falls in Parkinson disease. Neurology 2010, 75, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Matinolli, M.; Korpelainen, J.; Sotaniemi, K.; Myllylä, V.; Korpelainen, R. Recurrent falls and mortality in Parkinson’s disease: A prospective two-year follow-up study. Acta Neurol. Scand. 2011, 123, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Bloem, B.; Hausdorff, J.; Visser, J.; Giladi, N. Falls and freezing of gait in Parkinson’s disease: A review of two interconnected, episodic phenomena. Mov. Disord. 2004, 19, 871–884. [Google Scholar] [CrossRef]
- Voss, T.; Elm, J.; Wielinski, C.; Aminoff, M.; Bandyopadhyay, D.; Chou, K.; Sudarsky, L.; Tilley, B. Fall frequency and risk assessment in early Parkinson’s disease. Park. Relat. Disord. 2012, 18, 837–841. [Google Scholar] [CrossRef][Green Version]
- Bloem, B.; Grimbergen, Y.; Cramer, M.; Willemsen, M.; Zwinderman, A. Prospective assessment of falls in Parkinson’s disease. J. Neurol. 2001, 248, 950–958. [Google Scholar] [CrossRef]
- Lindholm, B.; Hagell, P.; Hansson, O.; Nilsson, M. Prediction of Falls and/or Near Falls in People with Mild Parkinson’s Disease. PLoS ONE 2015, 10, e0117018. [Google Scholar] [CrossRef]
- Wood, B. Incidence and prediction of falls in Parkinson’s disease: A prospective multidisciplinary study. J. Neurol. Neurosurg. Psychiatry 2002, 72, 721–725. [Google Scholar] [CrossRef]
- Bloem, B.; Munneke, M.; Carpenter, M.; Allum, J.; Pressley, J. The impact of comorbid disease and injuries on resource use and expenditures in parkinsonism. Neurology 2003, 61, 1023. [Google Scholar] [CrossRef]
- Gazibara, T.; Pekmezovic, T.; Tepavcevic, D.K.; Svetel, M.; Tomic, A.; Stankovic, I.; Kostic, V. Health-related quality of life in patients with Parkinson’s disease: Implications for falling. Park. Relat. Disord. 2015, 21, 573–576. [Google Scholar] [CrossRef] [PubMed]
- Walton, C.; Shine, J.; Hall, J.; O’Callaghan, C.; Mowszowski, L.; Gilat, M.; Szeto, J.; Naismith, S.; Lewis, S. The major impact of freezing of gait on quality of life in Parkinson’s disease. J. Neurol. 2002, 72, 721–725. [Google Scholar] [CrossRef] [PubMed]
- Parihar, R.; Mahoney, J.; Verghese, J. Relationship of Gait and Cognition in the Elderly. Curr. Transl. Geriatr. Exp. Gerontol. Rep. 2013, 2, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Eljamel, S. Overview of Deep Brain Stimulation Components. In Neurostimulation: Principles and Practice; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013; pp. 20–25. [Google Scholar] [CrossRef]
- Yadav, A.P.; Nicolelis, M.A. Electrical stimulation of the dorsal columns of the spinal cord for Parkinson’s disease. Mov. Disord. 2017, 32, 820–832. [Google Scholar] [CrossRef]
- Wang, Y.; Beuving, F.; Nonnekes, J.; Cohen, M.X.; Long, X.; Aarts, R.M.; Van Wezel, R. Freezing of gait detection in Parkinson’s disease via multimodal analysis of EEG and accelerometer signals. In Proceedings of the 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society, Montreal, QC, Canada, 20–24 July 2020; pp. 847–850. [Google Scholar]
- Haloi, R.; Hazarika, J.; Chanda, D. Selection of Appropriate Statistical Features of EEG Signals for Detection of Parkinson’s Disease. In Proceedings of the International Conference on Computational Performance Evaluation, Shillong, India, 2–4 July 2020; pp. 761–764. [Google Scholar]
- Handojoseno, A.M.A.; Shine, J.M.; Nguyen, T.N.; Tran, Y.; Lewis, S.J.G.; Nguyen, H.T. Analysis and Prediction of the Freezing of Gait Using EEG Brain Dynamics. IEEE Trans. Neural Syst. Rehabil. Eng. 2015, 23, 887–896. [Google Scholar] [CrossRef]
- Zhao, Y.; Tonn, K.; Niazmand, K.; Fietzek, U.M.; D’Angelo, L.T.; Ceballos-Baumann, A.; Lueth, T.C. Online FOG Identification in Parkinson’s disease with a time-frequency combined Algorithm. In Proceedings of the 2012 IEEE-EMBS International Conference on Biomedical and Health Informatics, Hong Kong, China, 5–7 January 2012; pp. 192–195. [Google Scholar]
- Pepa, L.; Ciabattoni, L.; Verdini, F.; Capecci, M.; Ceravolo, M.G. Smartphone based Fuzzy Logic freezing of gait detection in Parkinson’s Disease. In Proceedings of the 2014 IEEE/ASME 10th International Conference on Mechatronic and Embedded Systems and Applications (MESA), Senigallia, Italy, 10–12 September 2014; pp. 1–6. [Google Scholar]
- Saad, A.; Guerin, F.; Zaarour, I.; Ayache, M.; Lefebvre, D. Sensoring and features extraction for the detection of Freeze of Gait in Parkinson disease. In Proceedings of the 2014 IEEE 11th International Multi-Conference on Systems, Signals &Devices (SSD14), Barcelona, Spain, 11–14 February 2014; pp. 1–6. [Google Scholar]
- Punin, C.; Barzallo, B.; Huerta, M.; Bermeo, J.; Llumiguano, C.; Soto, A.; Clotet, R. Wireless system for detection of FOG in patients with Parkinson’s Disease. In Proceedings of the 2017 Global Medical Engineering Physics Exchanges/Pan American Health Care Exchanges (GMEPE/PAHCE), Tuxtla Gutierrez, Mexico, 20–25 March 2017; pp. 1–4. [Google Scholar]
- Barzallo, B.; Punin, C.; Llumiguano, C.; Huerta, M. Wireless Assistance System During Episodes of Freezing of Gait by Means Superficial Electrical Stimulation. In World Congress on Medical Physics and Biomedical Engineering; Springer: Singapore, 2018; pp. 865–870. [Google Scholar]
- Shalin, G.; Pardoel, S.; Nantel, J.; Lemaire, E.D.; Kofman, J. Prediction of Freezing of Gait in Parkinson’s Disease from Foot Plantar-Pressure Arrays using a Convolutional Neural Network. In Proceedings of the 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society, Montreal, QC, Canada, 20–24 July 2020; pp. 244–247. [Google Scholar]
- Soto, A.; Huerta, M.; Bermeo, J.-P.; Sagbay, G. Gait Modelling of People with Parkinson’s Disease. In Proceedings of the IEEE ANDESCON, Quito, Ecuador, 13–16 October 2020; pp. 1–6. [Google Scholar]
- Soumaya, Z.; Taoufiq, B.D.; Benayad, N.; Yunus, K.; Abdelkrim, A. The detection of Parkinson disease using the genetic algorithm and SVM classifier. Appl. Acoust. 2021, 171, 107528. [Google Scholar] [CrossRef]
- Chang, Y.; Ding, J.; Hu, H.; Yang, W.; Lin, K.; Wu, P. A real-time detection algorithm for freezing of gait in Parkinson’s disease. In Proceedings of the IEEE International Symposium on Circuits and Systems, Melbourne, VIC, Australia, 1–5 June 2014; pp. 1312–1315. [Google Scholar]
- Cole, B.T.; Roy, S.H.; Nawab, S.H. Detecting freezing-of-gait during unscripted and unconstrained activity. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; pp. 5649–5652. [Google Scholar]
- Punin, C.; Barzallo, B.; Clotet, R.; Bermeo, A.; Bravo, M.; Bermeo, J.P.; Llumiguano, C. A non-invasive medical device for parkinson’s patients with episodes of freezing of gait. Sensors 2019, 19, 737. [Google Scholar] [CrossRef]
- Velik, R.; Hoffmann, U.; Zabaleta, H.; Massó, J.F.M.; Keller, T. The effect of visual cues on the number and duration of freezing episodes in Parkinson’s patients. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; pp. 4656–4659. [Google Scholar]
- Niazmand, K.; Tonn, K.; Zhao, Y.; Fietzek, U.M.; Schroeteler, F.; Ziegler, K.; Ceballos-Baumann, A.O.; Lueth, T.C. Freezing of Gait detection in Parkinson’s disease using accelerometer based smart clothes. In Proceedings of the 2011 IEEE Biomedical Circuits and Systems Conference (BioCAS), San Diego, CA, USA, 10–12 November 2011; pp. 201–204. [Google Scholar]
- Sankaran, R.; Freeman, J.; Aravind, R. Design and implementation of a low-cost laser Parkinson’s Walker with safety and energy saving features. In Proceedings of the 2014 IEEE Global Humanitarian Technology Conference—South Asia Satellite (GHTC-SAS), Trivandrum, India, 26–27 September 2014; pp. 83–86. [Google Scholar]
- Winfree, K.N.; Pretzer-Aboff, I.; Hilgart, D.; Aggarwal, R.; Behari, M.; Agrawal, S.K. The Effect of Step-Synchronized Vibration on Patients With Parkinson’s Disease: Case Studies on Subjects With Freezing of Gait or an Implanted Deep Brain Stimulator. IEEE Trans. Neural Syst. Rehabil. Eng. 2013, 21, 806–811. [Google Scholar] [CrossRef]
- Mancini, M.; Priest, K.C.; Nutt, J.G.; Horak, F.B. Quantifying freezing of gait in Parkinson’s disease during the instrumented timed up and go test. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; pp. 1198–1201. [Google Scholar]
- Pan, J.I.; Huang, Y.C. Intelligent fall prevention for Parkinson’s disease patients based on detecting posture instabilily and freezing of gait. In Proceedings of the 2015 12th International Conference on Informatics in Control, Automation and Robotics (ICINCO), Colmar, France, 21–23 July 2015; pp. 608–613. [Google Scholar]
- Imbeault-Nepton, T.; Otis, M.J.D. Synchronized walking cadence for TUG in perturbed environments: Using Earcon or Tacton cues? In Proceedings of the 2014 IEEE International Symposium on Haptic, Audio and Visual Environments and Games (HAVE) Proceedings, Richardson, TX, USA, 10–11 October 2014; pp. 41–46. [Google Scholar]
- Janeh, O.; Fründt, O.; Schönwald, B.; Gulberti, A.; Buhmann, C.; Gerloff, C.; Steinicke, F.; Pötter-Nerger, M. Gait Training in Virtual Reality: Short-Term Effects of Different Virtual Manipulation Techniques in Parkinson’s Disease. Cells 2019, 8, 419. [Google Scholar] [CrossRef]
- Sijobert, B.; Azevedo, C.; Andreu, D.; Verna, C.; Geny, C. Effects of Sensitive Electrical Stimulation-Based Somatosensory Cueing in Parkinson’s Disease Gait and Freezing of Gait Assessment. Artif. Organs 2017, 41, E222–E232. [Google Scholar] [CrossRef]
- Rosenthal, L.; Sweeney, D.; Cunnington, A.L.; Quinlan, L.R.; ÓLaighin, G. Sensory Electrical Stimulation Cueing May Reduce Freezing of Gait Episodes in Parkinson’s Disease. J. Healthc. Eng. 2018, 2018, 4684925. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Jordana, L.; Stafford, J.; Peper, C.; Craig, C. Virtual footprints can improve walking performance in people with Parkinson’s disease. Front. Neurol. 2018, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Georgiades, M.; Gilat, M.; Martens, K.E.; Walton, C.; Bissett, P.; Shine, J.; Lewis, S. Investigating motor initiation and inhibition deficits in patients with Parkinson’s disease and freezing of gait using a virtual reality paradigm. Neuroscience 2016, 337, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Polat, K. Freezing of Gait (FoG) Detection Using Logistic Regression in Parkinson’s Disease from Acceleration Signals. In Proceedings of the 2019 Scientific Meeting on Electrical-Electronics & Biomedical Engineering and Computer Science (EBBT), Istanbul, Turkey, 24–26 April 2019. [Google Scholar]
- Stamatakis, J.; Crémers, J.; Maquet, D.; Macq, B.; Garraux, G. Gait feature extraction in Parkinson’s disease using low-cost accelerometers. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; pp. 7900–7903. [Google Scholar]
- Marcante, A.; Di Marco, R.; Gentile, G.; Pellicano, C.; Assogna, F.; Pontieri, F.E.; Spalletta, G.; Macchiusi, L.; Gatsios, D.; Giannakis, A.; et al. Foot Pressure Wearable Sensors for Freezing of Gait Detection in Parkinson’s Disease. Sensors 2021, 21, 128. [Google Scholar] [CrossRef] [PubMed]
- Rozo, L.; Pulgarin, J.; Morales, P.; Gallego, J. Wearable device intended for detection of fog episodes in Parkinson disease. Vis. Electron. 2019, 13, 10–16. [Google Scholar] [CrossRef]
- Barthel, C.; Nonnekes, J.; van Helvert, M.; Haan, R.; Janssen, A.; Delval, A.; Weerdesteyn, V.; Debû, B.; van Wezel, R.; Bloem, B.; et al. The laser shoes: A new ambulatory device to alleviate freezing of gait in Parkinson disease. Neurology 2017, 90, e164–e171. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).