Abstract
The mother and infant form a unique bond, with maternal mental health affecting the interactions with the infant and infant behaviours impacting maternal mental health. One of the possible mechanisms influencing maternal mental health is the manipulation of the gut-brain axis by consuming probiotic supplements. Probiotics can also have an indirect influence on maternal mental health via the modulation of the infant microbiome and consequently improving the infant’s health and thus, indirectly leading to an improvement in maternal mood. This systematic review evaluated the efficacy of probiotics on maternal mental health by searching for randomised controlled trials via international databases: Cochrane Library, PubMed, Scopus, ScienceDirect, and Web of Science until January 2022. A meta-analysis was performed using the Cochrane Collaboration methodology where possible. We found seven clinical trials that included the word probiotics and addressed maternal depression and/or anxiety. Of these, five trials investigated the influence of maternal probiotic supplementation on the gut-brain axis. Two trials investigated the indirect influence of probiotics on maternal depression via supplementation of probiotics by infants and subsequent influence on the crying of colicky infants. Meta-analysis of two studies of pregnant and postnatal women and two studies of infants consuming probiotics on the outcome of the Edinburgh Postnatal Depression Scale for mothers showed no statistical difference. The findings indicate that maternal depression is very complex and is influenced by various bidirectional factors. One of the factors that can improve maternal mental health is probiotics, however, careful consideration must be given to correct strain selection as strain-specific effectiveness was observed. Further well-designed, robust clinical studies are warranted.
1. Introduction
According to the World Health Organization (WHO), mental health is an integral part of health and well-being, which is described as: “a state of complete physical, mental and social well-being and not merely the absence of disease or infirmity” [1]. Anxiety is defined as “an emotion characterized by feelings of tension, worried thoughts and physical changes, such as increased blood pressure” according to the American Psychological Association. The WHO defines anxiety as a mental disorder characterised by feelings of anxiety and fear and depressive disorders are described as mental disorders characterised by sadness, loss of interest or pleasure, feelings of guilt or low self-worth, disturbed sleep or appetite, feelings of tiredness, and poor concentration. Depression can be long-lasting or recurrent, substantially impairing an individual’s ability to function at work or school or cope with daily life. At its most severe, depression can lead to suicide [2].
Increasing research into the microbiota-gut-brain axis may provide a new avenue of investigation to treat anxiety, depression, and cognition [3,4,5,6,7]. A range of potential mechanisms by which the gut microbiota affects central nervous system function have been proposed. These include altered microbial composition, immune inactivation, vagal nerve activation, tryptophan metabolism, gut hormone response, and through production of microbial neuroactive substances, bacterial cell wall sugars, or other metabolites [3,4,5,7]. There is evidence that less diversity of the intestinal microbial community is associated with neuropsychiatric disorders, including depression and anxiety in mothers and offspring. Assessing the maternal and child’s microbial communities may be an important missing component in mother-infant attachment-based therapies during the treatment of perinatal depression [8]. One approach to improving maternal mental health in the perinatal period is targeting the gut-brain axis by modulating the gut microbiota [9].
The administration of probiotics and prebiotics can achieve the microbiota-gut-brain axis homeostasis. According to the consensus statement by the International Scientific Association for Probiotics and Prebiotics, probiotics are defined as “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host” [10]. Prebiotics are defined as a substrate selectively utilised by host microorganisms conferring a health benefit [11]. The possible applications of probiotics based on well-designed clinical trials and meta-analyses are numerous, including treatment or prevention of several disorders, such as: antibiotic and Clostridium difficile-associated diarrhoea, irritable bowel syndrome and inflammatory bowel disease, to anxiety, wound healing, and even depression [12,13,14,15,16,17]. The influence of probiotics on the gut-brain axis has been investigated in several recent clinical trials with positive results, including the improvement of psychiatric and gut-barrier-associated traits [18] and cognitive functions [19] in patients with a major depressive disorder; the mitigation of anxiety symptoms in patients with anxiety disorders [20,21], and the improvement of mental state, sleep quality and the gut microbiota of healthy young adults under stressful conditions [22]. These findings support the importance of the interactions between the digestive system and the brain functions via the microbiota-gut-brain axis, thus indicating the significant role of the microbiota in the modulation of the central nervous system, including affective and cognitive functions [19]. Additionally, probiotics are among nutrients that are provisionally recommended for adjunctive use in mental disorders according to the World Federation of Societies of Biological Psychiatry (WFSBP) and the Canadian Network for Mood and Anxiety Disorders (CANMAT) [23]. Some researchers refer to probiotics administered with the purpose of supporting mental health wellbeing as psychobiotics [24,25,26,27]. However, the International Scientific Association for Probiotics and Prebiotics has published a consensus statement for the terms probiotics [10], prebiotics [11], synbiotics [28], postbiotics [29] and fermented foods [30], but not for pharmabiotics, immunobiotics, psychobiotics, etc. [31].
The mother and infant form a deep bond, and their interactions can be mediated by various biological factors, such as mother and infant characteristics, including mood, depression, maternal behavior style, age, biological risk, disability, temperament, irritable infant—for example, due to colic and other health and disease conditions [32,33,34,35]. Perinatal depression is a significant public health problem due to its negative impact on maternal well-being and long-term adverse effects on children. During the prenatal period, depression can result in poorer childbirth outcomes, including pre-term delivery and low birth weight or small for gestational age infants [8,36]. In the postpartum period, maternal suicides account for 20% of all postpartum deaths, making it one of the leading causes of maternal mortality [37]. Mother-infant interaction and maternal responsiveness and sensitivity and the rapidly developing infant brain are hypothesised mechanisms by which perinatal depression affects child development [8]. Postpartum depression creates an environment that is not conducive to the personal development of mothers or the optimal development of a child; therefore, it is important to detect and treat depression during the postnatal period as early as possible to avoid harmful consequences [38].
Published clinical trials have shown that certain probiotic strains are beneficial for either perinatal and postnatal maternal health or women’s health generally, including reducing gestational diabetes mellitus [39] mastitis [40,41], bacterial or yeast vaginosis [42,43,44] as well as improving vaginal health [45], allergic scores [46], immune factors [47] and the mental health-depression-anxiety symptoms [48,49] of mothers. There are also several ongoing clinical trials on maternal health and probiotics with published study protocols [50,51,52].
A recent review by Desai and co-authors [9] that included three randomised controlled trials investigated the effectiveness of probiotic, prebiotic, or synbiotic supplementation during pregnancy to improve perinatal maternal health. Desai and co-authors [9] focused only on probiotic supplementation during pregnancy and found that there is limited but promising evidence regarding the effectiveness of probiotics during pregnancy to reduce anxiety symptoms and reduce the proportion of women scoring above a cut-off depression score. Our review aimed to assess the influence of probiotics on improving maternal depression and anxiety, whether taken directly by pregnant women or after birth, as well as the indirect influence of probiotics on maternal mental health if consumed by infants, as all factors can influence maternal mental health.
2. Materials and Methods
We performed a systematic review using the method outlined by Higgens et al. of examination, analysis, synthesis of results and compilation [53]. We chose this type of review because it is more specific and addresses a focused range of outcomes [54]. Therefore, we included randomised controlled trials (RCTs), which examined the effect of probiotics or psychobiotics on the occurrence of depression during women’s pregnancy and in the postnatal period. We followed the PRISMA guidelines [55,56]. We formulated the research according to the PICO strategy (Population, Intervention, Comparison, Outcome) compilation [53].
2.1. Research Question
Are probiotics or psychobiotics (I) compared to placebo (C) given to the mother or infant (P) effective in reducing maternal depression during pregnancy and the postnatal period (O)?
2.2. Search Strategy
The search was performed using search terms in English: probiotics, psychobiotics, pregnant, depression and a clinical trial with their synonyms and Boolean operators (AND/OR). The final search term was: (probiotic* OR psychobiotic*) AND (mother OR pregnant) AND depression AND “clinical trial”). The systematic search was carried out in the following databases: Cochrane Library, PubMed, Scopus, ScienceDirect, and Web of Science. We used the following search limits: research articles published in English, German and/or Slovenian related to the research topic and all studies published up to January 2022. Data were collected between December 2021 and January 2022. We used the same search terms, search limits, inclusion, and exclusion criteria in all the databases.
2.3. Eligibility Criteria
Predetermined inclusion and exclusion criteria were applied as presented in Table 1.

Table 1.
Research strategy containing inclusion criteria, exclusion criteria and search limits.
2.4. Critical Assessment
The methodology of the studies was assessed using the Joanna Briggs Institute critical appraisal tool (JBI) for randomised controlled trials [57]. Based on Camp and Legge’s [58] recommendation, we evaluated the studies as medium-high quality (70–79%); high quality (80–90%) and excellent quality (90% or more). The authors S.F., S.K. and M.S. conducted the critical assessment.
2.5. Data Extraction and Synthesis of Data
The authors S.F. and M.S. conducted data extraction from the included clinical studies based on the predefined data extraction criteria. Both authors checked with each other and managed any queries and differences. The author J.F. entered data into Review Manager software (RevMan). We performed statistical analyses using Cochrane’s Review Manager [59]. We analysed continuous data using mean differences (MDs) and reported the 95% CI on all estimates. We used the random-effects model for all meta-analyses and assessed the heterogeneity between the included trials, using the I2 statistic. The degree of heterogeneity was graded as non-existent or minimal for an I2 value of less than 25%, low for an I2 value of 25–49%, moderate for an I2 value of 50–74%, and high for an I2 value of 75–100%. One author (S.F.) checked the data of the metanalysis.
3. Results
The search of the included databases (PubMed, Scopus, Cochrane Library, ScienceDirect, Web of Science) identified 195 records imported into the reference management program EndNote 20. We also conducted a hand-searching of the grey literature, and we identified one record. With the help of this program, we identified and eliminated 15 duplicates. The remaining search records (n = 181) were imported into an Excel 2016 file, where two authors (S.K. and S.F.) independently reviewed titles and abstracts. Those unrelated to our study’s topic and that did not meet the inclusion criteria (n = 174) were excluded. Ninety-nine studies found by the search did not investigate the effect of probiotics on depression during pregnancy or in the postnatal period and 53 studies did not display the effect of probiotics on the occurrence of depression during pregnancy or in the postnatal period. Twelve studies included participants, other than pregnant women or women during the postnatal period, three studies were not in English, Slovene, or German and seven papers were not clinical trials.
The authors reviewed the full-text papers for suitability (n = 7). Seven articles were included in the final analysis and synthesis [48,49,60,61,62,63,64]. Studies were conducted in Australia, New Zealand, China, the Netherlands, Finland, and Iran. One thousand two hundred fifty-six participants completed mental health evaluations using various tools. All studies were placebo-controlled and blinded. The selection of the studies was conducted according to the PRISMA statement and is presented in Figure 1 [56,65].
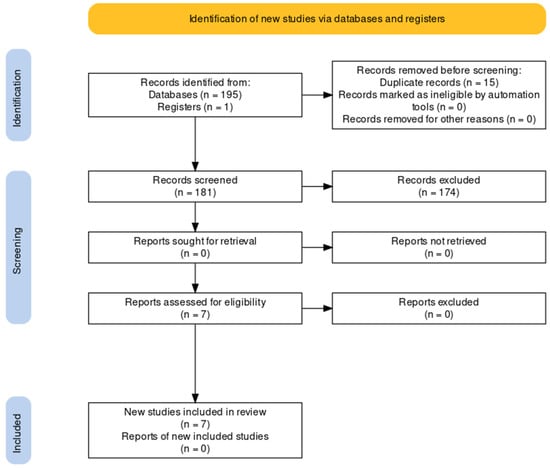
Figure 1.
PRISMA flow diagram of study selection.
3.1. Results of Critical Assessment
The clinical trials using the Joanna Briggs Institute critical appraisal tool for randomised controlled trials [57] are noted in Table 2. The clinical trial by Browne [60] and co-authors was conducted in the Netherlands and they have a previously published paper with full details about procedures, methods and outcome measures [66]. The clinical trial conducted by Dawe and co-authors [61] was a secondary analysis pertaining to antenatal mental health outcomes of the Healthy Mums and babies (HUMBA) trial with other data from the trial published elsewhere [67,68]. The clinical trial by Hulkkonen and co-authors [49] did not contain information regarding the randomisation process, concealment of allocation to treatment groups, or blinding of outcome assessors; however, another publication [69] form the same clinical trial contained this information. The clinical trial by Slykerman and co-authors [48] was part of the Probiotics in Pregnancy Study (PIP Study). It has a previously published protocol [3] and an additional paper published from the same clinical trial focusing on the influence of probiotics on gestational diabetes [70]. Details of the clinical trial by Sung and co-authors [64] are described in the published protocol [71].

Table 2.
Quality assessment checklist of included clinical trials using the Joanna Briggs Institute critical appraisal tool for randomised controlled trials.
The clinical trial by Mi and co-authors [62] was single-blinded. The parents were blinded, but no information was given on blinding to treatment assignment (question 5), or if outcome assessors were blind to treatment assignment (question 6). Therefore, all two critical appraisal questions were rated as unclear. Mirghafourvand and co-authors [63] described their study as a triple-blinded trial, as those involved in the sampling and data collection, the analysers, and the participants were unaware of the type of intervention received.
Question 3 (similar baseline characteristics at baseline) was rated unclear for Hulkkonen [49] because they did not divide their reported clinical characteristics separately for each treatment group. Question 11 was rated unclear for four studies [48,61,62,64] as either no information in the studies is given on the number of raters for statistical analysis in the text or no information on author contributions is given, or information is given that only one author conducted all statistical analysis.
All studies except the clinical trial by Mi and co-authors [62] were rated as superior quality according to Camp and Legge’s recommendation [58], as the percentage was 92% or 100%, whilst the study by Mi and co-authors [62] was 77% since it was single-blinded and three questions of the Joanna Briggs Institute critical appraisal tool assessed blinding.
3.2. Characteristics of Reviewed Articles
The characteristics of the reviewed articles are described in Table 3.

Table 3.
Characteristics and description of the seven clinical studies on the influence of probiotics on maternal depression and/or anxiety.
Two studies [62,64] did not assess improvement of maternal depression via the gut-brain axis, but as an indirect consequence of administration of probiotics (in both studies, Limosilactobacillus reuteri DSM 17938 was used) to babies with the intent to reduce infantile colic. In the study by Mi and co-authors [62], Limosilactobacillus reuteri DSM 17938 was a probiotic given to infants for 28 days. Throughout the study period, parents’ satisfaction was higher and improvement in maternal depression was observed as lower EPDS (Edinburgh Postnatal Depression Scale) scores in the probiotic group. On the other hand, although the clinical study by Sung and co-workers [64] assessed the same probiotic strain Limosilactobacillus reuteri DSM 17938 for effectiveness in reducing infant colic, no difference was observed in maternal mental health with the EPDS score. Additionally, no difference was observed for reduced colic, which could account for the indirect lack of difference in the EPDS score for maternal mental health.
Of the remaining five studies noted in Table 3, two found improved mental health outcomes [48,49]. The clinical study by Hulkkonen and co-authors [49] found a modest impact on the depressive symptoms in pregnant and postpartum women after supplementation with fish oil and Lacticaseibacillus rhamnosus HN001 and Bifidobacterium animalis subsp. lactis 420. This study focused on overweight women from early pregnancy until six months postpartum. The depressive and anxiety symptoms were assessed with the Edinburgh Postnatal Depression Scale (EPDS) and Anxiety subscale of Symptoms Checklist (SCL-90) at early and late pregnancy and three, six and 12 months postpartum. A statistically significant intervention effect was seen during pregnancy. The probiotics and fish oil group had lower EPDS scores at 12 months postpartum than the placebo group; however, they were not statistically significant. Therefore, the intervention had a modest impact on depressive symptoms. The diet, as well as obstetric events, also impacted depressive and anxiety symptoms. The study by Slykerman and co-authors [48] also used the strain Lacticaseibacillus rhamnosus HN001 and found that supplementation resulted in significantly lower postnatal anxiety scores. The probiotic was given during pregnancy and postpartum for up to 6 months. The EPDS was also used to assess maternal mood, and a six-month follow-up was conducted. This study demonstrated a significantly lower prevalence of symptoms of depression and anxiety postpartum in women supplemented with the probiotic Lacticaseibacillus rhamnosus HN001 during and after pregnancy than in those given a placebo. Furthermore, the number of women reporting clinically significant anxiety levels on screening was significantly lower in the probiotic group. Barthow and co-authors published the protocol for this study [3]. The primary outcomes were the development and severity of eczema and atopic sensitisation [72] in infants and a reduction in maternal gestational diabetes mellitus [70].
The last three clinical studies by Browne [60] Dawe [61], and Mirghafourvand [63] included pregnant women only. All three clinical studies focused on other outcomes and the mental health of pregnant women was a secondary outcome. In the pilot trial [60] no improvement in the mood of pregnant women after supplementation with a probiotic mixture (Bifidobacterium bifidum W23, Bifidobacterium lactis W51, Bifidobacterium lactis W52, Lactobacillus acidophilus W37, Levilactobacillus brevis W63, Lacticaseibacillus casei W56, Ligilactobacillus salivarius W24, Lactococcus lactis W19 and Lactococcus lactis W58) was found. There were also no differences in the mental health outcomes in obese pregnant women after supplementation with Lacticaseibacillus rhamnosus GG and Bifidobacterium animalis subsp. lactis Bb-12 [61] and no difference in the mental health of pregnant women after supplementation with conventional yoghurt containing two probiotics: Lactobacillus acidophilus La-5 and Bifidobacterium animalis subsp. lactis Bb-12 [63].
3.3. Outcome of EPDS Depression Score for Pregnant or Postnatal Women
A meta-analysis of two trials [48,49] involving a total of 512 women consuming probiotics during pregnancy and up to 6 months after birth was conducted to assess the outcome of EDPS depression scores at 12-months postpartum (Figure 2). The results showed no statistically significant difference between the probiotic and placebo groups (MD −0.35, 95% CI −2.29 to 1.58; I2 = 78%). However, the results show high heterogeneity (I2 = 78%) as the study by Slykerman and co-authors [48] included women with healthy pregnancies, whilst the study by Hulkkonen et al. and co-authors [49] included overweight pregnant women.
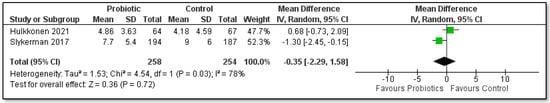
Figure 2.
Meta-analysis of the effect of probiotic supplementation for pregnant women and postnatal women on EPDS depression score.
The meta-analysis for the outcome of the EPDS depression scores by Browne and co-authors [60], Dawe and co-authors [61] and Mirghafourvand and co-authors [63] of pregnant women only was not possible due to different methods of reporting the outcomes. Browne and co-authors [60] observed no effect between the probiotic and placebo groups [F(2,38) = 0.8, p > 0.05] for the EPDS depression scores. Dawe and co-authors [61] assessed mental health outcomes at 36 weeks of pregnancy and found no effect between the probiotic and placebo group (p = 0.527) on the outcome of the EPDS depression scores. Mirghafourvand and co-authors [63] did not use the EPDS score; they used the SF-36 questionnaire which assessed the mental aspects of quality of life but found no differences between both groups.
The meta-analysis for the outcome of the EPDS depression scores of the mothers of infants consuming probiotics in two studies by Mi and co-authors [62] and Sung and co-workers [64] was also conducted (Figure 3). A total of 193 mothers were assessed after four weeks of supplementation of infants in order to treat infant colic, but no statistical difference was found between both groups MD −0.92, 95% CI −3.64 to 1.80; I2 = 72%). In both studies the infants received the same probiotic strain Limosilactobacillus reuteri DSM 17938 for one month; however, the infants in the Australian study by Sung and co-authors [64] were slightly older (mean age 7.5 weeks at beginning of the study) than the infants in the Chinese study (mean age 29.7 days) by Mi and co-authors [62].
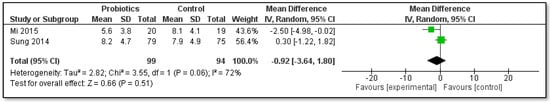
Figure 3.
Meta-analysis of the effect of probiotic supplementation of colicky infants on the EPDS depression score of mothers.
No other meta-analysis could be undertaken due to different measurement tools used and/or methods of reporting the outcomes.
4. Discussion
This systematic review focused on the efficacy of administering probiotics during pregnancy and in the postnatal period to improve mothers’ mental health. Our meta-analysis did not clearly prove an overall beneficial effect of the chosen probiotics, however, certain strains proved to have some impact on improving maternal mental health. Furthermore, certain probiotic strains that were consumed directly by the pregnant or postnatal women exhibited a slight impact on reducing depression. The review also showed that probiotic supplementation of infants leads to the improvement of colicky symptoms and indirectly influences the reduction of postnatal depression symptoms.
A similar systematic review and meta-analysis by Desai and co-authors [9] focused on the effectiveness of probiotic, prebiotic and synbiotic administration on perinatal maternal mental health; however, it focused only on supplementation during pregnancy. It is well known that probiotic supplementation of mothers during breastfeeding or direct supplementation to the infant has several benefits for the infant, including developing a healthy microbiome, reduction in colic and regurgitation, the development of a healthy immune system, and reduction in the risk of atopic eczema [73,74,75]. Several studies also found positive effects of probiotic supplementation for mothers, including preventing or treating mastitis [40], improving immunological factors [47] as well as reducing vaginosis and improving general vaginal health [42,45]. Poor maternal mental health has been associated with various pregnancy and child health complications. On the other hand, a recent review [76] warned that there could be an increased risk of pre-eclampsia with probiotic administration for the prevention of gestational diabetes, therefore, caution is necessary.
A systematic review and meta-analysis by Zhang and co-authors of non-pregnant healthy adults concluded that current evidence suggests that probiotics can reduce emotional stress levels in healthy volunteers and alleviate stress-related subthreshold anxiety/depression levels [77]. Psychological distress is a very real problem for new mothers and depressive symptoms and anxiety stress can potentially affect maternal mood and bonding to the offspring [60]; therefore, it could be beneficial for mothers taking probiotics during pregnancy to continue with probiotics supplementation after birth. Whilst probiotics are not intended to be a single management option for psychological distress, they can be an important supportive therapy.
According to the consensus statement of the International Probiotic and Prebiotic association [17], several probiotics have strain-specific health benefits. This was also obvious from the investigated clinical studies where one strain: Lacticaseibacillus rhamnosus HN001, used in the clinical studies by Hulkkonen et al. and Slykerman et al. [48,49] exhibited a beneficial effect on mental health, whilst another strain of the same species, namely Lacticaseibacillus rhamnosus GG used in the study by Dawe et al. [61] did not exhibit any beneficial effect on mental health compared to the placebo group.
Consideration of the possible synergistic effects of probiotic mixtures is also important. However, in our review, the beneficial effect of the Lacticaseibacillus rhamnosus HN001 strain on mental health was not greater when combined with Bifidobacterium animalis subsp. lactis 420 [49] compared to supplementation with only the HN001 strain [48]. Health specific benefits were also found for the two-strain probiotic containing Lactobacillus acidophilus La5 and Bifidobacterium animalis subsp. lactis Bb-12, used in the study by Mirghafourvand and co-authors [63]. Although this strain combination did not provide an improvement in mental health during pregnancy, reduced constipation was observed thus proving that this probiotic combination did indeed exhibit a health benefit, just not on maternal mental health.
It is known that infantile colic is associated with higher maternal depression symptoms scores and lower quality of life scores [78,79]. Two clinical studies included in our review by Mi and co-authors and Sung and co-authors [62,64] used the same strain of Limosilactobacillus reuteri DSM 17938, but statistically significant improvement in maternal mental health symptoms was found only in the study by Mi and co-authors [62]. Infantile colic, like regurgitation and subsequent infant crying, is very stressful for parents. However, these two studies investigated maternal depression based on distress due to infant colic. Therefore, the influence on maternal stress was indirect, indicating that maternal depression is tightly linked with infant well-being. Although both studies observed the same primary outcome using the same probiotic strain (Limosilactobacillus reuteri DSM 17938), the study conducted by Mi and co-authors was conducted on infants from China with a positive outcome for reduced colic and reduced maternal depression in the probiotic group, whilst in the Australian study by Sung and co-authors no statistically significant difference was found for reduction of colic or for maternal mental health. In the Chinese study, the average age of the infants enrolled in the study was 29.7 days for the probiotic group and 28.6 days for the placebo group, whilst in the Australian study the average age of the infants entering the study was 7.5 weeks for the probiotic group and 8.9 weeks for the placebo group, thus these infants were older and perhaps the opportunity for the probiotics to reduce infantile colic was distinct; thus leading to the difference in the consequent effect on EPDS depression score of mothers. The authors of the Australian study [64] also emphasised that they recruited infants from the ‘emergency or urgent care settings’ and so were already exhibiting symptoms of crying as the most common age for development of colic is about 3 to 6 weeks of age, with most resolved by three months of age [79]. Therefore, timely administration of probiotics to breastfeeding mothers or directly to infants, such as Limosilactobacillus reuteri DSM 17938 [80] could reduce infantile colic and thus benefit the infant and also indirectly influence maternal mental health. In the case of probiotic supplementation directly to infants, this benefit is not via the gut-brain axis of the mother. With mothers with mental health disorders found to be nearly five times as likely to have a baby admitted with GOR/GORD in the first year after birth [81] this is an important consideration. It is well known that the mode of birth, as well as breastfeeding, also influences the infant microbiota. Infants born via caesarian section have different gut microbiota compared to vaginally born infants. Infants born vaginally and fully breastfed infants have gut microbiota dominated by Bifidobacteria [82]. Thus, infant probiotic supplementation could be warranted when babies are born by caesarian section or when not exclusively breastfeeding.
Improvement of maternal mental health was observed in only three [48,49,62] of the seven clinical studies included in our review; the studies by Dawe and co-authors and Browne and co-authors [60,61] both investigated the influence of supplementation with probiotics only up to delivery and observed no beneficial effects on mental health, anxiety or depression. This could be due to two reasons, inappropriate strain selection, as not all probiotic strains influence mental health, as well as the impending birth and the natural elevation of anxiety connected with this [83]. However, since some of the clinical trials exhibited a positive effect of probiotics on reducing postnatal depression, the clinical environment can promote probiotics for reducing symptoms of postnatal depression.
5. Conclusions
Maternal mental health is very complex and can be influenced by the gut-brain axis or by other external influences, such as infant mood. Current evidence has shown that certain probiotic strains are better than placebo in exhibiting a positive health benefit for maternal mental health, based on individual clinical trials. The analysis of the clinical studies shows that strain selection is one of the most decisive factors in showing a statistically significant difference compared to placebo. The strain Lacticaseibacillus rhamnosus HN001 was effective in reducing maternal depressive symptoms when maternal supplementation was continued in the postnatal period; the supplementation of the probiotic Limosilactobacillus reuteri DSM 17938 to the infants was effective in reducing colic, and therefore, indirectly reducing maternal depressive symptoms if the probiotic was given to the infant before colicky symptoms peaked. However, more robust, well-designed clinical trials are needed. Future studies should include probiotic supplementation after birth and careful consideration should be given to measure outcomes in a reliable way. Accurate reporting of allocation concealment and blinding of participants and those delivering the treatment or assessing the outcomes is important in future trials. Importantly clinical trials should not be influenced by probiotic manufacturer funding.
Author Contributions
Conceptualization, S.F. and K.Č.T.; methodology, S.F., S.K., M.S. and J.P.F.; formal analysis, S.F. and S.K.; data curation, S.F., K.Č.T., S.K. and J.P.F.; writing—original draft preparation, S.F.; writing—review and editing, S.F., J.P.F., D.M.-T. and H.G.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- World Health Organization (Ed.) Comprehensive Mental Health Action Plan 2013–2030; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- WHO. Depression and Other Common Mental Disorders: Global Health Estimates. G; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Barthow, C.; Wickens, K.; Stanley, T.; Mitchell, E.A.; Maude, R.; Abels, P.; Purdie, G.; Murphy, R.; Stone, P.; Kang, J.; et al. The Probiotics in Pregnancy Study (PiP Study): Rationale and design of a double-blind randomised controlled trial to improve maternal health during pregnancy and prevent infant eczema and allergy. BMC Pregnancy Childbirth 2016, 16. [Google Scholar] [CrossRef] [PubMed]
- Marques, T.M.; Cryan, J.F.; Shanahan, F.; Fitzgerald, G.F.; Ross, R.P.; Dinan, T.G.; Stanton, C. Gut microbiota modulation and implications for host health: Dietary strategies to influence the gut–brain axis. Innov. Food Sci. Emerg. Technol. 2014, 22, 239–247. [Google Scholar] [CrossRef]
- Borre, Y.E.; O’Keeffe, G.W.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Microbiota and neurodevelopmental windows: Implications for brain disorders. Trends Mol. Med. 2014, 20, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.A.; McVey Neufeld, K.A. Gut-brain axis: How the microbiome influences anxiety and depression. Trends Neurosci. 2013, 36, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; Dinan, T.G. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Sanders, A.; Rackers, H.; Kimmel, M. A role for the microbiome in mother-infant interaction and perinatal depression. Int. Rev. Psychiatry 2019, 31, 280–294. [Google Scholar] [CrossRef] [PubMed]
- Desai, V.; Kozyrskyj, A.L.; Lau, S.; Sanni, O.; Dennett, L.; Walter, J.; Ospina, M.B. Effectiveness of Probiotic, Prebiotic, and Synbiotic Supplementation to Improve Perinatal Mental Health in Mothers: A Systematic Review and Meta-Analysis. Front. Psychiatry 2021, 12, 622181. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Bagga, D.; Reichert, J.L.; Koschutnig, K.; Aigner, C.S.; Holzer, P.; Koskinen, K.; Moissl-Eichinger, C.; Schöpf, V. Probiotics drive gut microbiome triggering emotional brain signatures. Gut Microbes 2018, 9, 486–496. [Google Scholar] [CrossRef]
- Galdeano, C.M.; Cazorla, S.I.; Dumit, J.M.L.; Vélez, E.; Perdigón, G. Beneficial Effects of Probiotic Consumption on the Immune System. Ann. Nutr. Metab. 2019, 74, 115–124. [Google Scholar] [CrossRef]
- Liu, Y.; Alookaran, J.; Rhoads, J. Probiotics in Autoimmune and Inflammatory Disorders. Nutrients 2018, 10, 1537. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, R.; Lau, C. Probiotics are effective at preventing Clostridium difficile-associated diarrhea: A systematic review and meta-analysis. Int. J. Gen. Med. 2016, 9, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Fijan, S.; Frauwallner, A.; Langerholc, T.; Krebs, B.; Younes, J.A.T.H.N.; Heschl, A.; Turk, D.M.; Rogelj, I. Efficacy of Using Probiotics with Antagonistic Activity against Pathogens of Wound Infections: An Integrative Review of Literature. BioMed Res. Int. 2019, 2019, 7585486. [Google Scholar] [CrossRef] [PubMed]
- Strauss, M.; Mičetić-Turk, D.; Pogačar, M.Š.; Fijan, S. Probiotics for the Prevention of Acute Respiratory-Tract Infections in Older People: Systematic Review. Healthcare 2021, 9, 690. [Google Scholar] [CrossRef]
- Karakula-Juchnowicz, H.; Rog, J.; Juchnowicz, D.; Łoniewski, I.; Skonieczna-Żydecka, K.; Krukow, P.; Futyma-Jedrzejewska, M.; Kaczmarczyk, M. The study evaluating the effect of probiotic supplementation on the mental status, inflammation, and intestinal barrier in major depressive disorder patients using gluten-free or gluten-containing diet (SANGUT study): A 12-week, randomized, double-blind, and placebo-controlled clinical study protocol. Nutr. J. 2019, 18, 50. [Google Scholar] [CrossRef]
- Rudzki, L.; Ostrowska, L.; Pawlak, D.; Małus, A.; Pawlak, K.; Waszkiewicz, N.; Szulc, A. Probiotic Lactobacillus Plantarum 299v decreases kynurenine concentration and improves cognitive functions in patients with major depression: A double-blind, randomized, placebo controlled study. Psychoneuroendocrinology 2019, 100, 213–222. [Google Scholar] [CrossRef]
- Gualtieri, P.; Marchetti, M.; Cioccoloni, G.; De Lorenzo, A.; Romano, L.; Cammarano, A.; Colica, C.; Condò, R.; Di Renzo, L. Psychobiotics Regulate the Anxiety Symptoms in Carriers of Allele A of IL-1β Gene: A Randomized, Placebo-Controlled Clinical Trial. Mediat. Inflamm. 2020, 2020, 2346126. [Google Scholar] [CrossRef]
- Zagórska, A.; Marcinkowska, M.; Jamrozik, M.; Wiśniowska, B.; Paśko, P. From probiotics to psychobiotics—The gut-brain axis in psychiatric disorders. Benef. Microbes 2020, 11, 717–732. [Google Scholar] [CrossRef]
- Nishida, K.; Sawada, D.; Kuwano, Y.; Tanaka, H.; Rokutan, K. Health Benefits of Lactobacillus gasseri CP2305 Tablets in Young Adults Exposed to Chronic Stress: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2019, 11, 1859. [Google Scholar] [CrossRef]
- Sarris, J.; Ravindran, A.; Yatham, L.N.; Marx, W.; Rucklidge, J.J.; McIntyre, R.S.; Akhondzadeh, S.; Benedetti, F.; Caneo, C.; Cramer, H.; et al. Clinician guidelines for the treatment of psychiatric disorders with nutraceuticals and phytoceuticals: The World Federation of Societies of Biological Psychiatry (WFSBP) and Canadian Network for Mood and Anxiety Treatments (CANMAT) Taskforce. World J. Biol. Psychiatry 2022, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Dinan, T.G.; Stanton, C.; Cryan, J.F. Psychobiotics: A novel class of psychotropic. Biol. Psychiatry 2013, 74, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Wasilewski, A.; Zielińska, M.; Storr, M.; Fichna, J. Beneficial Effects of Probiotics, Prebiotics, Synbiotics, and Psychobiotics in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2015, 21, 1674–1682. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.H.; Liu, Y.W.; Wu, C.C.; Wang, S.; Tsai, Y.C. Psychobiotics in mental health, neurodegenerative and neurodevelopmental disorders. J. Food Drug Anal. 2019, 27, 632–648. [Google Scholar] [CrossRef]
- Sarkar, A.; Lehto, S.M.; Harty, S.; Dinan, T.G.; Cryan, J.F.; Burnet, P.W.J. Psychobiotics and the Manipulation of Bacteria–Gut–Brain Signals. Trends Neurosci. 2016, 39, 763–781. [Google Scholar] [CrossRef]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Marco, M.L.; Sanders, M.E.; Gänzle, M.; Arrieta, M.C.; Cotter, P.D.; De Vuyst, L.; Hill, C.; Holzapfel, W.; Lebeer, S.; Merenstein, D.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on fermented foods. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 196–208. [Google Scholar] [CrossRef]
- Binda, S.; Hill, C.; Johansen, E.; Obis, D.; Pot, B.; Sanders, M.E.; Tremblay, A.; Ouwehand, A.C. Criteria to Qualify Microorganisms as “Probiotic” in Foods and Dietary Supplements. Front. Microbiol. 2020, 11, 1662. [Google Scholar] [CrossRef]
- Rocha, N.; Dos Santos Silva, F.P.; Dos Santos, M.M.; Dusing, S.C. Impact of mother-infant interaction on development during the first year of life: A systematic review. J. Child Health Care 2020, 24, 365–385. [Google Scholar] [CrossRef]
- Konijnenberg, C.; Sarfi, M.; Melinder, A. Mother-child interaction and cognitive development in children prenatally exposed to methadone or buprenorphine. Early Hum. Dev. 2016, 101, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Laing, S.; McMahon, C.; Ungerer, J.; Taylor, A.; Badawi, N.; Spence, K. Mother-child interaction and child developmental capacities in toddlers with major birth defects requiring newborn surgery. Early Hum. Dev. 2010, 86, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Fox, N.A.; Calkins, S.D. The Development of Self-Control of Emotion: Intrinsic and Extrinsic Influences. Motiv. Emot. 2003, 27, 7–26. [Google Scholar] [CrossRef]
- Smith, M.V.; Shao, L.; Howell, H.; Lin, H.; Yonkers, K.A. Perinatal Depression and Birth Outcomes in a Healthy Start Project. Matern. Child Health J. 2011, 15, 401–409. [Google Scholar] [CrossRef]
- Lindahl, V.; Pearson, J.L.; Colpe, L. Prevalence of suicidality during pregnancy and the postpartum. Arch. Womens Ment. Health 2005, 8, 77–87. [Google Scholar] [CrossRef]
- Slomian, J.; Honvo, G.; Emonts, P.; Reginster, J.-Y.; Bruyère, O. Consequences of maternal postpartum depression: A systematic review of maternal and infant outcomes. Womens Health 2019, 15, 174550651984404. [Google Scholar] [CrossRef]
- Luoto, R.; Laitinen, K.; Nermes, M.; Isolauri, E. Impact of maternal probiotic-supplemented dietary counselling on pregnancy outcome and prenatal and postnatal growth: A double-blind, placebo-controlled study. Br. J. Nutr. 2010, 103, 1792–1799. [Google Scholar] [CrossRef]
- Arroyo, R.; Martín, V.; Maldonado, A.; Jiménez, E.; Fernández, L.; Rodríguez, J.M. Treatment of infectious mastitis during lactation: Antibiotics versus oral administration of Lactobacilli isolated from breast milk. Clin. Infect. Dis. 2010, 50, 1551–1558. [Google Scholar] [CrossRef]
- Maldonado-Lobón, J.A.; Díaz-López, M.A.; Carputo, R.; Duarte, P.; Díaz-Ropero, M.P.; Valero, A.D.; Sañudo, A.; Sempere, L.; Ruiz-López, M.D.; Bañuelos, Ó.; et al. Lactobacillus fermentum CECT 5716 Reduces Staphylococcus Load in the Breastmilk of Lactating Mothers Suffering Breast Pain: A Randomized Controlled Trial. Breastfeed. Med. 2015, 10, 425–432. [Google Scholar] [CrossRef]
- Reznichenko, H.; Henyk, N.; Maliuk, V.; Khyzhnyak, T.; Tynna, Y.; Filipiuk, I.; Veresniuk, N.; Zubrytska, L.; Quintens, J.; Richir, K.; et al. Oral Intake of Lactobacilli Can Be Helpful in Symptomatic Bacterial Vaginosis: A Randomized Clinical Study. J. Low Genit. Tract Dis. 2020, 24, 284–289. [Google Scholar] [CrossRef]
- Yang, S.; Reid, G.; Challis, J.R.G.; Gloor, G.B.; Asztalos, E.; Money, D.; Seney, S.; Bocking, A.D. Effect of Oral Probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 on the Vaginal Microbiota, Cytokines and Chemokines in Pregnant Women. Nutrients 2020, 12, 368. [Google Scholar] [CrossRef] [PubMed]
- Pendharkar, S.; Brandsborg, E.; Hammarström, L.; Marcotte, H.; Larsson, P.G. Vaginal colonisation by probiotic lactobacilli and clinical outcome in women conventionally treated for bacterial vaginosis and yeast infection. BMC Infect. Dis. 2015, 15, 255. [Google Scholar] [CrossRef] [PubMed]
- Houttu, N.; Mokkala, K.; Koivuniemi, E.; Pellonperä, O.; Juhila, J.; Sorsa, T.; Laitinen, K. The Impacts of Fish Oil and/or Probiotic Intervention on Low-Grade Inflammation, IGFBP-1 and MMP-8 in Pregnancy: A Randomized, Placebo-Controlled, Double-Blind Clinical Trial. Biomolecules 2020, 11, 5. [Google Scholar] [CrossRef] [PubMed]
- Ou, C.Y.; Kuo, H.C.; Wang, L.; Hsu, T.Y.; Chuang, H.; Liu, C.A.; Chang, J.C.; Yu, H.R.; Yang, K.D. Prenatal and postnatal probiotics reduces maternal but not childhood allergic diseases: A randomized, double-blind, placebo-controlled trial. Clin. Exp. Allergy 2012, 42, 1386–1396. [Google Scholar] [CrossRef]
- Ortiz-Andrellucchi, A.; Sánchez-Villegas, A.; Rodríguez-Gallego, C.; Lemes, A.; Molero, T.; Soria, A.; Peña-Quintana, L.; Santana, M.; Ramírez, O.; García, J.; et al. Immunomodulatory effects of the intake of fermented milk with Lactobacillus casei DN114001 in lactating mothers and their children. Br. J. Nutr. 2008, 100, 834–845. [Google Scholar] [CrossRef]
- Slykerman, R.F.; Hood, F.; Wickens, K.; Thompson, J.M.D.; Barthow, C.; Murphy, R.; Kang, J.; Rowden, J.; Stone, P.; Crane, J.; et al. Effect of Lactobacillus rhamnosus HN001 in Pregnancy on Postpartum Symptoms of Depression and Anxiety: A Randomised Double-blind Placebo-controlled Trial. EBioMedicine 2017, 24, 159–165. [Google Scholar] [CrossRef]
- Hulkkonen, P.; Kataja, E.L.; Vahlberg, T.; Koivuniemi, E.; Houttu, N.; Pellonperä, O.; Mokkala, K.; Karlsson, H.; Laitinen, K. The efficacy of probiotics and/or n-3 long-chain polyunsaturated fatty acids intervention on maternal prenatal and postnatal depressive and anxiety symptoms among overweight and obese women. J. Affect. Disord. 2021, 289, 21–30. [Google Scholar] [CrossRef]
- Bond, D.M.; Morris, J.M.; Nassar, N. Study protocol: Evaluation of the probiotic Lactobacillus Fermentum CECT5716 for the prevention of mastitis in breastfeeding women: A randomised controlled trial. BMC Pregnancy Childbirth 2017, 17, 148. [Google Scholar] [CrossRef]
- Godfrey, K.M.; Cutfield, W.; Chan, S.Y.; Baker, P.N.; Chong, Y.S. Nutritional Intervention Preconception and During Pregnancy to Maintain Healthy Glucose Metabolism and Offspring Health (“NiPPeR”): Study protocol for a randomised controlled trial. Trials 2017, 18, 131. [Google Scholar] [CrossRef]
- Pirotta, M.; Gunn, J.; Chondros, P.; Grover, S.; Hurley, S.; Garland, S. The PAV trial: Does lactobacillus prevent post-antibiotic vulvovaginal candidiasis? Protocol of a randomised controlled trial [ISRCTN24141277]. BMC Fam. Pract. 2004, 5, 5. [Google Scholar] [CrossRef][Green Version]
- Higgins, J.P.T.; López-López, J.A.; Becker, B.J.; Davies, S.R.; Dawson, S.; Grimshaw, J.M.; McGuinness, L.A.; Moore, T.H.M.; Rehfuess, E.A.; Thomas, J.; et al. Synthesising quantitative evidence in systematic reviews of complex health interventions. BMJ Glob. Health 2019, 4, e000858. [Google Scholar] [CrossRef] [PubMed]
- Tsagris, M.; Fragkos, K.C. Umbrella Reviews, Overviews of Reviews, and Meta-epidemiologic Studies: Similarities and Differences. In Umbrella Reviews: Evidence Synthesis with Overviews of Reviews and Meta-Epidemiologic Studies; Biondi-Zoccai, G., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 43–54. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLoS Med. 2021, 18, e1003583. [Google Scholar] [CrossRef] [PubMed]
- Tufanaru, C.; Munn, Z.; Aromataris, E.; Campbell, J.; Hopp, L. Chapter 3: Systematic reviews of effectiveness. In JBI Manual for Evidence Synthesis; Aromataris, E.M.Z., Ed.; Joanna Briggs Institute, University of Adelaide: Adelaide, Australia, 2020. [Google Scholar]
- Camp, S.; Legge, T. Simulation as a Tool for Clinical Remediation: An Integrative Review. Clin. Simul. Nurs. 2018, 16, 48–61. [Google Scholar] [CrossRef]
- Higgins, J.P.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0; The Cochrane Collaboration: London, UK, 2017. [Google Scholar]
- Browne, P.D.; Bolte, A.C.; Besseling-van der Vaart, I.; Claassen, E.; de Weerth, C. Probiotics as a treatment for prenatal maternal anxiety and depression: A double-blind randomized pilot trial. Sci. Rep. 2021, 11, 3051. [Google Scholar] [CrossRef]
- Dawe, J.P.; McCowan, L.M.E.; Wilson, J.; Okesene-Gafa, K.A.M.; Serlachius, A.S. Probiotics and Maternal Mental Health: A Randomised Controlled Trial among Pregnant Women with Obesity. Sci. Rep. 2020, 10, 1291. [Google Scholar] [CrossRef] [PubMed]
- Mi, G.L.; Zhao, L.; Qiao, D.D.; Kang, W.Q.; Tang, M.Q.; Xu, J.K. Effectiveness of Lactobacillus reuteri in infantile colic and colicky induced maternal depression: A prospective single blind randomized trial. Antonie Leeuwenhoek 2015, 107, 1547–1553. [Google Scholar] [CrossRef]
- Mirghafourvand, M.; Rad, A.H.; Charandabi, S.M.A.; Fardiazar, Z.; Shokri, K. The Effect of Probiotic Yogurt on Constipation in Pregnant Women: A Randomized Controlled Clinical Trial. Iran. Red Crescent Med. J. 2016, 18, e39870. [Google Scholar] [CrossRef] [PubMed]
- Sung, V.; Hiscock, H.; Tang, M.L.; Mensah, F.K.; Nation, M.L.; Satzke, C.; Heine, R.G.; Stock, A.; Barr, R.G.; Wake, M. Treating infant colic with the probiotic Lactobacillus reuteri: Double blind, placebo controlled randomised trial. BMJ 2014, 348, g2107. [Google Scholar] [CrossRef]
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: An R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and Open Synthesis. Campbell Syst. Rev. 2022, 18, e1230. [Google Scholar] [CrossRef]
- Browne, P.D.; Bolte, A.; Claassen, E.; de Weerth, C. Probiotics in pregnancy: Protocol of a double-blind randomized controlled pilot trial for pregnant women with depression and anxiety (PIP pilot trial). Trials 2019, 20, 440. [Google Scholar] [CrossRef] [PubMed]
- Okesene-Gafa, K.A.M.; Li, M.; McKinlay, C.J.D.; Taylor, R.S.; Rush, E.C.; Wall, C.R.; Wilson, J.; Murphy, R.; Taylor, R.; Thompson, J.M.D.; et al. Effect of antenatal dietary interventions in maternal obesity on pregnancy weight-gain and birthweight: Healthy Mums and Babies (HUMBA) randomized trial. Am. J. Obstet. Gynecol. 2019, 221, 152.e1–152.e13. [Google Scholar] [CrossRef] [PubMed]
- Okesene-Gafa, K.; Li, M.; Taylor, R.S.; Thompson, J.M.D.; Crowther, C.A.; McKinlay, C.J.D.; McCowan, L.M.E. A randomised controlled demonstration trial of multifaceted nutritional intervention and or probiotics: The healthy mums and babies (HUMBA) trial. BMC Pregnancy Childbirth 2016, 16, 373. [Google Scholar] [CrossRef] [PubMed]
- Pellonperä, O.; Mokkala, K.; Houttu, N.; Vahlberg, T.; Koivuniemi, E.; Tertti, K.; Rönnemaa, T.; Laitinen, K. Efficacy of Fish Oil and/or Probiotic Intervention on the Incidence of Gestational Diabetes Mellitus in an At-Risk Group of Overweight and Obese Women: A Randomized, Placebo-Controlled, Double-Blind Clinical Trial. Diabetes Care 2019, 42, 1009–1017. [Google Scholar] [CrossRef]
- Wickens, K.L.; Barthow, C.A.; Murphy, R.; Abels, P.R.; Maude, R.M.; Stone, P.R.; Mitchell, E.A.; Stanley, T.V.; Purdie, G.L.; Kang, J.M.; et al. Early pregnancy probiotic supplementation with Lactobacillus rhamnosus HN001 may reduce the prevalence of gestational diabetes mellitus: A randomised controlled trial. Br. J. Nutr. 2017, 117, 804–813. [Google Scholar] [CrossRef]
- Sung, V.; Hiscock, H.; Tang, M.; Mensah, F.K.; Heine, R.G.; Stock, A.; York, E.; Barr, R.G.; Wake, M. Probiotics to improve outcomes of colic in the community: Protocol for the Baby Biotics randomised controlled trial. BMC Pediatr. 2012, 12, 135. [Google Scholar] [CrossRef]
- Wickens, K.; Barthow, C.; Mitchell, E.A.; Stanley, T.V.; Purdie, G.; Rowden, J.; Kang, J.; Hood, F.; Van Den Elsen, L.; Forbes-Blom, E.; et al. Maternal supplementation alone with Lactobacillus rhamnosus HN001 during pregnancy and breastfeeding does not reduce infant eczema. Pediatr. Allergy Immunol. 2018, 29, 296–302. [Google Scholar] [CrossRef]
- Younes, J.A.; Lievens, E.; Hummelen, R.; van der Westen, R.; Reid, G.; Petrova, M.I. Women and Their Microbes: The Unexpected Friendship. Trends Microbiol. 2018, 26, 16–32. [Google Scholar] [CrossRef]
- Foster, J.P.; Dahlen, H.G.; Fijan, S.; Badawi, N.; Schmied, V.; Thornton, C.; Smith, C.; Psaila, K. Probiotics for preventing and treating infant regurgitation: A systematic review and meta-analysis. Matern. Child Nutr. 2022, 18, e13290. [Google Scholar] [CrossRef]
- Janvier, A.; Malo, J.; Barrington, K.J. Cohort study of probiotics in a North American neonatal intensive care unit. J. Pediatr. 2014, 164, 980–985. [Google Scholar] [CrossRef]
- Davidson, S.J.; Barrett, H.L.; Price, S.A.; Callaway, L.K.; Nitert, M.D. Probiotics for preventing gestational diabetes. Cochrane Database Syst. Rev. 2021, 4, Cd009951. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zhang, Y.; Li, M.; Wang, W.; Liu, Z.; Xi, C.; Huang, X.; Liu, J.; Huang, J.; Tian, D.; et al. Efficacy of probiotics on stress in healthy volunteers: A systematic review and meta-analysis based on randomized controlled trials. Brain Behav. 2020, 10, e01699. [Google Scholar] [CrossRef] [PubMed]
- Abacı, F.B.; Gökçe, S.; Tuygun, N.; Karacan, C.D.; Öner, Ö. Psychosocial status and quality of life in mothers of infants with colic. Turk. J. Pediatr. 2013, 55, 391–395. [Google Scholar] [PubMed]
- Vik, T.; Grote, V.; Escribano, J.; Socha, J.; Verduci, E.; Fritsch, M.; Carlier, C.; von Kries, R.; Koletzko, B. Infantile colic, prolonged crying and maternal postnatal depression. Acta Paediatr. 2009, 98, 1344–1348. [Google Scholar] [CrossRef] [PubMed]
- Urbańska, M.; Szajewska, H. The efficacy of Lactobacillus reuteri DSM 17938 in infants and children: A review of the current evidence. Eur. J. Pediatr. 2014, 173, 1327–1337. [Google Scholar] [CrossRef]
- Dahlen, H.G.; Foster, J.P.; Psaila, K.; Spence, K.; Badawi, N.; Fowler, C.; Schmied, V.; Thornton, C. Gastro-oesophageal reflux: A mixed methods study of infants admitted to hospital in the first 12 months following birth in NSW (2000–2011). BMC Pediatr. 2018, 18, 30. [Google Scholar] [CrossRef]
- Sakwinska, O.; Foata, F.; Berger, B.; Brüssow, H.; Combremont, S.; Mercenier, A.; Dogra, S.; Soh, S.E.; Yen, J.C.K.; Heong, G.Y.S.; et al. Does the maternal vaginal microbiota play a role in seeding the microbiota of neonatal gut and nose? Benef. Microbes 2017, 8, 763–778. [Google Scholar] [CrossRef]
- Wu, N.; Huang, R.; Shan, S.; Li, Y.; Jiang, H. Effect of the labour roadmap on anxiety, labour pain, sense of control, and gestational outcomes in primiparas. Complement. Ther. Clin. Pract. 2022, 46, 101545. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).