Intestinal Permeability Associated with the Loss of Skeletal Muscle Strength in Middle-Aged and Older Adults in Rural Area of Beijing, China
Abstract
:1. Introduction
2. Participants, Materials and Methods
2.1. Participants and Study Design
2.2. Anthropometric Measurements
2.3. Intestinal Permeability
2.4. Gut Microbiota Analysis
2.5. Potential Covariates
2.6. Statistical Analysis
3. Results
3.1. Participants Characteristics
3.2. Correlation between Intestinal Permeability and Skeletal Muscle Strength
3.3. Association between Serum DAO and the Loss of Skeletal Muscle Strength in Middle-Aged and Older Adults
3.4. Association and Interaction among Indicators of Intestinal Permeability, GM and Skeletal Muscle Strength in Males and Females
4. Discussion
4.1. Intestinal Permeability and Skeletal Muscle Strength: The Potential Predicative Value of DAO for Sarcopenia?
4.2. Association and Interaction among GM, Intestinal Permeability, and Sarcopenia
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef]
- Anker, S.D.; Morley, J.E.; von Haehling, S. Welcome to the ICD-10 code for sarcopenia. J. Cachexia Sarcopenia Muscle 2016, 7, 512–514. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.K.; Liu, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Bahyah, K.S.; Chou, M.Y.; Chen, L.Y.; Hsu, P.S.; Krairit, O.; et al. Sarcopenia in Asia: Consensus report of the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 2014, 15, 95–101. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.-K.; Woo, J.; Assantachai, P.; Auyeung, T.-W.; Chou, M.-Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e302. [Google Scholar] [CrossRef]
- Ibrahim, K.; May, C.R.; Patel, H.P.; Baxter, M.; Sayer, A.A.; Roberts, H.C. Implementation of grip strength measurement in medicine for older people wards as part of routine admission assessment: Identifying facilitators and barriers using a theory-led intervention. BMC Geriatr. 2018, 18, 79. [Google Scholar] [CrossRef] [Green Version]
- Roberts, H.C.; Syddall, H.E.; Cooper, C.; Aihie Sayer, A. Is grip strength associated with length of stay in hospitalised older patients admitted for rehabilitation? Findings from the Southampton grip strength study. Age Ageing 2012, 41, 641–646. [Google Scholar] [CrossRef] [Green Version]
- Gopinath, B.; Kifley, A.; Liew, G.; Mitchell, P. Handgrip strength and its association with functional independence, depressive symptoms and quality of life in older adults. Maturitas 2017, 106, 92–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leong, D.P.; Teo, K.K.; Rangarajan, S.; Lopez-Jaramillo, P.; Avezum, A., Jr.; Orlandini, A.; Seron, P.; Ahmed, S.H.; Rosengren, A.; Kelishadi, R.; et al. Prognostic value of grip strength: Findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet 2015, 386, 266–273. [Google Scholar] [CrossRef]
- Celis-Morales, C.A.; Welsh, P.; Lyall, D.M.; Steell, L.; Petermann, F.; Anderson, J.; Iliodromiti, S.; Sillars, A.; Graham, N.; Mackay, D.F.; et al. Associations of grip strength with cardiovascular, respiratory, and cancer outcomes and all cause mortality: Prospective cohort study of half a million UK Biobank participants. BMJ 2018, 361, k1651. [Google Scholar]
- Wang, D.X.M.; Yao, J.; Zirek, Y.; Reijnierse, E.M.; Maier, A.B. Muscle mass, strength, and physical performance predicting activities of daily living: A meta-analysis. J. Cachexia Sarcopenia Muscle 2020, 11, 3–25. [Google Scholar] [CrossRef] [Green Version]
- Soysal, P.; Hurst, C.; Demurtas, J.; Firth, J.; Howden, R.; Yang, L.; Tully, M.A.; Koyanagi, A.; Ilie, P.C.; López-Sánchez, G.F.; et al. Handgrip strength and health outcomes: Umbrella review of systematic reviews with meta-analyses of observational studies. J. Sport Health Sci. 2021, 10, 290–295. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Kiesswetter, E.; Drey, M.; Sieber, C.C. Nutrition, frailty, and sarcopenia. Aging Clin. Exp. Res. 2017, 29, 43–48. [Google Scholar] [CrossRef]
- Ticinesi, A.; Nouvenne, A.; Cerundolo, N.; Catania, P.; Prati, B.; Tana, C.; Meschi, T. Gut Microbiota, Muscle Mass and Function in Aging: A Focus on Physical Frailty and Sarcopenia. Nutrients 2019, 11, 1633. [Google Scholar] [CrossRef] [Green Version]
- Thevaranjan, N.; Puchta, A.; Schulz, C.; Naidoo, A.; Szamosi, J.C.; Verschoor, C.P.; Loukov, D.; Schenck, L.P.; Jury, J.; Foley, K.P.; et al. Age-Associated Microbial Dysbiosis Promotes Intestinal Permeability, Systemic Inflammation, and Macrophage Dysfunction. Cell Host Microbe 2017, 21, 455–466.e454. [Google Scholar] [CrossRef] [Green Version]
- Gomes, A.C.; Hoffmann, C.; Mota, J.F. The human gut microbiota: Metabolism and perspective in obesity. Gut Microbes 2018, 9, 308–325. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Tan, Y.; Shi, Y.; Wang, X.; Liao, Z.; Wei, P. Diabetes and Sarcopenic Obesity: Pathogenesis, Diagnosis, and Treatments. Front. Endocrinol. 2020, 11, 568. [Google Scholar] [CrossRef]
- Lv, W.Q.; Lin, X.; Shen, H.; Liu, H.M.; Qiu, X.; Li, B.Y.; Shen, W.D.; Ge, C.L.; Lv, F.Y.; Shen, J.; et al. Human gut microbiome impacts skeletal muscle mass via gut microbial synthesis of the short-chain fatty acid butyrate among healthy menopausal women. J. Cachexia Sarcopenia Muscle 2021, 12, 1860–1870. [Google Scholar] [CrossRef]
- Lahiri, S.; Kim, H.; Garcia-Perez, I.; Reza, M.M.; Martin, K.A.; Kundu, P.; Cox, L.M.; Selkrig, J.; Posma, J.M.; Zhang, H.; et al. The gut microbiota influences skeletal muscle mass and function in mice. Sci. Transl. Med. 2019, 11, eaan5662. [Google Scholar] [CrossRef] [Green Version]
- Ni, Y.; Yang, X.; Zheng, L.; Wang, Z.; Wu, L.; Jiang, J.; Yang, T.; Ma, L.; Fu, Z. Lactobacillus and Bifidobacterium Improves Physiological Function and Cognitive Ability in Aged Mice by the Regulation of Gut Microbiota. Mol. Nutr. Food Res. 2019, 63, e1900603. [Google Scholar] [CrossRef]
- Hsu, Y.J.; Huang, W.C.; Lin, J.S.; Chen, Y.M.; Ho, S.T.; Huang, C.C.; Tung, Y.T. Kefir Supplementation Modifies Gut Microbiota Composition, Reduces Physical Fatigue, and Improves Exercise Performance in Mice. Nutrients 2018, 10, 862. [Google Scholar] [CrossRef] [Green Version]
- Fielding, R.A.; Reeves, A.R.; Jasuja, R.; Liu, C.; Barrett, B.B.; Lustgarten, M.S. Muscle strength is increased in mice that are colonized with microbiota from high-functioning older adults. Exp. Gerontol. 2019, 127, 110722. [Google Scholar] [CrossRef]
- Neto, J.V.; de Melo, C.M.; Ribeiro, S.M. Effects of three-month intake of synbiotic on inflammation and body composition in the elderly: A pilot study. Nutrients 2013, 5, 1276–1286. [Google Scholar] [CrossRef] [Green Version]
- Sovran, B.; Hugenholtz, F.; Elderman, M.; Van Beek, A.A.; Graversen, K.; Huijskes, M.; Boekschoten, M.V.; Savelkoul, H.F.J.; De Vos, P.; Dekker, J.; et al. Age-associated Impairment of the Mucus Barrier Function is Associated with Profound Changes in Microbiota and Immunity. Sci. Rep. 2019, 9, 1437. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Bhadra, R.; Schols, A.; Sambashivaiah, S. Gut microbial dysbiosis as a limiting factor in the management of primary and secondary sarcopenia: An Asian Indian perspective. Curr. Opin. Clin. Nutr. Metab. Care 2020, 23, 404–410. [Google Scholar] [CrossRef]
- Wilson, D.; Jackson, T.; Sapey, E.; Lord, J.M. Frailty and sarcopenia: The potential role of an aged immune system. Ageing Res. Rev. 2017, 36, 1–10. [Google Scholar] [CrossRef]
- Tang, G.; Du, Y.; Guan, H.; Jia, J.; Zhu, N.; Shi, Y.; Rong, S.; Yuan, W. Butyrate ameliorates skeletal muscle atrophy in diabetic nephropathy by enhancing gut barrier function and FFA2-mediated PI3K/Akt/mTOR signals. Br. J. Pharmacol. 2022, 179, 159–178. [Google Scholar] [CrossRef]
- Schmidt, W.U.; Sattler, J.; Hesterberg, R.; Röher, H.D.; Zoedler, T.; Sitter, H.; Lorenz, W. Human intestinal diamine oxidase (DAO) activity in Crohn’s disease: A new marker for disease assessment? Agents Actions 1990, 30, 267–270. [Google Scholar] [CrossRef]
- Honzawa, Y.; Nakase, H.; Matsuura, M.; Chiba, T. Clinical significance of serum diamine oxidase activity in inflammatory bowel disease: Importance of evaluation of small intestinal permeability. Inflamm. Bowel Dis. 2011, 17, E23–E25. [Google Scholar] [CrossRef]
- Grosicki, G.J.; Fielding, R.A.; Lustgarten, M.S. Gut Microbiota Contribute to Age-Related Changes in Skeletal Muscle Size, Composition, and Function: Biological Basis for a Gut-Muscle Axis. Calcif. Tissue Int. 2018, 102, 433–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levitt, M.D.; Levitt, D.G. Quantitative Evaluation of D-Lactate Pathophysiology: New Insights into the Mechanisms Involved and the Many Areas in Need of Further Investigation. Clin. Exp. Gastroenterol. 2020, 13, 321–337. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.Y.; Liu, C.S.; Lin, L.Y.; Lin, Y.C.; Sun, H.L.; Li, C.C.; Chen, H.W.; Wang, T.S.; Wang, J.; Liu, K.L. Borage oil supplementation decreases lipopolysaccharide-induced inflammation and skeletal muscle wasting in mice. Rsc Adv. 2016, 6, 100174–100185. [Google Scholar] [CrossRef]
- Ghosh, S.; Lertwattanarak, R.; Garduño Jde, J.; Galeana, J.J.; Li, J.; Zamarripa, F.; Lancaster, J.L.; Mohan, S.; Hussey, S.; Musi, N. Elevated muscle TLR4 expression and metabolic endotoxemia in human aging. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2015, 70, 232–246. [Google Scholar] [CrossRef]
- van Tongeren, S.P.; Slaets, J.P.; Harmsen, H.J.; Welling, G.W. Fecal microbiota composition and frailty. Appl. Environ. Microbiol. 2005, 71, 6438–6442. [Google Scholar] [CrossRef] [Green Version]
- Morita, E.; Yokoyama, H.; Imai, D.; Takeda, R.; Ota, A.; Kawai, E.; Hisada, T.; Emoto, M.; Suzuki, Y.; Okazaki, K. Aerobic Exercise Training with Brisk Walking Increases Intestinal Bacteroides in Healthy Elderly Women. Nutrients 2019, 11, 868. [Google Scholar] [CrossRef] [Green Version]
- Okamoto, T.; Morino, K.; Ugi, S.; Nakagawa, F.; Lemecha, M.; Ida, S.; Ohashi, N.; Sato, D.; Fujita, Y.; Maegawa, H. Microbiome potentiates endurance exercise through intestinal acetate production. Am. J. Physiol. Endocrinol. Metab. 2019, 316, e956–e966. [Google Scholar] [CrossRef]

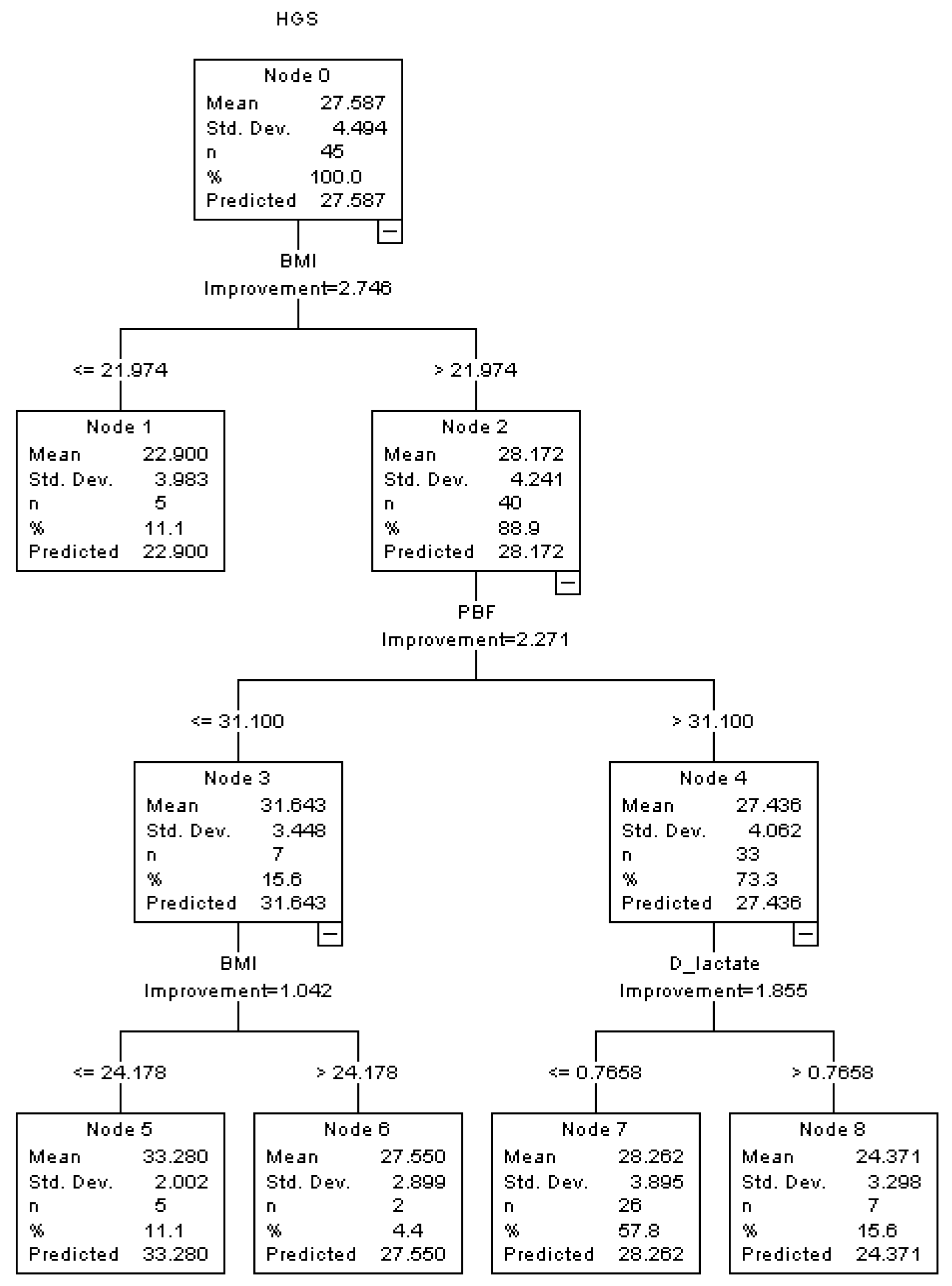
| Characteristic | Total | Male | Female | |
|---|---|---|---|---|
| n = 92 | n = 47 | n = 45 | p Value | |
| Age (y) | 58.3 ± 6.6 | 60.1 ± 7.4 | 56.4 ± 5.1 | 0.006 |
| Body mass (kg) | 68.3 ± 10.5 | 71.4 ± 10.9 | 65.0 ± 9.1 | 0.003 |
| BMI (kg/m2) | 25.4 ± 3.2 | 25.1 ± 3.1 | 25.7 ± 3.4 | 0.453 |
| PBF (%) | 31.0 ± 8.7 | 25.9 ± 5.7 | 36.3 ± 8.2 | <0.001 |
| ASMI (kg/m2) | 7.1 ± 1.0 | 7.5 ± 1.2 | 6.7 ± 0.7 | <0.001 |
| HGS (kg) | 33.0 ± 8.8 | 38.2 ± 8.7 | 27.6 ± 4.5 | <0.001 |
| HGSW | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.4 ± 0.1 | <0.001 |
| HGSB | 1.3 ± 0.4 | 1.5 ± 0.3 | 1.1 ± 0.2 | <0.001 |
| Daily exercise level | ||||
| Light (n, %) | 2 (2.2) | 2 (4.3) | 0 (0.0) | 0.002 |
| Moderate (n, %) | 31 (33.7) | 22 (46.8) | 9 (20.0) | |
| Vigorous (n, %) | 57 (61.9) | 21 (44.6) | 36 (80.0) | |
| No response (n, %) | 2 (2.2) | 2 (4.3) | 0 (0.0) | |
| Chronic diseases | ||||
| Hypertension (n, %) | 44 (47.8) | 25 (53.2) | 19 (42.2) | 0.292 |
| T2Ds (n, %) | 17 (18.5) | 9 (19.2) | 8 (17.8) | 0.866 |
| Indicator of | Gender | HGS | HGSW | HGSB | |||
|---|---|---|---|---|---|---|---|
| Intestinal Permeability | r | p | r | p | r | p | |
| DAO | Male | −0.396 | 0.006 | −0.263 | 0.074 | −0.307 | 0.036 |
| Female | 0.057 | 0.710 | −0.027 | 0.859 | 0.025 | 0.870 | |
| LPS | Male | 0.041 | 0.783 | 0.084 | 0.573 | 0.038 | 0.799 |
| Female | 0.199 | 0.189 | 0.115 | 0.452 | 0.102 | 0.504 | |
| D-lactate | Male | −0.089 | 0.551 | −0.186 | 0.211 | −0.210 | 0.157 |
| Female | −0.083 | 0.589 | 0.060 | 0.697 | 0.088 | 0.567 | |
| Model | HGS | HGSW | HGSB | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β Value | 95% CI | p Value | β Value | 95% CI | p Value | β Value | 95% CI | p Value | ||||
| Model 1 | −1.862 | −3.158 | −0.566 | 0.006 | −0.015 | −0.032 | 0.002 | 0.074 | −0.058 | −0.111 | −0.004 | 0.036 |
| Model 2 | −1.206 | −2.226 | −0.186 | 0.022 | −0.015 | −0.028 | −0.001 | 0.037 | −0.051 | −0.092 | −0.010 | 0.015 |
| Model 3 | −1.401 | −2.566 | −0.235 | 0.020 | −0.017 | −0.032 | −0.001 | 0.034 | −0.058 | −0.105 | −0.012 | 0.016 |
| Model | HGS | HGSW | HGSB | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β Value | 95% CI | p Value | β Value | 95% CI | p Value | β Value | 95% CI | p Value | ||||
| Model 1 | 0.167 | −0.730 | 1.064 | 0.710 | −0.001 | −0.018 | 0.015 | 0.859 | 0.004 | −0.041 | 0.049 | 0.870 |
| Model 2 | −0.203 | −1.219 | 0.814 | 0.689 | 0.000 | −0.016 | 0.015 | 0.954 | −0.005 | −0.046 | 0.035 | 0.791 |
| Model 3 | −0.292 | −1.343 | 0.759 | 0.577 | −0.004 | −0.020 | 0.013 | 0.646 | −0.010 | −0.052 | 0.033 | 0.650 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Li, Y.; Wang, N.; Ge, Z.; Shi, Z.; Wang, J.; Ding, B.; Bi, Y.; Wang, Y.; Hong, Z. Intestinal Permeability Associated with the Loss of Skeletal Muscle Strength in Middle-Aged and Older Adults in Rural Area of Beijing, China. Healthcare 2022, 10, 1100. https://doi.org/10.3390/healthcare10061100
Li C, Li Y, Wang N, Ge Z, Shi Z, Wang J, Ding B, Bi Y, Wang Y, Hong Z. Intestinal Permeability Associated with the Loss of Skeletal Muscle Strength in Middle-Aged and Older Adults in Rural Area of Beijing, China. Healthcare. 2022; 10(6):1100. https://doi.org/10.3390/healthcare10061100
Chicago/Turabian StyleLi, Cheng, Yaru Li, Nan Wang, Zhiwen Ge, Zhengli Shi, Jia Wang, Bingjie Ding, Yanxia Bi, Yuxia Wang, and Zhongxin Hong. 2022. "Intestinal Permeability Associated with the Loss of Skeletal Muscle Strength in Middle-Aged and Older Adults in Rural Area of Beijing, China" Healthcare 10, no. 6: 1100. https://doi.org/10.3390/healthcare10061100
APA StyleLi, C., Li, Y., Wang, N., Ge, Z., Shi, Z., Wang, J., Ding, B., Bi, Y., Wang, Y., & Hong, Z. (2022). Intestinal Permeability Associated with the Loss of Skeletal Muscle Strength in Middle-Aged and Older Adults in Rural Area of Beijing, China. Healthcare, 10(6), 1100. https://doi.org/10.3390/healthcare10061100






