Prognosis of the Ipsilesional Corticospinal Tracts with Preserved Integrities at the Early Stage of Cerebral Infarction: Follow Up Diffusion Tensor Tractography Study
Abstract
:1. Introduction
2. Methods
2.1. Subjects
2.2. Clinical Evaluation
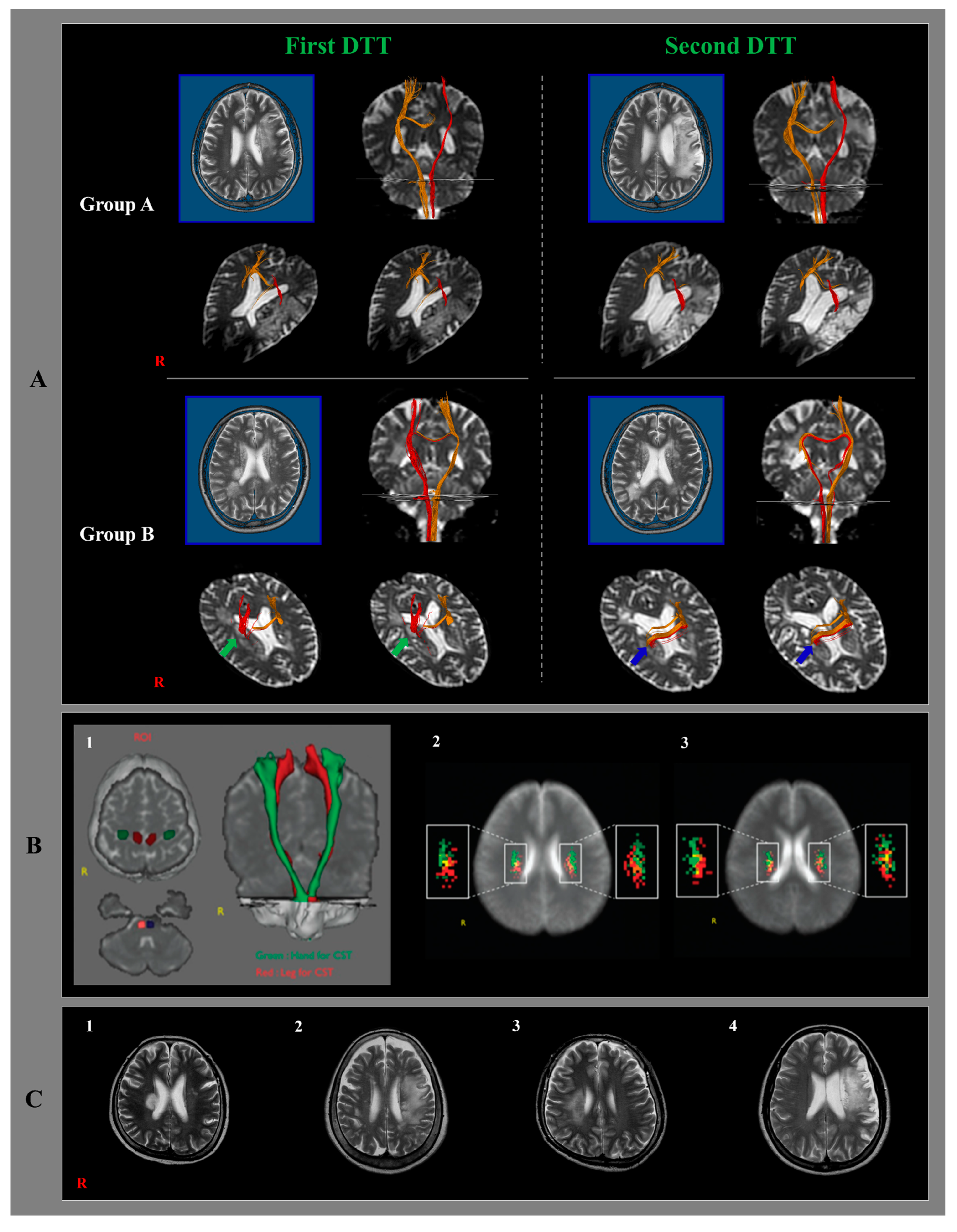
2.3. Diffusion Tensor Imaging and Tractography
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Heart, Lung, and Blood Institute Homepage. Available online: https://web.archive.org/web/20150218230259/http:/www.nhlbi.nih.gov/health/health-topics/topics/stroke/ (accessed on 26 February 2015).
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart disease and stroke statistics-2022 update: A report from the american heart association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef] [PubMed]
- Bogiatzi, C.; Hackam, D.G.; McLeod, A.I.; Spence, J.D. Secular trends in ischemic stroke subtypes and stroke risk factors. Stroke 2014, 45, 3208–3213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anwer, S.; Waris, A.; Gilani, S.O.; Iqbal, J.; Shaikh, N.; Pujari, A.N.; Niazi, I.K. Rehabilitation of upper limb motor impairment in stroke: A narrative review on the prevalence, risk factors, and economic statistics of stroke and state of the art therapies. Healthcare 2022, 10, 190. [Google Scholar] [CrossRef] [PubMed]
- Rathore, S.; Hinn, A.; Cooper, L.; Tyroler, H.; Rosamond, W. Characterization of incident stroke signs and symptoms: Findings from the atherosclerosis risk in communities study. Stroke 2002, 33, 2718–2721. [Google Scholar] [CrossRef] [Green Version]
- Thomalla, G.; Glauche, V.; Koch, M.A.; Beaulieu, C.; Weiller, C.; Rother, J. Diffusion tensor imaging detects early wallerian degeneration of the pyramidal tract after ischemic stroke. Neuroimage 2004, 22, 1767–1774. [Google Scholar] [CrossRef]
- Saposnik, G.; Levin, M. Virtual reality in stroke rehabilitation: A meta-analysis and implications for clinicians. Stroke 2011, 42, 1380–1386. [Google Scholar] [CrossRef]
- Singh, R.J.; Chen, S.; Ganesh, A.; Hill, M.D. Long-term neurological, vascular, and mortality outcomes after stroke. Int. J. Stroke 2018, 13, 787–796. [Google Scholar] [CrossRef]
- Strilciuc, S.; Grad, D.A.; Radu, C.; Chira, D.; Stan, A.; Ungureanu, M.; Gheorghe, A.; Muresanu, F.D. The economic burden of stroke: A systematic review of cost of illness studies. J. Med. Life 2021, 14, 606–619. [Google Scholar] [CrossRef]
- Ovbiagele, B.; Goldstein, L.B.; Higashida, R.T.; Howard, V.J.; Johnston, S.C.; Khavjou, O.A.; Lackland, D.T.; Lichtmat, J.H.; Mohl, S.; Sacco, R.L.; et al. Forecasting the future of stroke in the united states: A policy statement from the american heart association and american stroke association. Stroke 2013, 44, 2361–2375. [Google Scholar] [CrossRef] [Green Version]
- Jang, S.H. The corticospinal tract from the viewpoint of brain rehabilitation. J. Rehabil. Med. 2014, 46, 193–199. [Google Scholar] [CrossRef] [Green Version]
- Duncan, P.W.; Goldstein, L.B.; Matchar, D.; Divine, G.W.; Feussner, J. Measurement of motor recovery after stroke. Outcome assessment and sample size requirements. Stroke 1992, 23, 1084–1089. [Google Scholar] [CrossRef] [Green Version]
- Jang, S.H. A review of motor recovery mechanisms in patients with stroke. NeuroRehabilitation 2007, 22, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Macdonell, R.A.; Jackson, G.D.; Curatolo, J.M.; Abbott, D.F.; Berkovic, S.F.; Carey, L.M.; Syngeniotin, A.; Fabinyi, G.C.; Scheffer, I.E. Motor cortex localization using functional MRI and transcranial magnetic stimulation. Neurology 1999, 53, 1462–1467. [Google Scholar] [CrossRef] [PubMed]
- Diana, M.; Raij, T.; Melis, M.; Nummenmaa, A.; Leggio, L.; Bonci, A. Rehabilitating the addicted brain with transcranial magnetic stimulation. Nat. Rev. Neurosci. 2017, 18, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Kunimatsu, A.; Aoki, S.; Masutani, Y.; Abe, O.; Hayashi, N.; Mori, H.; Masumoto, T.; Ohtomo, K. The optimal trackability threshold of fractional anisotropy for diffusion tensor tractography of the corticospinal tract. Magn. Reson. Med. Sci. 2004, 3, 11–17. [Google Scholar] [CrossRef] [Green Version]
- Seo, J.P.; Kwon, Y.H.; Jang, S.H. Mini-review of studies reporting the repeatability and reproducibility of diffusion tensor imaging. Investig. Magn. Reson. Imaging 2019, 23, 26–33. [Google Scholar] [CrossRef]
- Yu, C.; Zhu, C.; Zhang, Y.; Chen, H.; Qin, W.; Wang, M.; Li, K. A longitudinal diffusion tensor imaging study on Wallerian degeneration of corticospinal tract after motor pathway stroke. Neuroimage 2009, 47, 451–458. [Google Scholar] [CrossRef]
- Puig, J.; Pedraza, S.; Blasco, G.; Daunis-I-Estadella, J.; Prats, A.; Prados, F.; Boada, I.; Castellanos, M.; Sanchez-Gonzalez, J.; Remollo, S.; et al. Wallerian degeneration in the corticospinal tract evaluated by diffusion tensor imaging correlates with motor deficit 30 days after middle cerebral artery ischemic stroke. AJNR Am. J. Neuroradiol. 2010, 31, 1324–1330. [Google Scholar] [CrossRef] [Green Version]
- Liang, Z.; Zeng, J.; Zhang, C.; Liu, S.; Ling, X.; Xu, A.; Ling, L.; Wang, F.; Pei, Z. Longitudinal investigations on the anterograde and retrograde degeneration in the pyramidal tract following pontine infarction with diffusion tensor imaging. Cerebrovasc. Dis. 2008, 25, 209–216. [Google Scholar] [CrossRef]
- Ma, C.; Liu, A.; Li, Z.; Zhou, X.; Zhou, S. Longitudinal study of diffusion tensor imaging properties of affected cortical spinal tracts in acute and chronic hemorrhagic stroke. J. Clin. Neurosci. 2014, 21, 1388–1392. [Google Scholar] [CrossRef]
- Shaheen, H.A.; Sayed, S.S.; Magdy, M.M.; Saad, M.A.; Magdy, A.M.; Daker, L.I. Prediction of motor recovery after ischemic stroke: Clinical and diffusion tensor imaging study. J. Clin. Neurosci. 2022, 96, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Zolkefley, M.K.I.; Firwana, Y.M.S.; Hatta, H.Z.M.; Rowbin, C.; Nassir, C.; Hanafi, M.H.; Abdullah, M.S.; Mustapha, M. An overview of fractional anisotropy as a reliable quantitative measurement for the corticospinal tract (CST) integrity in correlation with a fugl-meyer assessment in stroke rehabilitation. J. Phys. Ther. Sci. 2021, 33, 75–83. [Google Scholar] [CrossRef]
- Gong, Z.; Zhang, R.; Jiang, W.; Fu, Z. Integrity of the hand fibers of the corticospinal tract shown by diffusion tensor imaging predicts hand function recovery after hemorrhagic stroke. J. Stroke Cerebrovasc. Dis. 2021, 30, 105447. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.H.; Ahn, S.H.; Sakong, J.; Byun, W.M.; Choi, B.Y.; Chang, C.H.; Bai, D.; Son, S.M. Comparison of TMS and DTT for predicting motor outcome in intracerebral hemorrhage. J. Neurol. Sci. 2010, 290, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Kim, D.I.; Kim, J.; Kim, D.J.; Kim, H.D.; Kim, D.S.; Mori, S. Diffusion-tensor MR imaging and fiber tractography: A new method of describing aberrant fiber connections in developmental CNS anomalies. Radiographics 2005, 25, 53–65. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, Y.; Man, Y.; Hu, Z.; Zhang, N.; Pan, S. Clinical features, risk factors, and early prognosis for Wallerian degeneration in the descending pyramidal tract after acute cerebral infarction. J. Stroke Cerebrovasc. Dis. 2021, 30, 105480. [Google Scholar] [CrossRef]
- Jung, Y.J.; Jang, S.H. The fate of injured corticospinal tracts in patients with intracerebral hemorrhage: Diffusion tensor imaging study. AJNR Am. J. Neuroradiol. 2012, 33, 1775–1778. [Google Scholar] [CrossRef] [Green Version]
- Demeurisse, G.; Demol, O.; Robaye, E. Motor evaluation in vascular hemiplegia. Eur. Neurol. 1980, 19, 382–389. [Google Scholar] [CrossRef]
- Brunnstrom, S. Motor testing procedures in hemiplegia: Based on sequential recovery stages. Phys. Ther. 1966, 46, 357–375. [Google Scholar] [CrossRef] [Green Version]
- Cunha, I.T.; Lim, P.A.; Henson, H.; Monga, T.; Qureshy, H.; Protas, E.J. Performance-based gait tests for acute stroke patients. Am. J. Phys. Med. Rehabil. 2002, 81, 848–856. [Google Scholar] [CrossRef]
- Jang, S.H. A review of corticospinal tract location at corona radiata and posterior limb of the internal capsule in human brain. NeuroRehabilitation 2009, 24, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.G.; Yang, J.H.; Park, J.B.; Kim, M.H.; Choi, S.H.; Yang, D.S. Anatomical location and somatotopic organization of the corticospinal tract in the corona radiata of the normal human brain: A diffusion tensor tractography study. Neuroreport 2014, 25, 710–714. [Google Scholar] [CrossRef] [PubMed]
| A | B | |||
|---|---|---|---|---|
| No | 24 | 7 | - | |
| Sex | (M/F) | 19/5 | 5/2 | p > 0.05 |
| Age | 59.12 (11.89) | 47.71 (16.16) | p > 0.05 | |
| Infarct side | (Right/Left) | 11/13 | 4/3 | p > 0.05 |
| Infarct location | 24 | 7 | p > 0.05 | |
| MCA | 7 | 3 | ||
| Cortex | 1 | - | ||
| Centrum semiovale | 1 | - | ||
| Corona radiate | 13 | 4 | ||
| Posterior limb | 2 | - | ||
| Group A | Group B | |||||||
|---|---|---|---|---|---|---|---|---|
| 1st | 2nd | 1st | 2nd | |||||
| MI | 53.72 (22.83) | 80.30 (13.93) | 35.42 (25.76) | 68.97 (16.15) | p > 0.05a | p > 0.05 b | p < 0.05 c* | p < 0.05 d* |
| MBC | 2.95 (2.15) | 4.83 (1.30) | 2.10 (1.95) | 3.71 (1.70) | p > 0.05 a | p > 0.05 b | p < 0.05 c* | p < 0.05 d* |
| FAC | 1.45 (1.35) | 4.20 (0.65) | 0.21 (0.56) | 3.71 (0.95) | p < 0.05 a* | p > 0.05 b | p < 0.05 c* | p < 0.05 d* |
| Total | Group A | Group B | |
|---|---|---|---|
| Patient No. | 31 | 24 | 7 |
| (100%) | (77.4%) | (22.6%) | |
| Both | 17 | 16 | 1 |
| (54.8%) | (51.6%) | (3.2%) | |
| Hand | 4 | 2 | 2 |
| (12.9%) | (6.4%) | (6.4%) | |
| Gait | 7 | 5 | 2 |
| (22.5%) | (16.1%) | (6.4%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, S.H.; Seo, H.R.; Byun, D.H. Prognosis of the Ipsilesional Corticospinal Tracts with Preserved Integrities at the Early Stage of Cerebral Infarction: Follow Up Diffusion Tensor Tractography Study. Healthcare 2022, 10, 1096. https://doi.org/10.3390/healthcare10061096
Jang SH, Seo HR, Byun DH. Prognosis of the Ipsilesional Corticospinal Tracts with Preserved Integrities at the Early Stage of Cerebral Infarction: Follow Up Diffusion Tensor Tractography Study. Healthcare. 2022; 10(6):1096. https://doi.org/10.3390/healthcare10061096
Chicago/Turabian StyleJang, Sung Ho, Hye Rin Seo, and Dong Hyun Byun. 2022. "Prognosis of the Ipsilesional Corticospinal Tracts with Preserved Integrities at the Early Stage of Cerebral Infarction: Follow Up Diffusion Tensor Tractography Study" Healthcare 10, no. 6: 1096. https://doi.org/10.3390/healthcare10061096
APA StyleJang, S. H., Seo, H. R., & Byun, D. H. (2022). Prognosis of the Ipsilesional Corticospinal Tracts with Preserved Integrities at the Early Stage of Cerebral Infarction: Follow Up Diffusion Tensor Tractography Study. Healthcare, 10(6), 1096. https://doi.org/10.3390/healthcare10061096





