Treatment of Knee Osteochondral Fractures
Abstract
:1. Introduction
2. General Presentation of the Knee Osteochondral Fractures
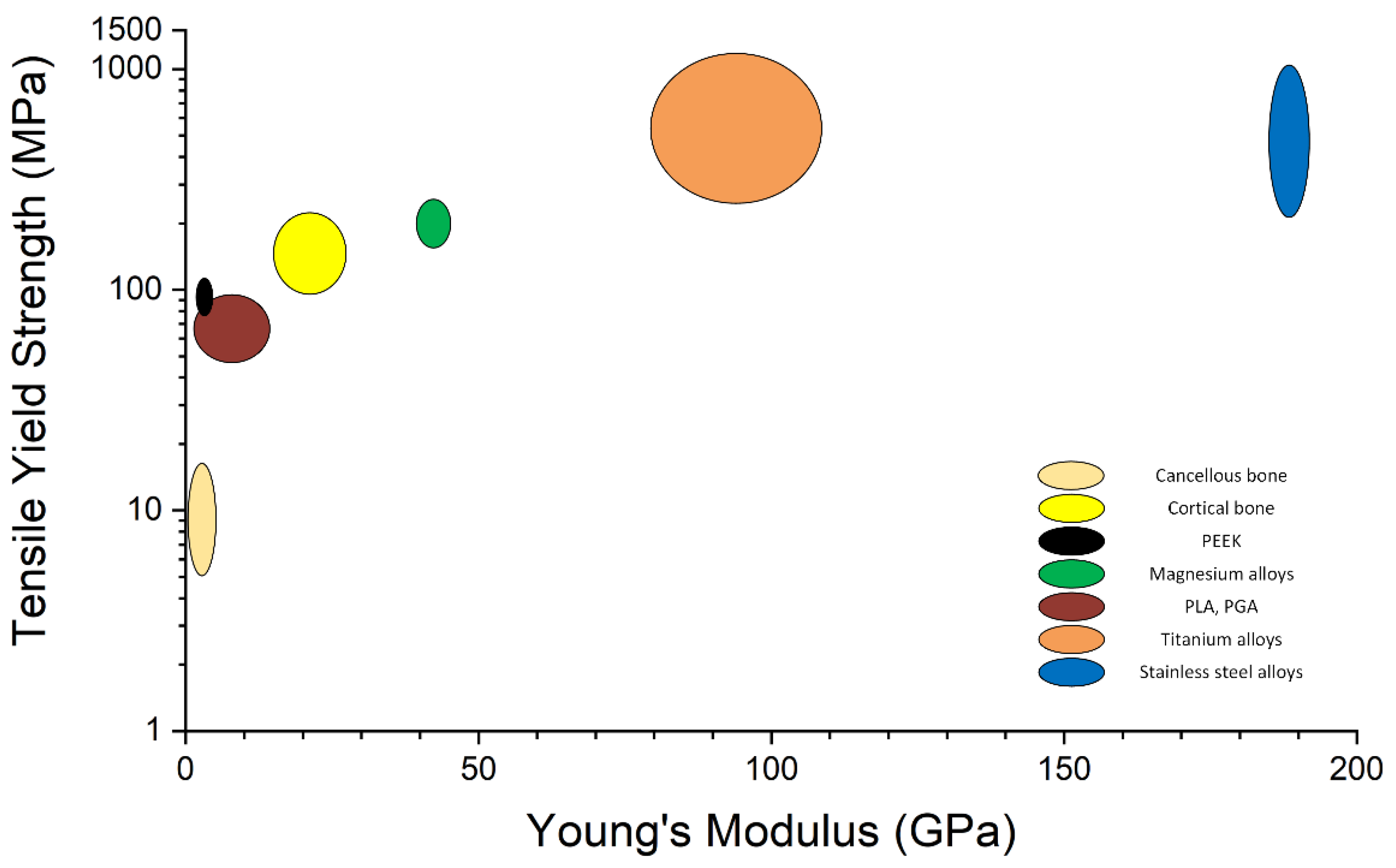
3. Biocompatible Implants Used in Pediatric Osteochondral Fracture Treatment
4. Case Report
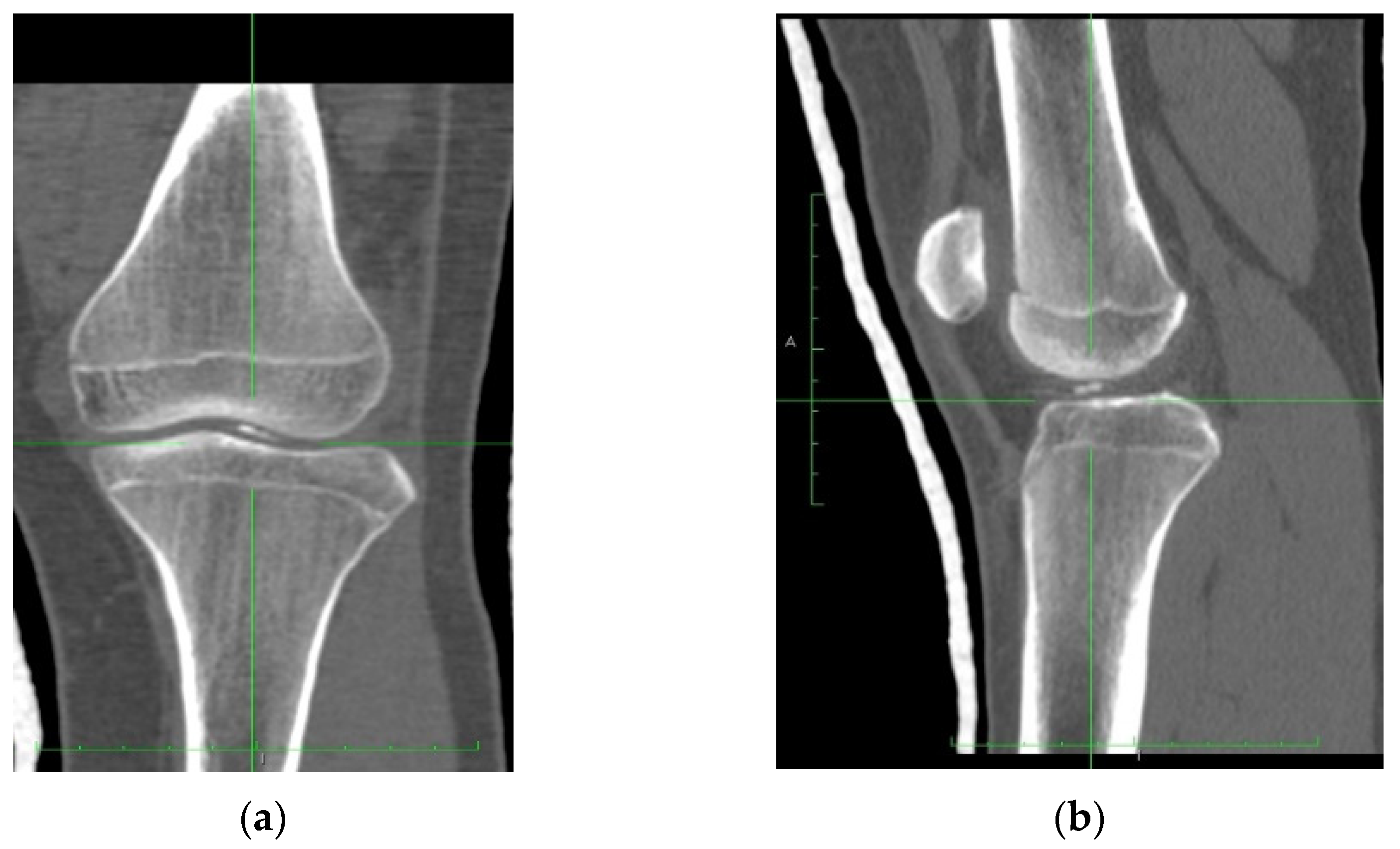
4.1. Clinical History
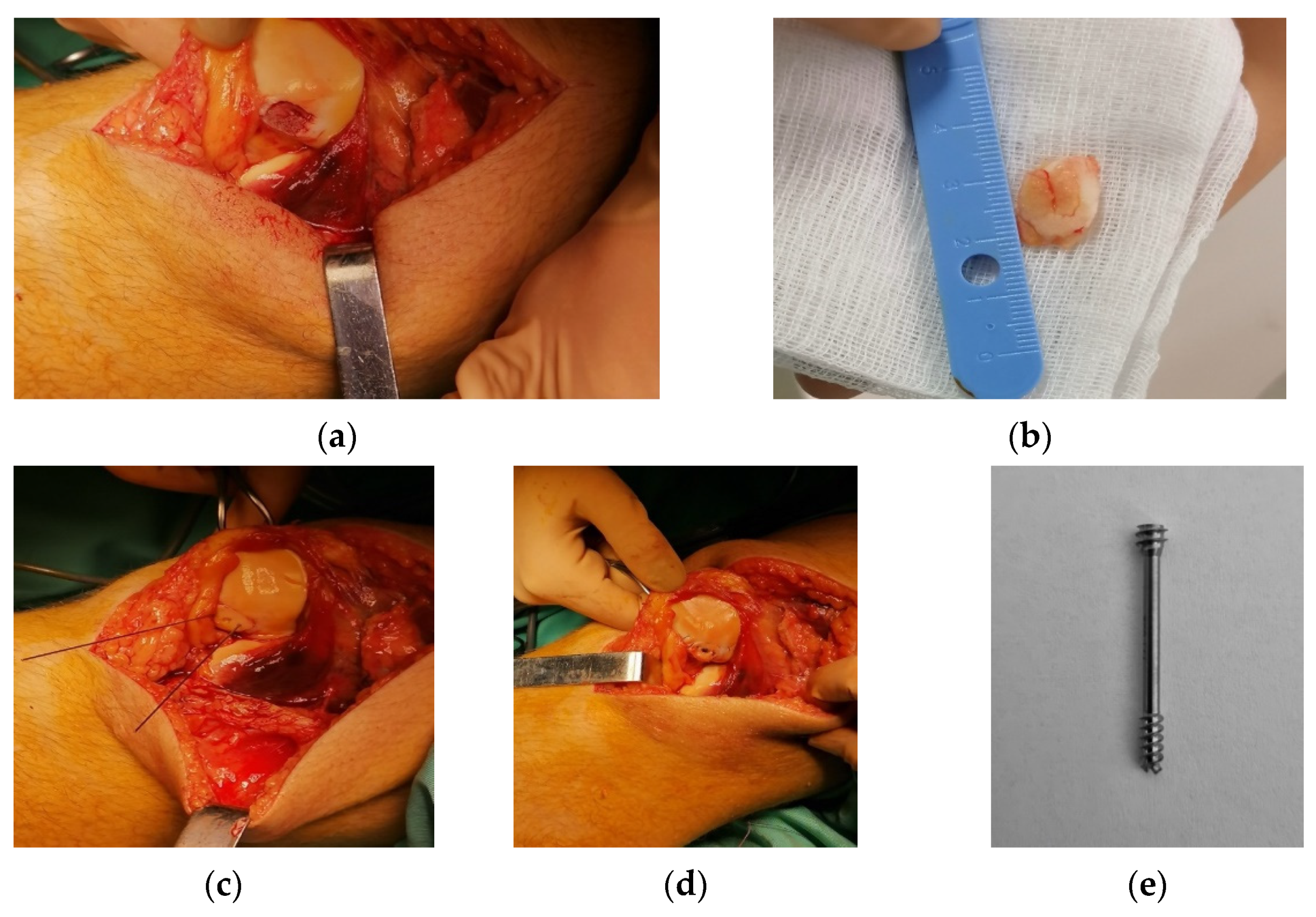
4.2. Surgical Technique
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Juneja, P.; Munjal, A.; Hubbard, J.B. Anatomy, Joints. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Karam, M.D.; Marsh, J.L. Clasification of Fractures. In Rockwood and Green’s Fractures in Adults, 9th ed.; Tornetta, P., Ricci, W.M., Ostrum, R.F., McQueen, M.M., McKee, M.D., Court-Brown, C.M., Eds.; Wolters Kluwer: Philadelfia, PA, USA, 2019; Volume 1, pp. 104–122. [Google Scholar]
- Kramer, D.E.; Kocher, M.S. Intra-Articular Injuries of the Knee. In Rockwood and Wilkins’ Fractures in Children, 9th ed.; Waters, P.M., Skaggs, D.L., Flynn, J.M., Eds.; Wolters Kluwer: Philadelfia, PA, USA, 2019; pp. 1011–1076. ISBN 978-1-4698-7158-5. [Google Scholar]
- Pedersen, M.E.; DaCambra, M.P.; Jibri, Z.; Dhillon, S.; Jen, H.; Jomha, N.M. Acute Osteochondral Fractures in the Lower Extremities—Approach to Identification and Treatment. Open Orthop. J. 2015, 9, 463–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kennedy, J.C.; Grainger, R.W.; McGraw, R.W. Osteochondral Fractures of the Femoral Condyles. J. Bone Jt. Surg. 1966, 48, 436–440. [Google Scholar] [CrossRef]
- Gianotti, S.M.; Marshall, S.W.; Hume, P.A.; Bunt, L. Incidence of Anterior Cruciate Ligament Injury and Other Knee Ligament Injuries: A National Population-Based Study. J. Sci. Med. Sport 2009, 12, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.B.; Bollen, S.R. Bollen’s Jig and Anterior Cruciate Ligament Reconstruction. J. R. Coll. Surg. Edinb. 2000, 45, 318–320. [Google Scholar] [PubMed]
- Johnson, D.L.; Urban, W.P.; Caborn, D.N.; Vanarthos, W.J.; Carlson, C.S. Articular Cartilage Changes Seen with Magnetic Resonance Imaging-Detected Bone Bruises Associated with Acute Anterior Cruciate Ligament Rupture. Am. J. Sports Med. 1998, 26, 409–414. [Google Scholar] [CrossRef]
- Lahm, A.; Erggelet, C.; Steinwachs, M.; Reichelt, A. Articular and Osseous Lesions in Recent Ligament Tears: Arthroscopic Changes Compared with Magnetic Resonance Imaging Findings. Arthroscopy 1998, 14, 597–604. [Google Scholar] [CrossRef]
- Elias, D.A.; White, L.M.; Fithian, D.C. Acute Lateral Patellar Dislocation at MR Imaging: Injury Patterns of Medial Patellar Soft-Tissue Restraints and Osteochondral Injuries of the Inferomedial Patella. Radiology 2002, 225, 736–743. [Google Scholar] [CrossRef]
- Terry, G.C.; Flandry, F.; Van Manen, J.W.; Norwood, L.A. Isolated Chondral Fractures of the Knee. Clin. Orthop. Relat. Res. 1988, 234, 170–177. [Google Scholar] [CrossRef]
- Bauer, K. Osteochondral Injuries of the Knee in Pediatric Patients. J. Knee Surg. 2018, 31, 382–391. [Google Scholar] [CrossRef]
- Badekas, T.; Takvorian, M.; Souras, N. Treatment Principles for Osteochondral Lesions in Foot and Ankle. Int. Orthop. 2013, 37, 1697–1706. [Google Scholar] [CrossRef] [Green Version]
- Boffa, A.; Previtali, D.; Di Laura Frattura, G.; Vannini, F.; Candrian, C.; Filardo, G. Evidence on Ankle Injections for Osteochondral Lesions and Osteoarthritis: A Systematic Review and Meta-Analysis. Int. Orthop. 2021, 45, 509–523. [Google Scholar] [CrossRef] [PubMed]
- Maheu, E.; Bannuru, R.R.; Herrero-Beaumont, G.; Allali, F.; Bard, H.; Migliore, A. Why We Should Definitely Include Intra-Articular Hyaluronic Acid as a Therapeutic Option in the Management of Knee Osteoarthritis: Results of an Extensive Critical Literature Review. Semin. Arthritis Rheum. 2019, 48, 563–572. [Google Scholar] [CrossRef]
- Zhao, J.; Huang, H.; Liang, G.; Zeng, L.-F.; Yang, W.; Liu, J. Effects and Safety of the Combination of Platelet-Rich Plasma (PRP) and Hyaluronic Acid (HA) in the Treatment of Knee Osteoarthritis: A Systematic Review and Meta-Analysis. BMC Musculoskelet. Disord. 2020, 21, 224. [Google Scholar] [CrossRef] [Green Version]
- Ghahremani, S.; Griggs, R.; Hall, T.; Motamedi, K.; Boechat, M. Osteochondral Lesions in Pediatric and Adolescent Patients. Semin. Musculoskelet. Radiol. 2014, 18, 505–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, C.D.; Migliorini, F.; Hildebrand, F. Reconstruction of Large Osteochondral Lesions in the Knee: Focus on Fixation Techniques. Life 2021, 11, 543. [Google Scholar] [CrossRef] [PubMed]
- Roberti di Sarsina, T.; Fiore, M.; Coco, V.; Govoni, M.; Vivarelli, L.; Rani, N.; Del Piccolo, N.; Dallari, D. Fresh Osteochondral Allograft Transplantation in Osteochondritis Dissecans in the Knee Joint. Life 2021, 11, 1205. [Google Scholar] [CrossRef] [PubMed]
- Schuette, H.B.; Kraeutler, M.J.; McCarty, E.C. Matrix-Assisted Autologous Chondrocyte Transplantation in the Knee: A Systematic Review of Mid- to Long-Term Clinical Outcomes. Orthop. J. Sports Med. 2017, 5, 2325967117709250. [Google Scholar] [CrossRef] [Green Version]
- Long-Term Results After Hyaluronan-Based MACT for the Treatment of Cartilage Lesions of the Patellofemoral Joint. Available online: https://pubmed.ncbi.nlm.nih.gov/26755690/ (accessed on 27 May 2022).
- Zak, L.; Aldrian, S.; Wondrasch, B.; Albrecht, C.; Marlovits, S. Ability to Return to Sports 5 Years after Matrix-Associated Autologous Chondrocyte Transplantation in an Average Population of Active Patients. Am. J. Sports Med. 2012, 40, 2815–2821. [Google Scholar] [CrossRef]
- Wondrasch, B.; Risberg, M.-A.; Zak, L.; Marlovits, S.; Aldrian, S. Effect of Accelerated Weightbearing after Matrix-Associated Autologous Chondrocyte Implantation on the Femoral Condyle: A Prospective, Randomized Controlled Study Presenting MRI-Based and Clinical Outcomes after 5 Years. Am. J. Sports Med. 2015, 43, 146–153. [Google Scholar] [CrossRef]
- Hantes, M.E.; Fyllos, A.H. Management of Knee Cartilage Defects with the Autologous Matrix-Induced Chondrogenesis (AMIC) Technique; IntechOpen: London, UK, 2017; ISBN 978-953-51-3789-4. [Google Scholar]
- Gomoll, A.H.; Gillogly, S.D.; Cole, B.J.; Farr, J.; Arnold, R.; Hussey, K.; Minas, T. Autologous Chondrocyte Implantation in the Patella: A Multicenter Experience. Am. J. Sports Med. 2014, 42, 1074–1081. [Google Scholar] [CrossRef]
- Whyte, G.P.; Gobbi, A.; Sadlik, B. Dry Arthroscopic Single-Stage Cartilage Repair of the Knee Using a Hyaluronic Acid-Based Scaffold With Activated Bone Marrow-Derived Mesenchymal Stem Cells. Arthrosc. Tech. 2016, 5, e913–e918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, S.-M.; Kwon, H.-N. Dry Arthroscopy with a Simple Retraction Technique for Knee Joint Cartilage Repair Using Allogenic Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells. Arthrosc. Tech. 2021, 10, e2747–e2752. [Google Scholar] [CrossRef]
- Sadlik, B.; Wiewiorski, M. Implantation of a Collagen Matrix for an AMIC Repair during Dry Arthroscopy. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 2349–2352. [Google Scholar] [CrossRef] [PubMed]
- Navarro, M.; Michiardi, A.; Castaño, O.; Planell, J.A. Biomaterials in Orthopaedics. J. R. Soc. Interface 2008, 5, 1137–1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Poh, C.K. Titanium Alloys in Orthopaedics. In Titanium Alloys—Advances in Properties Control; Sieniawski, J., Ziaja, W., Eds.; InTech: London, UK, 2013; ISBN 978-953-51-1110-8. [Google Scholar]
- IntechOpen. Evaluation of the Biotribological Behavior and Cytotoxicity of Laser-Textured ISO 5832-1 Stainless Steel for Use in Orthopedic Implants. Available online: https://www.intechopen.com/chapters/58961 (accessed on 5 March 2022).
- Gosal, H.S.; Singh, P.; Field, R.E. Clinical Experience of Patellar Fracture Fixation Using Metal Wire or Non-Absorbable Polyester—A Study of 37 Cases. Injury 2001, 32, 129–135. [Google Scholar] [CrossRef]
- Larson, A.N. Effect of Metallosis on Tissues and Serum Metal Levels in Children With Orthopedic Implants; ClinicalTrials.gov: Bathesda, MD, USA, 2021.
- Loder, R.T.; Feinberg, J.R. Orthopaedic Implants in Children: Survey Results Regarding Routine Removal by the Pediatric and Nonpediatric Specialists. Yearb. Orthop. 2007, 2007, 53–54. [Google Scholar] [CrossRef]
- Maher, S.; Linklater, D.; Rastin, H.; Le Yap, P.; Ivanova, E.P.; Losic, D. Tailoring Additively Manufactured Titanium Implants for Short-Time Pediatric Implantations with Enhanced Bactericidal Activity. ChemMedChem 2022, 17, e202100580. [Google Scholar] [CrossRef]
- Sherman, S.L.; Calcei, J.; Ray, T.; Magnussen, R.A.; Musahl, V.; Kaeding, C.C.; Clatworthy, M.; Bergfeld, J.A.; Arnold, M.P. ACL Study Group Presents the Global Trends in ACL Reconstruction: Biennial Survey of the ACL Study Group. J. ISAKOS 2021, 6, 322–328. [Google Scholar] [CrossRef]
- Mugnai, R.; Tarallo, L.; Capra, F.; Catani, F. Biomechanical Comparison between Stainless Steel, Titanium and Carbon-Fiber Reinforced Polyetheretherketone Volar Locking Plates for Distal Radius Fractures. Orthop. Traumatol. Surg. Res. 2018, 104, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Araoye, I.B.; Chodaba, Y.E.; Smith, K.S.; Hadden, R.W.; Shah, A.B. Use of Intramedullary Carbon Fiber Nail in Hindfoot Fusion: A Small Cohort Study. Foot Ankle Surg. 2019, 25, 2–7. [Google Scholar] [CrossRef]
- Caforio, M.; Perugia, D.; Colombo, M.; Calori, G.M.; Maniscalco, P. Preliminary Experience with Piccolo Composite™, a Radiolucent Distal Fibula Plate, in Ankle Fractures. Injury 2014, 45, S36–S38. [Google Scholar] [CrossRef]
- Guzzini, M.; Lanzetti, R.M.; Lupariello, D.; Morelli, F.; Princi, G.; Perugia, D.; Ferretti, A. Comparison between Carbon-Peek Plate and Conventional Stainless Steal Plate in Ankle Fractures. A Prospective Study of Two Years Follow Up. Injury 2017, 48, 1249–1252. [Google Scholar] [CrossRef] [PubMed]
- Perugia, D.; Guzzini, M.; Mazza, D.; Iorio, C.; Civitenga, C.; Ferretti, A. Comparison between Carbon-Peek Volar Locking Plates and Titanium Volar Locking Plates in the Treatment of Distal Radius Fractures. Injury 2017, 48, S24–S29. [Google Scholar] [CrossRef]
- Bizenjima, T.; Takeuchi, T.; Seshima, F.; Saito, A. Effect of Poly (Lactide-Co-Glycolide) (PLGA)-Coated Beta-Tricalcium Phosphate on the Healing of Rat Calvarial Bone Defects: A Comparative Study with Pure-Phase Beta-Tricalcium Phosphate. Clin. Oral Impl. Res. 2016, 27, 1360–1367. [Google Scholar] [CrossRef] [PubMed]
- Feeley, A.; Feeley, I.; Ni Fhoghlú, C.; Sheehan, E.; Kennedy, M. Use of Biomaterials in Scaphoid Fracture Fixation, a Systematic Review. Clin. Biomech. 2021, 89, 105480. [Google Scholar] [CrossRef] [PubMed]
- Vogel, L.A.; Fitzsimmons, K.P.; Pace, J.L. Osteochondral Fracture Fixation With Fragment Preserving Suture Technique. Arthrosc. Tech. 2020, 9, e761–e767. [Google Scholar] [CrossRef]
- Poircuitte, J.M.; Popkov, P.; Huber, D.H.; Polirsztok, E.; Lascombes, P.; Journeau, P. Resorbable Osteosynthetic Devices in Pediatric Traumatology: A Prospective Series of 24 Cases. Eur. J. Orthop. Surg. Traumatol. 2015, 25, 997–1004. [Google Scholar] [CrossRef]
- Laurencin, C.T.; Badon, M.A. Regenerative Engineering in the Field of Orthopedic Surgery. In Biologics in Orthopaedic Surgery; Elsevier: Amsterdam, The Netherlands, 2019; pp. 201–213. ISBN 978-0-323-55140-3. [Google Scholar]
- Adam, R.; Orban, H.; Dragomir, L.; Milea, C.; Antoniac, I.; Barbilian, A. Investigation of Biodegradation Behavior of an Mg-1Ca Alloy during In Vivo Testing. Key Eng. Mater. 2017, 752, 87–92. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, C.; Wang, J.; Liu, F.; Chau, K.W.; Qin, L.; Wang, J. Clinical Translation and Challenges of Biodegradable Magnesium-Based Interference Screws in ACL Reconstruction. Bioact. Mater. 2021, 6, 3231–3243. [Google Scholar] [CrossRef]
- Antoniac, I.; Miculescu, M.; Mănescu, V.; Stere, A.; Quan, P.H.; Păltânea, G.; Robu, A.; Earar, K. Magnesium-Based Alloys Used in Orthopedic Surgery. Materials 2022, 15, 1148. [Google Scholar] [CrossRef]
- Angrisani, N.; Seitz, J.-M.; Meyer-Lindenberg, A.; Reifenrath, J. Rare Earth Metals as Alloying Components in Magnesium Implants for Orthopaedic Applications. In New Features on Magnesium Alloys; IntechOpen: London, UK, 2012; ISBN 978-953-51-0668-5. [Google Scholar]
- Quan, P.H.; Antoniac, I.; Miculescu, F.; Antoniac, A.; Mănescu, V.; Robu, A.; Bița, A.-I.; Miculescu, M.; Saceleanu, A.; Bodog, A.D.; et al. Fluoride Treatment and In Vitro Corrosion Behavior of Mg-Nd-Y-Zn-Zr Alloys Type. Materials 2022, 15, 566. [Google Scholar] [CrossRef] [PubMed]
- Pichler, K.; Kraus, T.; Martinelli, E.; Sadoghi, P.; Musumeci, G.; Uggowitzer, P.J.; Weinberg, A.M. Cellular Reactions to Biodegradable Magnesium Alloys on Human Growth Plate Chondrocytes and Osteoblasts. Int. Orthop. 2014, 38, 881–889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ezechieli, M.; Ettinger, M.; König, C.; Weizbauer, A.; Helmecke, P.; Schavan, R.; Lucas, A.; Windhagen, H.; Becher, C. Biomechanical Characteristics of Bioabsorbable Magnesium-Based (MgYREZr-Alloy) Interference Screws with Different Threads. Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 3976–3981. [Google Scholar] [CrossRef] [PubMed]
- Bita, A.-I.; Antoniac, I.; Ciuca, I. Potential Use of Mg-Ca Alloys for Orthopedic Applications. Univ. Politech. Buchar. Sci. Bull. Series B-Chem. Mater. Sci. 2016, 78, 173–184. [Google Scholar]
- Bita, A.I.; Antoniac, A.; Cotrut, C.; Vasile, E.; Ciuca, I.; Niculescu, M.; Antoniac, I. In Vitro Degradation and Corrosion Evaluation of Mg-Ca Alloys for Biomedical Applications. J. Optoelectron. Adv. Mater. 2016, 18, 394–398. [Google Scholar]
- Kraus, T.; Fischerauer, S.; Treichler, S.; Martinelli, E.; Eichler, J.; Myrissa, A.; Zötsch, S.; Uggowitzer, P.J.; Löffler, J.F.; Weinberg, A.M. The Influence of Biodegradable Magnesium Implants on the Growth Plate. Acta Biomater. 2018, 66, 109–117. [Google Scholar] [CrossRef]
- Bita, A.-I.; Stan, G.E.; Niculescu, M.; Ciuca, I.; Vasile, E.; Antoniac, I. Adhesion Evaluation of Different Bioceramic Coatings on Mg–Ca Alloys for Biomedical Applications. J. Adhes. Sci. Technol. 2016, 30, 1968–1983. [Google Scholar] [CrossRef]
- Grün, N.G.; Holweg, P.L.; Donohue, N.; Klestil, T.; Weinberg, A.-M. Resorbable Implants in Pediatric Fracture Treatment. Innov. Surg. Sci. 2018, 3, 119–125. [Google Scholar] [CrossRef]
- Agarwal, S.; Curtin, J.; Duffy, B.; Jaiswal, S. Biodegradable Magnesium Alloys for Orthopaedic Applications: A Review on Corrosion, Biocompatibility and Surface Modifications. Mater. Sci. Eng. C 2016, 68, 948–963. [Google Scholar] [CrossRef] [Green Version]
- Hermawan, H. Biodegradable Metals; SpringerBriefs in Materials; Springer: Berlin/Heidelberg, Germany, 2012; ISBN 978-3-642-31169-7. [Google Scholar]
- Rau, J.V.; Antoniac, I.; Filipescu, M.; Cotrut, C.; Fosca, M.; Nistor, L.C.; Birjega, R.; Dinescu, M. Hydroxyapatite Coatings on Mg-Ca Alloy Prepared by Pulsed Laser Deposition: Properties and Corrosion Resistance in Simulated Body Fluid. Ceram. Int. 2018, 44, 16678–16687. [Google Scholar] [CrossRef]
- Rau, J.V.; Antoniac, I.; Fosca, M.; De Bonis, A.; Blajan, A.I.; Cotrut, C.; Graziani, V.; Curcio, M.; Cricenti, A.; Niculescu, M.; et al. Glass-Ceramic Coated Mg-Ca Alloys for Biomedical Implant Applications. Mater. Sci. Eng. C 2016, 64, 362–369. [Google Scholar] [CrossRef]
- Istrate, B.; Munteanu, C.; Lupescu, S.; Antoniac, I.V.; Sindilar, E. Structural Characterization of Mg-0.5Ca-XY Biodegradable Alloys. Key Eng. Mater. 2018, 782, 129–135. [Google Scholar] [CrossRef]
- Antoniac, I.; Miculescu, F.; Cotrut, C.; Ficai, A.; Rau, J.V.; Grosu, E.; Antoniac, A.; Tecu, C.; Cristescu, I. Controlling the Degradation Rate of Biodegradable Mg–Zn-Mn Alloys for Orthopedic Applications by Electrophoretic Deposition of Hydroxyapatite Coating. Materials 2020, 13, 263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakraborty Banerjee, P.; Al-Saadi, S.; Choudhary, L.; Harandi, S.E.; Singh, R. Magnesium Implants: Prospects and Challenges. Materials 2019, 12, 136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- May, H.; Alper Kati, Y.; Gumussuyu, G.; Yunus Emre, T.; Unal, M.; Kose, O. Bioabsorbable Magnesium Screw versus Conventional Titanium Screw Fixation for Medial Malleolar Fractures. J. Orthop. Traumatol. 2020, 21, 9. [Google Scholar] [CrossRef]
- Windhagen, H.; Radtke, K.; Weizbauer, A.; Diekmann, J.; Noll, Y.; Kreimeyer, U.; Schavan, R.; Stukenborg-Colsman, C.; Waizy, H. Biodegradable Magnesium-Based Screw Clinically Equivalent to Titanium Screw in Hallux Valgus Surgery: Short Term Results of the First Prospective, Randomized, Controlled Clinical Pilot Study. Biomed. Eng. Online 2013, 12, 62. [Google Scholar] [CrossRef] [Green Version]
- Baldini, M.; Coppa, V.; Falcioni, D.; Senigagliesi, E.; Marinelli, M.; Gigante, A.P. Use of Resorbable Magnesium Screws in Children: Systematic Review of the Literature and Short-Term Follow-up from Our Series. J. Child. Orthop. 2021, 15, 194–203. [Google Scholar] [CrossRef]
- Jungesblut, O.D.; Moritz, M.; Spiro, A.S.; Stuecker, R.; Rupprecht, M. Fixation of Unstable Osteochondritis Dissecans Lesions and Displaced Osteochondral Fragments Using New Biodegradable Magnesium Pins in Adolescents. Cartilage 2021, 13, 302S–310S. [Google Scholar] [CrossRef]
- Gigante, A.; Setaro, N.; Rotini, M.; Finzi, S.S.; Marinelli, M. Intercondylar Eminence Fracture Treated by Resorbable Magnesium Screws Osteosynthesis: A Case Series. Injury 2018, 49, S48–S53. [Google Scholar] [CrossRef] [Green Version]
- Lidder, S.; Thomas, M.; Desai, A.; Skyrme, A.; Armitage, A.; Rajaratnam, S. Osteochondral Fractures of the Knee in Skeletally Immature Patients: Short-Term Results of Operative Fixation Using Omnitech Screws. Acta Chir. Orthop. Traumatol. Cech. 2016, 83, 16–20. [Google Scholar]
- Yousefi, A.-M.; Hoque, M.E.; Prasad, R.G.S.V.; Uth, N. Current Strategies in Multiphasic Scaffold Design for Osteochondral Tissue Engineering: A Review. J. Biomed. Mater. Res. Part A 2015, 103, 2460–2481. [Google Scholar] [CrossRef] [PubMed]
- Fabricant, P.D.; Yen, Y.-M.; Kramer, D.E.; Kocher, M.S.; Micheli, L.J.; Lawrence, J.T.R.; Ganley, T.J.; Heyworth, B.E. Fixation of Traumatic Chondral-Only Fragments of the Knee in Pediatric and Adolescent Athletes: A Retrospective Multicenter Report. Orthop. J. Sports Med. 2018, 6, 2325967117753140. [Google Scholar] [CrossRef] [Green Version]
- Seitz, J.-M.; Durisin, M.; Goldman, J.; Drelich, J.W. Recent Advances in Biodegradable Metals for Medical Sutures: A Critical Review. Adv. Healthc. Mater. 2015, 4, 1915–1936. [Google Scholar] [CrossRef] [PubMed]
- Seitz, J.-M.; Lucas, A.; Kirschner, M. Magnesium-Based Compression Screws: A Novelty in the Clinical Use of Implants. JOM 2016, 68, 1177–1182. [Google Scholar] [CrossRef]
- Könneker, S.; Krockenberger, K.; Pieh, C.; von Falck, C.; Brandewiede, B.; Vogt, P.M.; Kirschner, M.H.; Ziegler, A. Comparison of SCAphoid Fracture Osteosynthesis by MAGnesium-Based Headless Herbert Screws with Titanium Herbert Screws: Protocol for the Randomized Controlled SCAMAG Clinical Trial. BMC Musculoskelet. Disord. 2019, 20, 357. [Google Scholar] [CrossRef] [PubMed]



| Diagnosis | Age/Sex | Treatment and Follow up in Months | Complications |
|---|---|---|---|
| Tibial spine avulsion fracture | 12/Female | Arthroscopic reduction internal fixation (ARIF) with two cannulated screws/26 months | None |
| Fracture–dislocation of patella | 13/M | Open reduction internal fixation (ORIF) with three cannulated screws/18 months | None |
| Medial epicondyle avulsion | 11/M | Open reduction internal fixation (ORIF) with two cannulated screws/14 months | Detachment of the screw head |
| Tibial distal epiphysis fracture | 12/M | Closed reduction internal fixation (CRIF) (one all-epiphyseal cannulated screw and one trans-physeal Kirschner wire)/16 months | None |
| Flat foot and hallux valgus | 8/F | Calcaneal notch filler (CNF) and hallux proximal physis emiepiphysiodesis (one cannulated screw)/12 months | None |
| Osteochondritis dissecans of the knee | 14/M | Anterograde drilling and fixation (one cannulated screw)/12 months | None |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cordunianu, M.A.; Antoniac, I.; Niculescu, M.; Paltanea, G.; Raiciu, A.D.; Dura, H.; Forna, N.; Carstoc, I.D.; Cristea, M.B. Treatment of Knee Osteochondral Fractures. Healthcare 2022, 10, 1061. https://doi.org/10.3390/healthcare10061061
Cordunianu MA, Antoniac I, Niculescu M, Paltanea G, Raiciu AD, Dura H, Forna N, Carstoc ID, Cristea MB. Treatment of Knee Osteochondral Fractures. Healthcare. 2022; 10(6):1061. https://doi.org/10.3390/healthcare10061061
Chicago/Turabian StyleCordunianu, Mihai Alexandru, Iulian Antoniac, Marius Niculescu, Gheorghe Paltanea, Anca Daniela Raiciu, Horatiu Dura, Norin Forna, Ioana Dana Carstoc, and Mihai Bogdan Cristea. 2022. "Treatment of Knee Osteochondral Fractures" Healthcare 10, no. 6: 1061. https://doi.org/10.3390/healthcare10061061
APA StyleCordunianu, M. A., Antoniac, I., Niculescu, M., Paltanea, G., Raiciu, A. D., Dura, H., Forna, N., Carstoc, I. D., & Cristea, M. B. (2022). Treatment of Knee Osteochondral Fractures. Healthcare, 10(6), 1061. https://doi.org/10.3390/healthcare10061061








