Efficacy of High-Resolution Preoperative 3D Reconstructions for Lesion Localization in Oncological Colorectal Surgery—First Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patient Selection
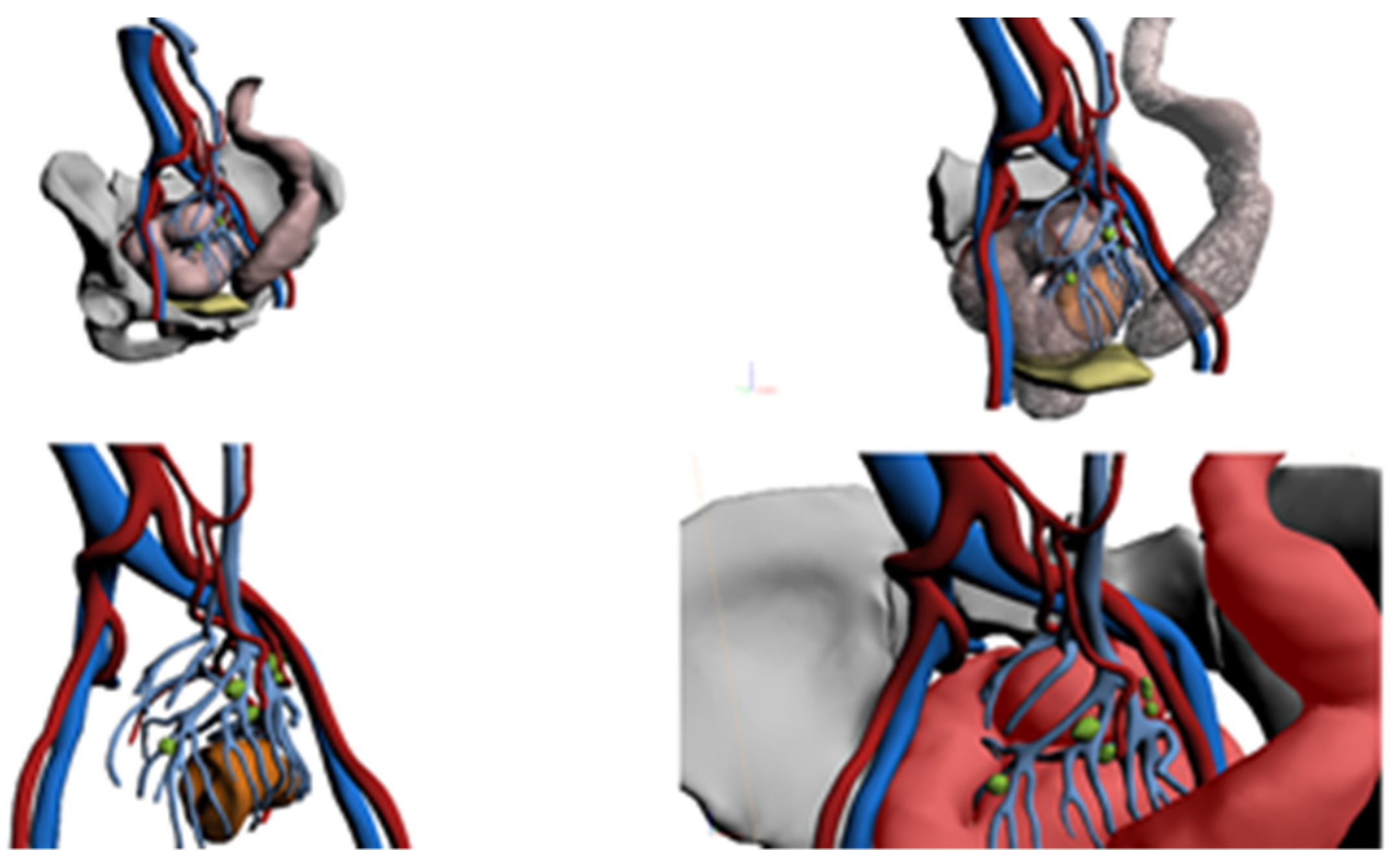
2.3. The 3D Reconstruction Software
2.4. Research Protocol
- SB planning vs. intraoperative and histopathological findings: The localization of the lesion performed by SB (based on the 3D models) was compared with intraoperative and histopathological findings (macroscopic characteristics).
- SB planning vs. SA planning: The localization of the lesion performed by SB was compared with SA’s 2D image-based localization.
- SB planning vs. SA surgical procedure: SB’s surgical pre-operative planning was compared with the intra-operative surgical procedure performed by SA.
2.5. Statistical Analysis
2.6. Study Limitations
- ✓
- Limited number of cases;
- ✓
- Limited number of surgeons who have tested the software;
- ✓
- No specific training of surgeons in viewing radiological images;
- ✓
- The prospective observational nature of the study.
3. Results
4. Case Descriptions
4.1. Case 1
- -
- Substenosing neoplasm located in the distal portion of the sigma in a dolichosigma framework.
- -
- Vascularization of the neoplasm came from the sigmoid arterial branches, which individually originate below the outlet of the left colic artery. This one was of moderate length.
- -
- Suspicious lymph nodes were evident in the area surrounding the neoplasm only.
4.2. Case 2
- -
- A large lesion of the sigma rectum joint, occupying a large portion of the pelvic excavation and in close contact with the contiguous organs (especially the uterus and bladder) and the iliac vessels, without signs of infiltration.
- -
- The vascular reconstruction highlighted the inferior mesenteric artery, while the left colic artery was not visible.
- -
- The presence of pathological lymph nodes in the Douglas and in the iliac area, especially on the left.
4.3. Case 3
- -
- A neoplastic lesion located between the proximal and middle third of the ascending colon.
- -
- Vascularization was ensured by the right ileocolic and colic arteries.
- -
- Multiple mesenteric lymph nodes were noticed.
4.4. Case 4
- -
- A heteroplastic lesion of the splenic flexure in the setting of a dolichocolon.
- -
- Vascularization by the middle colic artery.
- -
- Some mesenteric lymph nodes.
4.5. Case 5
- -
- Voluminous neoplastic mass of the middle rectum.
- -
- Vascularization by the inferior mesenteric artery.
- -
- Some locoregional lymph nodes.
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gao, Y.; Lu, Y. Variations of Gastrocolic Trunk of Henle and Its Significance in Gastrocolic Surgery. Gastroenterol. Res. Pract. 2018, 2018, 3573680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peltrini, R.; Luglio, G.; Pagano, G.; Sacco, M.; Sollazzo, V.; Bucci, L. Gastrocolic trunk of Henle and its variants: Review of the literature and clinical relevance in colectomy for right-sided colon cancer. Surg. Radiol. Anat. SRA 2019, 41, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Costi, R.; Ricco’, M.; Negrini, G.; Wind, P.; Violi, V.; Le Bian, A.Z. Is CT Scan more Accurate than Endoscopy in Identifying Distance from the Anal Verge for Left Sided Colon Cancer? A Comparative Cohort Analysis. J. Investig. Surg. Off. J. Acad. Surg. Res. 2020, 33, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Gallo, G. Preoperative Colorectal-Cancer Detection: Do We Need Anything Else? An Invited Brief Commentary on Is CT Scan More Accurate than Endoscopy in Identifying Distance from the Anal Verge for Left-sided Colon Cancer? A Comparative Cohort Analysis. J. Investig. Surg. Off. J. Acad. Surg. Res. 2020, 33, 281–282. [Google Scholar] [CrossRef] [Green Version]
- Guerriero, L.; Quero, G.; Diana, M.; Soler, L.; Agnus, V.; Marescaux, J.; Corcione, F. Virtual Reality Exploration and Planning for Precision Colorectal Surgery. Dis. Colon Rectum 2018, 61, 719–723. [Google Scholar] [CrossRef]
- Beets-Tan, R.; Lambregts, D.; Maas, M.; Bipat, S.; Barbaro, B.; Curvo-Semedo, L.; Fenlon, H.M.; Gollub, M.J.; Gourtsoyianni, S.; Halligan, S.; et al. Magnetic resonance imaging for clinical management of rectal cancer: Updated recommendations from the 2016 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur. Radiol. 2018, 28, 1465–1475. [Google Scholar] [CrossRef] [Green Version]
- Schoening, W.N.; Denecke, T.; Neumann, U.P. Präoperative Bildgebung/Operationsplanung für die Leberchirurgie [Preoperative imaging/operation planning for liver surgery]. Chir. Z. Alle Geb. Oper. Medizen 2015, 86, 1167–1181. [Google Scholar] [CrossRef]
- Solon, J.G.; Al-Azawi, D.; Hill, A.; Deasy, J.; McNamara, D.A. Colonoscopy and computerized tomography scan are not sufficient to localize right-sided colonic lesions accurately. Colorectal Dis. Off. J. Assoc. Coloproctology Great Br. Irel. 2010, 12, e267–e272. [Google Scholar] [CrossRef]
- D’Agostino, J.; Diana, M.; Vix, M.; Soler, L.; Marescaux, J. Three-dimensional virtual neck exploration before parathyroidectomy. NEJM 2012, 367, 1072–1073. [Google Scholar] [CrossRef]
- Szura, M.; Pasternak, A.; Solecki, R.; Matyja, M.; Szczepanik, A.; Matyja, A. Accuracy of preoperative tumor localization in large bowel using 3D magnetic endoscopic imaging: Randomized clinical trial. Surg. Endosc. 2017, 31, 2089–2095. [Google Scholar] [CrossRef] [Green Version]
- Porpiglia, F.; Bertolo, R.; Checcucci, E.; Amparore, D.; Autorino, R.; Dasgupta, P.; Wiklund, P.; Tewari, A.; Liatsikos, E.; Fiori, C. ESUT Research Group. Development and validation of 3D printed virtual models for robot-assisted radical prostatectomy and partial nephrectomy: Urologists’ and patients’ perception. World J. Urol. 2018, 36, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Porpiglia, F.; Amparore, D.; Checcucci, E.; Autorino, R.; Manfredi, M.; Iannizzi, G.; Fiori, C.; ESUT Research Group. Current Use of Three-dimensional Model Technology in Urology: A Road Map for Personalised Surgical Planning. Eur. Urol. Focus 2018, 4, 652–656. [Google Scholar] [CrossRef] [PubMed]
- Porpiglia, F.; Fiori, C.; Checcucci, E.; Amparore, D.; Bertolo, R. Hyperaccuracy Three-dimensional Reconstruction Is Able to Maximize the Efficacy of Selective Clamping During Robot-assisted Partial Nephrectomy for Complex Renal Masses. Eur. Urol. 2018, 74, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Aprato, A.; Olivero, M.; Iannizzi, G.; Bistolfi, A.; Sabatini, L.; Masse, A. Pelvic discontinuity in acetabular revisions: Does CT scan overestimate it? A comparative study of diagnostic accuracy of 3D-modeling and traditional 3D CT scan. Musculoskelet. Surg. 2020, 104, 171–177. [Google Scholar] [CrossRef]
- Marano, L.; Ricci, A.; Savelli, V.; Verre, L.; Di Renzo, L.; Biccari, E.; Costantini, G.; Marrelli, D.; Roviello, F. From digital world to real life: A robotic approach to the esophagogastric junction with a 3D printed model. BMC Surg. 2019, 19, 153. [Google Scholar] [CrossRef] [Green Version]
- Bailer, R.; Martin, R., II. The effectiveness of using 3D reconstruction software for surgery to augment surgical education. Am. J. Surg. 2019, 218, 1016–1021. [Google Scholar] [CrossRef]
- Volonté, F.; Pugin, F.; Bucher, P.; Sugimoto, M.; Ratib, O.; Morel, P. Augmented reality and image overlay navigation with OsiriX in laparoscopic and robotic surgery: Not only a matter of fashion. J. Hepato-Biliary-Pancreat. Sci. 2011, 18, 506–509. [Google Scholar] [CrossRef]
- Ellis, K.K.; Fennerty, M.B. Marking and identifying colon lesions. Tattoos, clips, and radiology in imaging the colon. Gastrointest. Endosc. Clin. N. Am. 1997, 7, 401–4111. [Google Scholar] [CrossRef]
- Klang, E.; Eifer, M.; Kopylov, U.; Belsky, V.; Raskin, S.; Konen, E.; Amitai, M.M. Pitfalls in diagnosing colon cancer on abdominal CT. Clin. Radiol. 2017, 72, 858–863. [Google Scholar] [CrossRef]
- Conaghan, P.J.; Maxwell-Armstrong, C.A.; Garrioch, M.V.; Hong, L.; Acheson, A.G. Leaving a mark: The frequency and accuracy of tattoing prior to laparoscopic colorectal surgery. Colorectal Dis. 2011, 13, 1184–1187. [Google Scholar] [CrossRef]
- Chen, J.K.; Johnson, P.T.; Horton, K.M.; Fishman, E.K. Unsuspected mesenteric arterial abnormality: Comparison of MDCT axial sections to interactive 3D rendering. AJR Am. J. Roentgenol. 2007, 189, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Kubo, N.; Furusawa, N.; Imai, S.; Terada, M. A case of laparoscopic high anterior resection of rectosigmoid colon cancer associated with a horseshoe kidney using preoperative 3D-CT angiography. Surg. Case Rep. 2018, 4, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penza, V.; Soriero, D.; Barresi, G.; Pertile, D.; Scabini, S.; Mattos, L.S. The GPS for surgery: A user-centered evaluation of a navigation system for laparoscopic surgery. Int. J. Med. Robot. Comput. Assist. Surg. MRCAS 2020, 16, 1–13. [Google Scholar] [CrossRef] [PubMed]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soriero, D.; Batistotti, P.; Malinaric, R.; Pertile, D.; Massobrio, A.; Epis, L.; Sperotto, B.; Penza, V.; Mattos, L.S.; Sartini, M.; et al. Efficacy of High-Resolution Preoperative 3D Reconstructions for Lesion Localization in Oncological Colorectal Surgery—First Pilot Study. Healthcare 2022, 10, 900. https://doi.org/10.3390/healthcare10050900
Soriero D, Batistotti P, Malinaric R, Pertile D, Massobrio A, Epis L, Sperotto B, Penza V, Mattos LS, Sartini M, et al. Efficacy of High-Resolution Preoperative 3D Reconstructions for Lesion Localization in Oncological Colorectal Surgery—First Pilot Study. Healthcare. 2022; 10(5):900. https://doi.org/10.3390/healthcare10050900
Chicago/Turabian StyleSoriero, Domenico, Paola Batistotti, Rafaela Malinaric, Davide Pertile, Andrea Massobrio, Lorenzo Epis, Beatrice Sperotto, Veronica Penza, Leonardo S. Mattos, Marina Sartini, and et al. 2022. "Efficacy of High-Resolution Preoperative 3D Reconstructions for Lesion Localization in Oncological Colorectal Surgery—First Pilot Study" Healthcare 10, no. 5: 900. https://doi.org/10.3390/healthcare10050900
APA StyleSoriero, D., Batistotti, P., Malinaric, R., Pertile, D., Massobrio, A., Epis, L., Sperotto, B., Penza, V., Mattos, L. S., Sartini, M., Cristina, M. L., Nencioni, A., & Scabini, S. (2022). Efficacy of High-Resolution Preoperative 3D Reconstructions for Lesion Localization in Oncological Colorectal Surgery—First Pilot Study. Healthcare, 10(5), 900. https://doi.org/10.3390/healthcare10050900








