Evaluating Patterns and Factors Related to Sleep Disturbances in Prostate Cancer Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedure
2.3. Measures
2.3.1. Background Data
2.3.2. Subjective Sleep Measures
2.3.3. Objective Sleep Measures
Actigraphy
2.4. Statistical Analysis
3. Results
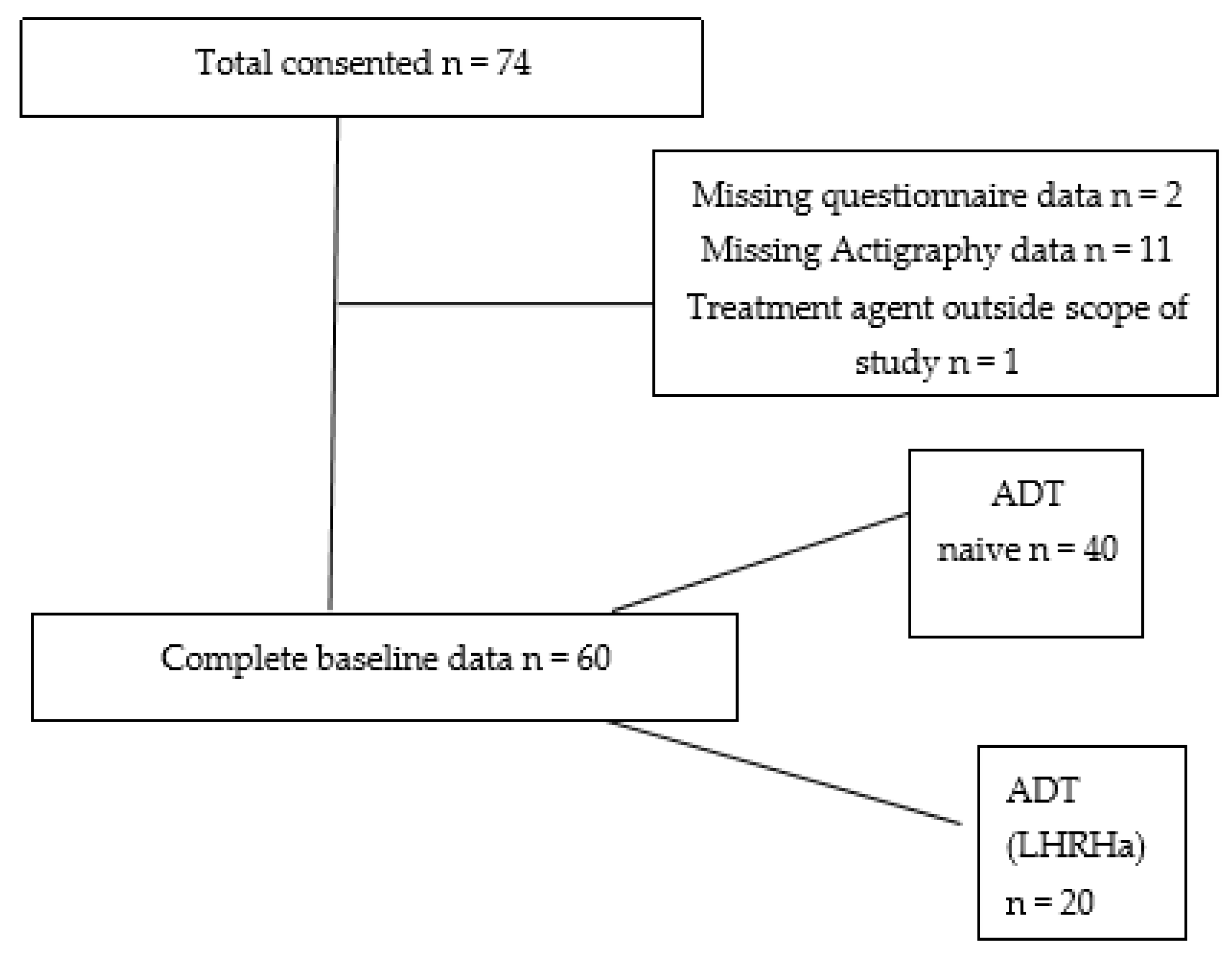
3.1. Missing Data
3.2. Demographic
3.3. Prevalence of Sleep Problems
3.4. Correlation between Actigraphy and Questionnaire Data
3.5. Differences in Sleep Outcomes According to Treatment
3.5.1. Sleep–Wake Parameters from Questionnaire Data
3.5.2. Sleep–Wake Parameters from Actigraphic Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garland, S.N.; Johnson, J.A.; Savard, J.; Gehrman, P.; Perlis, M.; Carlson, L.; Campbell, T. Sleeping well with cancer: A systematic review of cognitive behavioural therapy for insomnia in cancer patients. Neuropsychiatr. Dis. Treat. 2014, 10, 1113–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ancoli-Israel, S.; Cole, R.; Alessi, C.; Chambers, M.; Moorcroft, W.; Pollak, C.P. The role of actigraphy in the study of sleep and circadian rhythms. Sleep 2003, 26, 342–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haack, M.; Simpson, N.; Sethna, N.; Kaur, S.; Mullington, J. Sleep deficiency and chronic pain: Potential underlying mechanisms and clinical implications. Neuropsychopharmacology 2020, 45, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Wennberg, A.M.V.; Wu, M.N.; Rosenberg, P.B.; Spira, A.P. Sleep Disturbance, Cognitive Decline, and Dementia: A Review. Semin. Neurol. 2017, 37, 395–406. [Google Scholar] [PubMed]
- Chaudhary, S.; Zhornitsky, S.; Roy, A.; Summers, C.; Ahles, T.; Li, C.R.; Chao, H.H. The effects of androgen deprivation on working memory and quality of life in prostate cancer patients: The roles of hypothalamic connectivity. Cancer Med. 2022, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Savard, J.; Simard, S.; Hervouet, S.; Ivers, H.; Lacombe, L.; Fradet, Y. Insomnia in men treated with radical prostatectomy for prostate cancer. Psychooncology 2005, 14, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Connolly, R.M.; A Carducci, M.; Antonarakis, E.S. Use of androgen deprivation therapy in prostate cancer: Indications and prevalence. Asian J. Androl. 2012, 14, 177–186. [Google Scholar] [CrossRef] [Green Version]
- Andersen, M.L.; Tufik, S. The effects of testosterone on sleep and sleep-disordered breathing in men: Its bidirectional interaction with erectile function. Sleep Med. Rev. 2008, 12, 365–379. [Google Scholar] [CrossRef]
- Wijk, M.V.; Garland, S.N.; Rodriguez, N.; Scurrey, S.; Thoms, J.; Laing, K. 0825 The Effect of Androgen Deprivation Therapy on Insomnia Symptoms, Fatigue, Mood, and Hot Flashes in Men with Non-Metastatic Prostate Cancer. Sleep 2019, 42 (Suppl. 1), A331. [Google Scholar] [CrossRef]
- Berger, A.M.; Mooney, K.; Alvarez-Perez, A.; Breitbart, W.S.; Carpenter, K.M.; Cella, D.; Cleeland, C.; Dotan, E.; Eisenberger, M.A.; Escalante, C.P.; et al. Cancer-Related Fatigue, Version 2.2015’. J. Natl. Compr. Cancer Netw. 2015, 13, 1012–1039. [Google Scholar] [CrossRef]
- Buysse, D.J.; Hall, M.L.; Strollo, P.J.; Kamarck, T.W.; Owens, J.; Lee, L.; Reis, S.E.; Matthews, K.A. Relationships Between the Pittsburgh Sleep Quality Index (PSQI), Epworth Sleepiness Scale (ESS), and Clinical/Polysomnographic Measures in a Community Sample. J. Clin. Sleep Med. 2008, 4, 563–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, M.T.; McCrae, C.S.; Cheung, J.; Martin, J.L.; Harrod, C.G.; Heald, J.L.; Carden, K.A. Use of Actigraphy for the Evaluation of Sleep Disorders and Circadian Rhythm Sleep-Wake Disorders: An American Academy of Sleep Medicine Systematic Review, Meta-Analysis, and GRADE Assessment. J. Clin. Sleep Med. 2018, 14, 1209–1230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buysse, D.J.; Reynolds, C.F., III; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Johns, M.W. Daytime sleepiness, snoring, and obstructive sleep apnea. The Epworth Sleepiness Scale. Chest 1993, 103, 30–36, Online. [Google Scholar] [CrossRef] [PubMed]
- CAMNTECH Motionwatch 8 user manual. Pdf. Download ManualsLib. Online. Available online: https://www.manualslib.com/manual/1299199/Camntech-Motionwatch-8.html (accessed on 27 February 2022).
- Hanisch, L.J.; Gooneratne, N.S.; Soin, K.; Gehrman, P.R.; Vaughn, D.J.; Coyne, J.C. Sleep and daily functioning during androgen deprivation therapy for prostate cancer. Eur. J. Cancer Care Engl. 2011, 20, 549–554. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, B.D.; Small, B.J.; Cases, M.G.; Williams, N.L.; Fishman, M.N.; Jacobsen, P.B.; Jim, H.S.L. Sleep Disturbance in Men Receiving Androgen Deprivation Therapy for Prostate Cancer: The Role of Hot Flashes and Nocturia. Cancer 2018, 124, 499–506. [Google Scholar] [CrossRef]
- Landry, G.J.; Best, J.R.; Liu-Ambrose, T. Measuring sleep quality in older adults: A comparison using subjective and objective methods. Front. Aging Neurosci. 2015, 7, 166. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.-L.; Lin, C.-C. Relationships Among Daytime Napping and Fatigue, Sleep Quality, and Quality of Life in Cancer Patients. Cancer Nurs. 2016, 39, 383–392. [Google Scholar] [CrossRef]
- Miaskowski, C.; Paul, S.M.; Cooper, B.A.; Lee, K.; Dodd, M.; West, C.; Aouizerat, B.E.; Dunn, L.; Swift, P.S.; Wara, W. Predictors of the trajectories of self-reported sleep disturbance in men with prostate cancer during and following radiation therapy. Sleep 2011, 34, 171–179. [Google Scholar] [CrossRef] [Green Version]
- Smurra, M.V.; Dury, M.; Aubert, G.; Rodenstein, D.O.; Liistro, G. Sleep fragmentation: Comparison of two definitions of short arousals during sleep in OSAS patients. Eur. Respir. J. 2001, 17, 723–727. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Vitiello, M.V.; Gooneratne, N.S. Sleep in Normal Aging. Sleep Med. Clin. 2018, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- van Hees, V.T.; Sabia, S.; Jones, S.E.; Wood, A.R.; Anderson, K.N.; Kivimäki, M.; Frayling, T.M.; Pack, A.I.; Bucan, M.; Trenell, M.T.; et al. Estimating sleep parameters using an accelerometer without sleep diary. Sci. Rep. 2018, 8, 12975. [Google Scholar] [CrossRef] [PubMed]
- Savard, J.; Ivers, H.; Savard, M.-H.; Morin, C.M. Cancer treatments and their side effects are associated with aggravation of insomnia: Results of a longitudinal study. Cancer 2015, 121, 1703–1711. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, J.S. The Hot Flash Related Daily Interference Scale: A tool for assessing the impact of hot flashes on quality of life following breast cancer. J. Pain Symptom Manag. 2001, 22, 979–989. [Google Scholar] [CrossRef]
| Total Participants (n = 60) | ADT Naïve (n = 40) | ADT (LHRHa) (n = 20) |
|---|---|---|
| Characteristic | Mean ± S.D. Median (IQR) | Mean ± S.D. Median (IQR) |
| Current Age | 71.8 ± 6.9 | 79.9 ± 5.6 ** |
| Age at diagnosis | 68.0 ± 8.1 | 73.4 ± 7.5 * |
| Ethnicity | Number (%) | |
| Caucasian | 23 (57.5%) | 10 (50%) |
| Other ethnicities | 17 (42.5%) | 10 (50%) |
| Years since diagnosis | 2.4 (4.3) | 4.9 (7.48) * |
| PSA at diagnosis (ng/mL) | 9.0 (8.2) | 132.9 (408.7) ** |
| Gleason Score | Number (%) | |
| 6–7 | 26 (65%) | 6 (30%) * |
| 8–9 | 14 (35%) | 12 (60%) |
| No histological confirmation | 0 | 2 (10%) |
| TNM Staging | Number (%) | |
| T1 | 9 (23.1%) | 0 ** |
| T2 | 14 (35.9%) | 4 (23.5%) |
| T3 | 15 (38.5%) | 5 (29.4%) |
| T4 | 1 (2.6%) | 8 (47.1%) |
| N1 | 4 (11.1%) | 10 (55.6%) ** |
| M1 | 3 (9.1%) | 12 (66.7%) ** |
| ADT duration (years) | - | 3.2 (2.4) |
| WHO performance status | 0 (1.0) | 1.0 (1.0) |
| Body weight # | 84.5 ± 15.4 | 86.0 ± 17.1 |
| Body Mass Index BMI # | 26.5 ± 4.7 | 27.7 ± 4.2 |
| Other co-morbidities | Number (%) | |
| Diabetes | 6 (15%) | 5 (20%) |
| Hypertension | 22 (55%) | 13 (65%) |
| Ischemic heart disease | 10 (25%) | 4 (20%) |
| Smoking | Number (%) | |
| Past | 10 (25%) | 8 (40%) |
| Current | 2 (5%) | 3 (15%) |
| Alcohol | Number (%) | |
| Past | 12 (40%) | 8 (40%) |
| Current | 11 (28%) | 8 (40%) |
| Parameter Score | Total Participants (n = 60) | ADT Naïve (n = 40) | ADT (LHRHa) (n = 20) |
|---|---|---|---|
| Total ESS score Number (%) | |||
| ≤10 | 50 (83.3%) | 34 (85%) | 16 (80%) |
| >10 | 10 (16.7%) | 6 (15%) | 4 (20%) |
| Global PSQI score Number (%) | |||
| ≤5 | 30 (50%) | 24 (60%) | 6 (30%) * |
| >5 | 30 (50%) | 16 (40%) | 14 (70%) |
| Actigraphy parameters Number (%) | |||
| Sleep Efficiency (SE) | |||
| ≥80% | 37 (61.6%) | 23 (57.5%) | 14 (70%) |
| <80% | 23 (38.4%) | 17 (42.5%) | 6 (30%) |
| Fragmentation Index (FI) | |||
| ≤40 | 25 (41.6%) | 21 (52.5%) | 4 (20%) * |
| >40 | 35 (58.4%) | 19 (47.5%) | 16 (80%) |
| Actual Sleep Time (AST) | |||
| ≥7 h | 34 (56.6%) | 20 (50%) | 14 (70%) |
| <7 h | 26 (43.4%) | 20 (50%) | 6 (30%) |
| Daytime Napping duration | |||
| ≤60 min | 34 (56.6%) | 26 (65%) | 8 (40%) |
| >60 min | 26 (43.4%) | 14 (35%) | 12 (60%) |
| Total Participants (n = 60) | ADT Naïve (n = 40) | ADT (LHRHa) (n = 20) |
|---|---|---|
| PSQI Scoring | ||
| Subjective Sleep quality | Number (%) | |
| Very good | 10 (25%) | 2 (10%) * |
| Fairly good | 28 (70%) | 12 (60%) |
| Fairly bad | 2 (5%) | 5 (25%) |
| Very bad | 0 | 1 (5%) |
| Sleep latency score | Number (%) | |
| 0 | 16 (40%) | 2 (10%) ** |
| 1–2 | 16 (40%) | 6 (30%) |
| 3–4 | 2 (5%) | 8 (40%) |
| 5–6 | 6 (15%) | 4 (20%) |
| Sleep duration | Number (%) | |
| >7 h | 16 (40%) | 5 (25%) |
| 6–7 h | 15 (37.5%) | 11 (55) |
| 5–6 h | 6 (15%) | 3 (15%) |
| <5 h | 3 (7.5%) | 1 (5%) |
| Habitual sleep efficiency | Number (%) | |
| >85% | 21 (52.5%) | 2 (10%) ** |
| 75–84% | 7 (17.5%) | 10 (50%) |
| 65–74% | 7 (17.5%) | 3 (15%) |
| <65% | 5 (12.5%) | 5 (25%) |
| Sleep disturbance score | Number (%) | |
| 0 | 5 (12.5%) | 0 |
| 1–9 | 28 (70%) | 13 (65%) |
| 10–18 | 7 (17.5%) | 7 (35%) |
| 19–27 | - | - |
| Use of sleep medication | Number (%) | |
| Not during the past month | 39 (97.5%) | 20 (100%) |
| Less than once a week | - | - |
| Once or twice a week | - | - |
| Three or more times a week | 1 (2.5%) | 0 |
| Daytime functioning score | Number (%) | |
| 0 | 31 (77.5%) | 16 (80%) |
| 1–2 | 7 (17.5%) | 2 (10%) |
| 3–4 | 1 (2.5%) | 2 (10%) |
| 5–6 | 1 (2.5%) | 0 |
| Global PSQI score | 4 (4) | 7 (3.8) ** |
| ESS Scoring Median (IQR) | ||
| Total ESS Score | 5 (6) | 8 (5.8) |
| Selected Actigraphy Parameters | ||
|---|---|---|
| Total Participants (n = 60) | ADT Naïve (n = 40) | ADT (LHRHa) (n = 20) |
| Parameters | Median (IQR) | |
| Actual Sleep Time (h) | 6.8 ± 1.3 | 7.7 ± 1.4 * |
| Sleep efficiency (%) | 81.5 (14.3) | 81.4 (7.4) |
| Wake-bout frequency | 39.2 (15.7) | 46.2 (15.9) * |
| Wake-bout duration (min) | 100.5 (64.0) | 114.5 (34.8) |
| Fragmentation index | 37.4 (23.2) | 48.3 (17.9) ** |
| Nap duration (min) | 45.2 (43.0) | 64.1 (74.5) * |
| Nap frequency | 4.4 (5.3) | 7.6 (6.9) * |
| Actigraphic Variables | B | SE | β | p Value | R2 | F (5,50) | Model p Value |
|---|---|---|---|---|---|---|---|
| Total sleep time | 0.695 | 0.520 | 0.235 | 0.187 | 0.088 | 0.966 | 0.447 |
| Sleep efficiency | 0.388 | 3.863 | 0.018 | 0.920 | 0.034 | 0.357 | 0.875 |
| Fragmentation index | 10.626 | 5.838 | 0.303 | 0.075 | 0.184 | 2.260 | 0.063 |
| Nap duration | 27.283 | 16.627 | 0.282 | 0.107 | 0.128 | 1.466 | 0.218 |
| Nap frequency | 2.709 | 1.600 | 0.287 | 0.097 | 0.152 | 1.787 | 0.133 |
| Sleep latency | −1.105 | 6.154 | −0.032 | 0.858 | 0.074 | 0.798 | 0.556 |
| Wake-bout frequency | 11.629 | 4.893 | 0.385 | 0.021 | 0.225 | 2.904 | 0.022 |
| Wake-bout duration | 4.542 | 15.260 | 0.054 | 0.298 | 0.037 | 0.379 | 0.861 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mondal, S.; Edwards, S.; Wibowo, E.; Ahmed, H.; Wassersug, R.J.; Ellis, J.; Isaac, M.; Dimitriou, D.; Mangar, S. Evaluating Patterns and Factors Related to Sleep Disturbances in Prostate Cancer Patients. Healthcare 2022, 10, 832. https://doi.org/10.3390/healthcare10050832
Mondal S, Edwards S, Wibowo E, Ahmed H, Wassersug RJ, Ellis J, Isaac M, Dimitriou D, Mangar S. Evaluating Patterns and Factors Related to Sleep Disturbances in Prostate Cancer Patients. Healthcare. 2022; 10(5):832. https://doi.org/10.3390/healthcare10050832
Chicago/Turabian StyleMondal, Shalini, Steve Edwards, Erik Wibowo, Hashim Ahmed, Richard J. Wassersug, Jason Ellis, Maximus Isaac, Dagmara Dimitriou, and Stephen Mangar. 2022. "Evaluating Patterns and Factors Related to Sleep Disturbances in Prostate Cancer Patients" Healthcare 10, no. 5: 832. https://doi.org/10.3390/healthcare10050832
APA StyleMondal, S., Edwards, S., Wibowo, E., Ahmed, H., Wassersug, R. J., Ellis, J., Isaac, M., Dimitriou, D., & Mangar, S. (2022). Evaluating Patterns and Factors Related to Sleep Disturbances in Prostate Cancer Patients. Healthcare, 10(5), 832. https://doi.org/10.3390/healthcare10050832






