Comparing the Effectiveness of Multicomponent Sleep-Promoting Interventions on the Sleep Quality of Menopausal Women: A Quasi-Experimental Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design
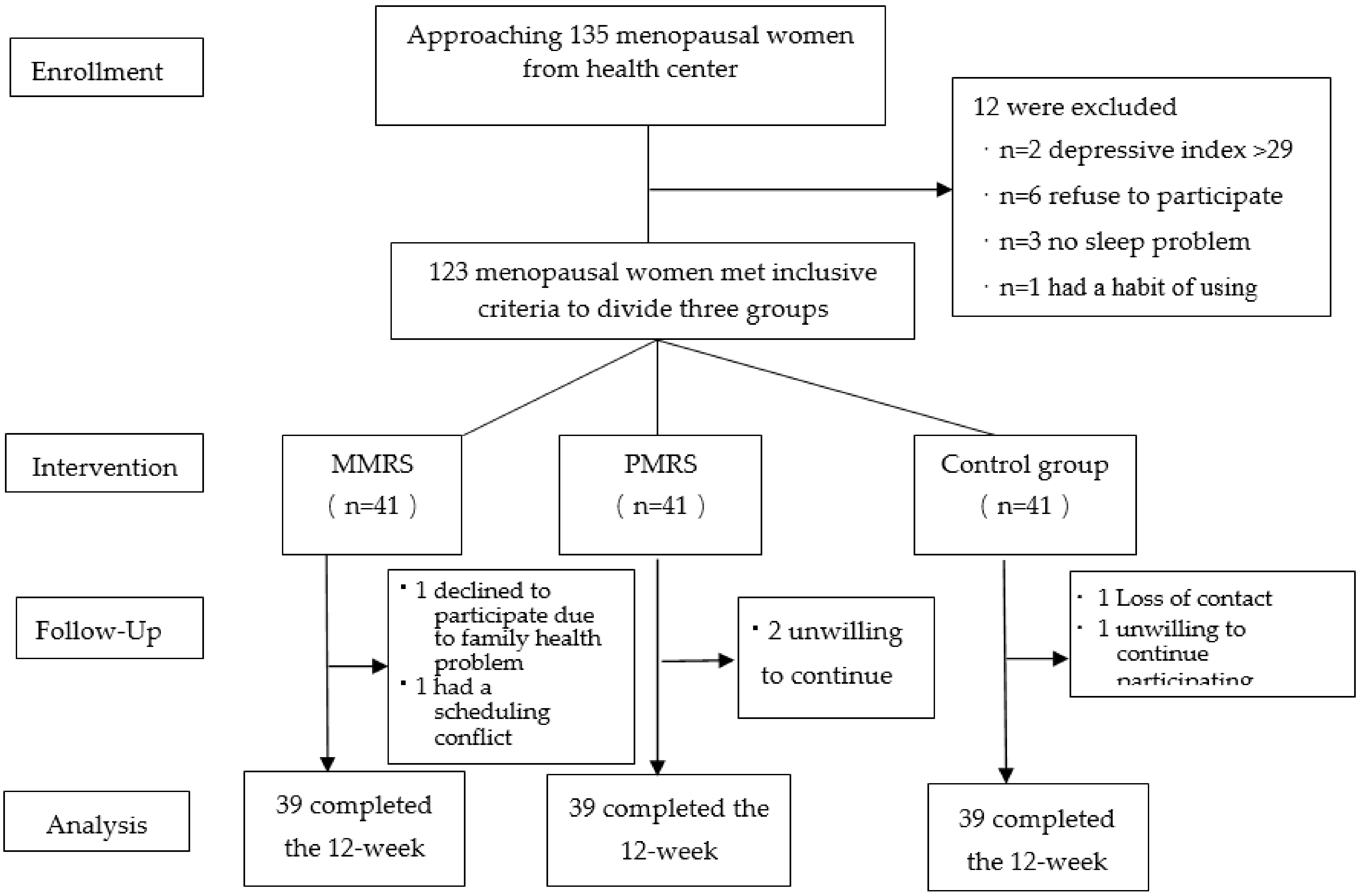
2.2. Participants and Sampling
2.3. Instruments
2.3.1. Demographic Data
2.3.2. The Chinese Version of Pittsburgh Sleep Quality Index (CPSQI)
2.3.3. Actigraphy
2.3.4. Intervention
2.4. Ethical Consideration
2.5. Data Collection
2.6. Data Analyses
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prairie, B.A.; Klein-Patel, M.; Lee, M.; Wisner, K.L.; Balk, J.L. What midlife Women Want from gynecologists: A survey of patients in specialty and private practices. J. Womens Health 2014, 23, 513–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalmbach, D.A.; Cheng, P.; Arnedt, J.T.; Anderson, J.R.; Roth, T.; Fellman-Couture, C.; Williams, R.A.; Drake, C.L. Treating insomnia improves depression, maladaptive thinking, and hyperarousal in postmenopausal women: Comparing cognitive-behavioral therapy for insomnia (CBTI), sleep restriction therapy, and sleep hygiene education. Sleep Med. 2019, 55, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Attarian, H.; Hachul, H.; Guttuso, T.; Barbara, P. Treatment of chronic insomnia disorder in menopause: Evaluation of literature. Menopause 2015, 22, 674e84. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Han, Y.; Cho, H.H.; Kim, M.R. Sleep disorders and menopause. J. Menopausal Med. 2019, 25, 83–87. [Google Scholar] [CrossRef]
- Halpern, J.; Cohen, M.; Kennedy, G.; Reece, J.; Cahan, C.; Baharav, A. Yoga for improving sleep quality and quality of life for older adults. Altern. Ther. Health Med. 2014, 20, 37–46. [Google Scholar]
- Ong, J.C.; Manber, R.; Segal, Z.; Xia, Y.; Shapiro, S.; Wyatt, J.K. A randomized controlled trial of mindfulness meditation for chronic insomnia. Sleep 2014, 37, 1553–1563. [Google Scholar] [CrossRef] [Green Version]
- Rubio-Arias, J.Á.; Marín-Cascale, E.; Ramos-Campo, D.J.; Hernandez, A.V.; Pérez-López, F.R. Effect of exercise on sleep quality and insomnia in middle-aged women: A systematic review and meta-analysis of randomized controlled trials. Maturitas 2017, 100, 49–56. [Google Scholar] [CrossRef] [Green Version]
- Hsu, H.C.; Tsao, L.I.; Lin, M.H. Improving sleep quality interventions among menopausal women with sleep disturbances in Taiwan: A preliminary study. Appl. Nurs. Res. 2015, 28, 374–380. [Google Scholar] [CrossRef]
- Irish, L.A.; Kline, C.E.; Gunn, H.E.; Buysse, D.J.; Hall, M.H. The role of sleep hygiene in promoting public health: A review of empirical evidence. Sleep Med. Rev. 2015, 22, 23–36. [Google Scholar] [CrossRef] [Green Version]
- Larkey, L.; Jahnke, R.; Etnier, J.; Gonzalez, J. Meditative movement as a category of exercise: Implications for research. J. Phys. Act. Health 2009, 6, 230–238. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.W.; Kwong, E.; Lan, X.Y.; Jiang, X.Y. The effect of a meditative movement intervention on quality of sleep in the elderly: A systematic review and meta-Analysis. J. Altern. Complement. Med. 2015, 21, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.H.; Kruse, A. A randomized controlled trial of Tai chi for balance, sleep quality and cognitive performance in elderly Vietnamese. Clin. Interv. Aging 2012, 7, 185–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aksu, N.T.; Erdogan, A.; Ozgur, N. Effects of progressive muscle relaxation training on sleep and quality of life in patients with pulmonary resection. Sleep Breath. 2018, 22, 695–702. [Google Scholar] [CrossRef]
- Luberto, C.M.; Hall, D.L.; Park, E.R.; Haramati, A.; Cotton, S. A perspective on the similarities and differences between mindfulness and relaxation. Glob. Adv. Health Med. 2020, 9, 2164956120905597. [Google Scholar] [CrossRef] [PubMed]
- Dolbier, C.L.; Rush, E.T. Efficacy of Abbreviated Progressive Muscle Relaxation in a High-stress College Sample. Int. J. Stress Manag. 2012, 19, 48–68. [Google Scholar] [CrossRef]
- Akmese, Z.B.; Oran, N.T. Effects of progressive muscle relaxation exercises accompanied by music on low back pain and quality of life during pregnancy. J. Midwifery Women’s Health 2014, 59, 503e9. [Google Scholar] [CrossRef]
- Blanaru, M.; Bloch, B.; Vadas, L.; Arnon, Z.; Ziv, N.; Kremer, I.; Haimov, I. The effects of music relaxation and muscle relaxation techniques on sleep quality and emotional measures among individuals with posttraumatic stress dis-order. Ment. Illn. 2018, 4, e13. [Google Scholar] [CrossRef]
- Golmakani, N.; Seyed, A.; Mohammad, T.; Asghari, P.N. Comparing the effects of progressive muscle relaxation and guided imagery on sleep quality in primigravida women referring to Mashhad health care centers-1393. J. Midwifery Reprod. Health 2015, 3, 335–342. [Google Scholar]
- Zarbakhsh, M.; Sayed Raisi, L. The efficacy of progressive muscular relaxation upon nurses sleep quality. Int. Clin. Neurosci. J. 2018, 5, 164–168. [Google Scholar] [CrossRef]
- National Sleep Foundation. How Much Sleep Do We Really Need? 2019. Available online: https://www.health.harvard.edu/staying-healthy/how-much-sleep-do-we-really-need (accessed on 1 October 2020).
- Everitt, H.; McDermott, L.; Leydon, G.M.; Yules, H.; Baldwin, D.; Little, P. GPs’ management strategies for patients with insomnia: A survey and qualitative interview study. Br. J. Gen. Pract. 2014, 64, e112–e119. [Google Scholar] [CrossRef] [Green Version]
- Bilsbury, C.D.; Rajda, M. What’s wrong with sleep hygiene? Sleep Med. 2004, 5, 513. [Google Scholar] [CrossRef]
- Kaul, P.; Passafiume, J.; Sargent, R.C.; O’Hara, B.F. Meditation acutely improves psychomotor vigilance, and may decrease sleep need. Behav. Brain Funct. 2010, 6, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, P.-S.; Wang, S.-Y.; Wang, M.-Y.; Su, C.-T.; Yang, T.-T.; Huang, C.-J.; Fang, S.-C. Psychometric evaluation of the Chinese version of the Pittsburgh Sleep Quality Index (CPSQI) in primary insomnia and control subjects. Qual. Life Res. 2005, 14, 1943–1952. [Google Scholar] [CrossRef] [PubMed]
- Sternfeld, B.; Guthrie, K.A.; Ensrud, K.E.; LaCroix, A.Z.; Larson, J.C.; Dunn, A.L.; Anderson, G.L.; Seguin, R.A.; Carpenter, J.S.; Caan, B.J.; et al. Efficacy of exercise for menopausal symptoms: A randomized controlled trial. Menopause 2014, 21, 330–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kredlow, M.A.; Capozzoli, M.C.; Hearon, B.A.; Calkins, A.W.; Otto, M.W. The effects of physical activity on sleep: A meta—Analytic review. J. Behav. Med. 2015, 38, 427–449. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, K.A.; Larson, J.C.; Ensrud, K.; Anderson, G.L.; Carpenter, J.S.; Freeman, E.W.; Joffe, H.; Lacroix, A.Z.; Manson, J.; Morin, C.M.; et al. Effects of pharmacologic and nonpharmacologic interventions on insomnia symptoms and self-reported sleep quality in women with hot flashes: A pooled analysis of individual participant data from four MsFLASH trials. Sleep 2018, 41, zsx190. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Cho, J.; Ahn, Y.; Yim, G.; Park, H.Y. Association between physical activity and menopausal symptoms in perimenopausal women. BMC Women Health 2014, 14, 122. [Google Scholar] [CrossRef] [Green Version]
- Nagendra, R.P.; Maruthai, N.; Kutty, B.M. Meditation and Its Regulatory Role on Sleep. Front. Neurol. 2012, 3, 54. [Google Scholar] [CrossRef] [Green Version]
- Goyal, M.; Singh, S.; Sibinga, E.M.S.; Gould, N.F.; Rowland-Seymour, A.; Sharma, R.; Berger, Z.; Sleicher, D.; Maron, D.D.; Shihab, H.M.; et al. Meditation programs for psychological stress and well-being: A systematic review and meta-analysis. JAMA Intern. Med. 2014, 174, 357–368. [Google Scholar] [CrossRef] [Green Version]
| Groups | MMRS (n = 39) | PMRS (n = 39) | Control (n = 39) |
|---|---|---|---|
| Frequency of sessions | 2 h per week * four sessions | 2 h per week * four sessions | None |
| Key content of sessions | First session: 1. Instructed on issues relating to the sleep problems of menopausal women. 2. Instructed on topics relating to sleep hygiene, and given a brochure to participants. 3. Shared and discussed sleep problems of participants. 4. Provided practice log and explained how to record the duration and frequency of their practice at home. Second session: 1. Instructed and demonstrated the eight-move exercise’ meditation with diaphragmatic breathing skills 2. Had participants to immediately practice they have learned and encouraged sharing experience. 3. Issued the handouts outlining the contents of the MMR session and asked them to practice at least 30 min every day. Third session: 1. Assessed how the participants had learned in the previous teaching session. 2. Encouraged participants to practice what they had learned with a positive reinforcement. Fourth session: 1. Assessed the helpfulness of what they had learned in that particular session, and their thoughts. 2. Practiced the MMR and answered participants’ problems. 3. Discussed participants’ experiences.4. Emphasized the importance of daily practice and log keeping. | First session: 1. Instructed on issues relating to the sleep problems of menopausal women. 2. Instructed on topics relating to sleep hygiene and gave a brochure to participants. 3. Shared and discussed sleep problems of participants. 4. Provided practice log and explained how to record the duration and frequency of their practice at home. Second session: 1. Explained and demonstrated the procedures of the PMR 2. Asked participants to immediately returned demonstration. 3. Shared and discussed participants’ experiences. 4. Provided CDs with PMR instructions to the participants and asked them to practice at least 30 min every night lying down to fall asleep. Third session: 1. Assessed how the participants had learned the previous teaching session, and encouraged participants to practice what they had learned with a positive reinforcement. 2. Answered and discussed participants’ experiences. Fourth session: 1. Assessed the helpfulness of what participants had learned in that particular session, and their thoughts. 2. Practiced the PMR and answered problems. 3. Discussed participants’ experiences 4. Emphasized the importance of daily practice and log keeping. | 1. Received no intervention. 2. Provided the same sleep-promoting brochure given to the experimental groups at the end of intervention. |
| Characteristic | Total (n = 117) | PMRS (n = 39) | MMRS (n = 39) | Control (n = 39) | F/χ2/Fisher’s Exact |
|---|---|---|---|---|---|
| Age, mean (SD) | 52.74 (3.9) | 53.8 (4.3) | 51.8 (3.4) | 52.6 (4.09) | 2.55 |
| Menopausal status, n (%) | 7.43 | ||||
| Pre-menopause | 32 (27.1) | 6 (15.4) | 10 (25.0) | 16 (41.0) | |
| Peri-menopause | 21 (17.8) | 7 (17.9) | 9 (22.5) | 5 (12.8) | |
| Post-menopause | 64 (55.1) | 26 (66.7) | 20 (52.5) | 18 (46.2) | |
| Employment, n (%) | 33.47 *** | ||||
| No | 50 (42.4) | 28 (71.8) | 19 (48.6) | 3 (7.7) | |
| Yes | 67 (57.6) | 11 (28.2) | 20 (51.4) | 36 (92.3) | |
| Hypnotic use, n (%) | 0.96 | ||||
| No | 89 (75.4) | 27 (69.2) | 27 (69.3) | 35 (89.7) | |
| Yes | 28 (24.6) | 12 (30.8) | 12 (30.7) | 4 (10.3) | |
| Hormone replacement use, n (%) | 4.09 | ||||
| No | 111 (94.9) | 37 (94.9) | 35 (89.6) | 39 (100) | |
| Yes | 6 (5.1) | 2 (5.1) | 4 (10.4) | 0 (0) | |
| Education, n (%) | 28.37 ** | ||||
| Elementary | 23 (20.3) | 15 (38.5) | 6 (17.5) | 2 (5.1) | |
| Junior or senior high school | 49 (41.5) | 19 (48.7) | 19 (47.5) | 11 (28.2) | |
| college or above | 45 (38.2) | 5 (12.8) | 14 (35.0) | 26 (66.7) | |
| Marital status, n (%) | 7.72 | ||||
| Married | 91 (78.0) | 32 (82.1) | 31 (79.4) | 28 (71.8) | |
| Divorced/widowed | 18 (15.3) | 7 (17.9) | 6 (15.4) | 5 (12.8) | |
| Single | 8 (6.7) | 0 (0) | 2 (5.2) | 6 (15.4) | |
| Exercise, n (%) | 2.89 | ||||
| No | 68 (58.5) | 20 (51.3) | 21 (53.8) | 27 (69.2) | |
| Yes | 49 (41.5) | 19 (48.7) | 18 (46.2) | 12 (30.8) | |
| Chronic illnesses, n (%) | 8.54 | ||||
| No | 70 (60.2) | 21 (53.8) | 20 (51.4) | 29 (74.4) | |
| Yes | 47 (39.8) | 18 (38.3) | 19 (48.6) | 10 (25.6) | |
| Subjective sleep quality, (mean, SD) | 10.81 (.28) | 11.4 (56) | 11.0 ± 0.47 | 10.03 ± 0.38 | 2.23 |
| Objective sleep parameter Onset Latency (min) | 20.19 (18.6) | 21.03 (22.4) | 23.74 (19.1) | 15.80 (12.1) | 1.87 |
| Snooze (min) | 15.23 (16.4) | 15.36 (16.7) | 14.03 (17.5) | 16.29 (15.2) | 0.18 |
| Sleep efficiency (%) | 80 (8.1) | 81.05 (8.1) | 81.20 (7.6) | 78.07 (8.2) | 1.90 |
| WASO (%) | 13.49 (6.59) | 11.75 (6.3) | 12.29 (5.6) | 16.42 (6.9) | 6.42 ** |
| TST (h) | 6.27 (1.1) | 5.94 (1.1) | 6.55 (0.99) | 6.320 (1.2) | 2.23 |
| Variables | PMRS (n = 39) | MMRS (n = 39) | Control (n = 39) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre (Mean ± SD) | Post (Mean ± SD) | 8-Weeks F/U (Mean ± SD) | 12-Weeks F/U(Mean ± SD) | Pre (Mean ± SD) | Post (Mean ± SD) | 8-Weeks F/U (Mean ± SD) | 12-Weeks F/U (Mean ± SD) | Pre (Mean ± SD) | Post (Mean ± SD) | 8-Weeks F/U (Mean ± SD) | 12-Weeks F/U (Mean ± SD) | |
| Subjective sleep quality | 2.0 ± 0.82 | 1.36 ± 0.67 | 1.38 ± 0.71 | 1.33 ± 0.74 | 1.93 ± 0.73 | 1.28 ± 0.60 | 1.23 ± 0.71 | 0.90 ± 0.60 | 1.56 ± 0.85 | 1.59 ± 0.72 | 1.36 ± 0.78 | 1.54 ± 0.72 |
| Sleep latency | 41.41 ± 31.64 | 34.49 ± 21.97 | 35.13 ± 29.03 | 25.18 ± 12.80 | 42.87 ± 30.25 | 30.51 ± 27.62 | 28.85 ± 17.82 | 27.31 ± 17.62 | 23.13 ± 16.44 | 24.56 ± 19.77 | 22.62 ± 17.16 | 21.72 ± 20.60 |
| Sleep duration (h) | 5.41 ± 1.27 | 5.60 ± 1.24 | 5.78 ± 1.33 | 5.95 ± 1.09 | 5.73 ± 0.74 | 6.18 ± 0.94 | 6.63 ± 0.94 | 6.59 ± 0.95 | 6.05 ± 0.96 | 5.78 ± 1.07 | 6.00 ± 1.25 | 5.99 ± 1.06 |
| Habitual sleep efficiency (%) | 73.80 ± 14.62 | 79.91 ± 13.06 | 78.27 ± 14.86 | 82.55 ± 11.11 | 77.87 ± 11.67 | 81.47 ± 11.60 | 82.16 ± 10.83 | 82.96 ± 10.31 | 81.15 ± 12.07 | 79.72 ± 14.39 | 80.05 ± 15.32 | 77.87 ± 17.54 |
| Sleep disturbance | 1.51 ± 0.56 | 1.41 ± 0.50 | 1.38 ± 0.71 | 1.31 ± 0.61 | 1.50 ± 0.56 | 1.31 ± 0.47 | 1.26 ± 0.50 | 1.10 ± 0.45 | 1.36 ± 0.54 | 1.31 ± 0.52 | 1.28 ± 0.56 | 1.21 ± 0.47 |
| Use of sleeping pills | 1.15 ± 1.24 | 0.74 ± 1.11 | 0.92 ± 1.18 | 0.79 ± 1.08 | 1.05 ± 1.26 | 0.33 ± 0.86 | 0.51 ± 0.85 | 0.44 ± 0.79 | 0.31 ± 0.69 | 0.31 ± 0.66 | 0.23 ± 0.63 | 0.23 ± 0.48 |
| Daytime dysfunction | 1.38 ± 0.63 | 1.00 ± 0.83 | 0.97 ± 0.81 | 0.77 ± 0.67 | 1.13 ± 0.72 | 0.69 ± 0.69 | 0.67 ± 0.53 | 0.38 ± 0.54 | 0.74 ± 0.59 | 0.64 ± 0.63 | 0.67 ± 0.66 | 0.64 ± 0.62 |
| Global sleep quality | 11.41 ± 3.48 | 8.56 ± 3.42 | 8.41 ± 3.60 | 7.41 ± 3.46 | 10.95 ± 2.92 | 7.28 ± 2.71 | 6.41 ± 3.03 | 6.67 ± 2.91 | 7.67 ± 3.18 | 8.43 ± 3.21 | 7.90 ± 3.16 | 8.10 ± 2.79 |
| Variables | PMRS (n = 39) | MMRS (n = 39) | Control (n = 39) | |||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | |
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| Onset Latency (min) | 21.03 ± 22.43 | 14.37 ± 12.08 | 23.74 ± 19.16 | 15.81 ± 12.61 | 15.80 ± 12.12 | 16.79 ± 13.33 |
| Sleep efficiency (%) | 81.05 ± 8.13 | 82.37 ± 6.66 | 81.20 ± 7.65 | 84.15 ± 7.57 | 78.07 ± 8.21 | 77.36 ± 10.13 |
| WASO (%) | 13.75 ± 6.30 | 10.01 ± 3.71 | 12.29 ± 5.64 | 9.72 ± 5.06 | 12.42 ± 6.90 | 14.05 ± 9.36 |
| Snooze (min) | 15.36 ± 14.75 | 13.24 ± 12.39 | 14.03 ± 17.57 | 9.88 ± 9.39 | 16.29 ± 15.16 | 9.62 ± 8.12 |
| TST | 5.94 ± 1.05 | 6.12 ± 1.08 | 6.55 ± 0.99 | 6.42 ± 1.34 | 6.32 ± 1.17 | 5.89 ± 1.08 |
| TST (%) | 88.33 ± 6.38 | 87.55 ± 7.83 | 87.71 ± 5.64 | 88.33 ± 7.14 | 83.35 ± 7.27 | 82.03 ± 9.36 |
| Parameter | Β (Estimate) | S E | Wald x2 | p-Value |
|---|---|---|---|---|
| Global PSQI | ||||
| Intercept | 8.54 | 1.02 | 69.67 | <0.001 |
| Group | ||||
| PMRS vs. control | 3.23 | 0.91 | 12.38 | <0.001 |
| MMRS vs. control | 3.20 | 0.76 | 17.46 | <0.001 |
| Time | ||||
| T2 vs. T1 | 0.18 | 0.46 | 0.15 | 0.69 |
| T3 vs. T1 | −0.54 | 0.49 | 1.19 | 0.28 |
| T4 vs. T1 | −0.15 | 0.41 | 0.14 | 0.71 |
| Group × Time | ||||
| Group(PMRS) × T 2 † | −2.51 | 0.67 | 14.03 | <0.001 |
| Group(PMRS) × T3 † | −1.69 | 0.71 | 5.61 | 0.02 |
| Group(PMRS) × T4 † | −3.05 | 0.67 | 20.72 | <0.001 |
| Group(MMRS) × T2 † | −3.97 | 0.68 | 34.21 | <0.001 |
| Group(MMRS) × T3 † | −3.54 | 0.70 | 25.92 | <0.001 |
| Group(MMRS) × T4 † | −4.92 | 0.64 | 59.79 | <0.001 |
| Group(MMRS) × T2 †† | −1.46 | 0.70 | 4.40 | 0.04 |
| Group(MMRS) × T3 †† | −1.85 | 0.71 | 6.75 | 0.01 |
| Group(MMRS) × T4 †† | −1.92 | 0.72 | 7.11 | 0.01 |
| Education level § | ||||
| Junior or senior high school | −0.45 | 0.83 | 0.29 | 0.59 |
| College or above | −0.95 | 0.86 | 1.34 | 0.28 |
| Employment status * | ||||
| No | −0.1 | 0.66 | 0.05 | 0.83 |
| Actigraphic parameters | ||||
| 1. Sleep Onset Latency | ||||
| Intercep | 11.62 | 3.22 | 13.02 | <0.001 |
| Group | ||||
| PMRS vs. control | 7.23 | 4.06 | 3.17 | 0.15 |
| MMRS vs. control | 8.93 | 3.49 | 6.56 | 0.03 |
| Time | ||||
| T2 vs. T1 | 0.99 | 1.96 | 0.26 | 0.61 |
| Group × Time | ||||
| PMRS × T2 † | −7.66 | 3.67 | 4.36 | 0.04 |
| MMRS × T2 † | −8.93 | 3.63 | 6.05 | 0.01 |
| MMRS × T2 †† | −1.27 | 4.35 | 0.09 | 0.77 |
| Education level § | ||||
| Junior or senior high school | 3.20 | 3.32 | 0.93 | 0.34 |
| College or above | 4.93 | 3.08 | 2.56 | 0.11 |
| Employment status * | ||||
| No | −3.50 | 2.70 | 1.69 | 0.20 |
| 2. Wake time (%) | ||||
| Intercep | 14.93 | 1.61 | 95.52 | <0.001 |
| Group | ||||
| PMRS vs. control | −3.95 | 1.59 | 7.96 | 0.01 |
| MMRS vs. control | −3.74 | 1.47 | 7.72 | 0.01 |
| Time | ||||
| T2 vs. T1 | 4.45 | 7.09 | 0.39 | 0.53 |
| Group × Time | ||||
| PMRS × T2 † | −3.36 | 1.85 | 3.29 | 0.07 |
| MMRS × T2 † | −4.20 | 1.78 | 5.55 | 0.02 |
| MMRS × T2 †† | −0.83 | 1.36 | 0.37 | 0.54 |
| Education level § | ||||
| Junior or senior high school | 0.86 | 1.14 | 0.57 | 0.45 |
| College or above | 0.68 | 1.31 | 0.27 | 0.61 |
| Employment status * | ||||
| No | 1.08 | 0.94 | 1.34 | 0.25 |
| 3. Sleep Efficiency | ||||
| Intercep | 81.50 | 2.16 | 1791.39 | <0.001 |
| Group | ||||
| PMRS vs. control | 2.11 | 1.96 | 1.16 | 0.28 |
| MMRS vs. control | 2.80 | 1.83 | 2.35 | 0.13 |
| Time | ||||
| T2 vs. T1 § | −0.70 | 1.64 | 0.18 | 0.67 |
| Group × Time | ||||
| PMRS × T2 † | 2.02 | 2.11 | 0.92 | 0.34 |
| MMRS × T2 † | 3.64 | 2.06 | 3.14 | 0.08 |
| MMRS × T2 †† | 1.62 | 1.80 | 0.81 | 0.37 |
| Education level § | ||||
| Junior or senior high school | −2.67 | 1.51 | 3.12 | 0.08 |
| College or above | −2.65 | 1.63 | 2.65 | 0.10 |
| Employment status * | ||||
| No | −1.21 | 1.43 | 0.71 | 0.40 |
| 4. Snooze Time | ||||
| Intercep | 11.70 | 3.81 | 9.42 | <0.01 |
| Group | ||||
| PMRS vs. control | −0.51 | 3.77 | 0.02 | 0.91 |
| MMRS vs. control | −1.71 | 3.65 | 0.44 | 0.64 |
| Time | −6.68 | 2.56 | 6.79 | <0.01 |
| T2 vs. T1 § | ||||
| Group × Time | ||||
| PMR × T2 † | 4.56 | 3.62 | 1.58 | 0.21 |
| MMRS × T2 † | 2.53 | 4.06 | 0.388 | 0.53 |
| MMRS × T2 †† | −2.03 | 4.06 | 0.25 | 0.62 |
| Education level § | ||||
| Junior or senior high school | 4.74 | 2.77 | 2.93 | 0.09 |
| College or above | 2.61 | 2.46 | 1.12 | 0.29 |
| Employment status * | ||||
| No | 2.06 | 2.99 | 0.48 | 0.49 |
| 5. Total sleep time (min) | ||||
| Intercep | 395.62 | 19.27 | 421.34 | <0.001 |
| Group | ||||
| PMRS vs. control | −34.76 | 16.58 | 4.40 | 0.04 |
| MMRS vs. control | 6.46 | 16.06 | 0.16 | 0.69 |
| Time | ||||
| T2 vs. T1 § | −25.82 | 11.70 | 5.48 | 0.02 |
| Group × Time | ||||
| PMRS × T2 † | 36.66 | 16.07 | 5.21 | 0.02 |
| MMRS × T2 † | 26.38 | 16.55 | 2.53 | 0.11 |
| MMRS × T2 †† | −10.28 | 16.55 | 0.39 | 0.53 |
| Education level § | −0.45 | 1.48 | 0.09 | 0.76 |
| Junior or senior high school | ||||
| College or above | −0.13 | 1.59 | 0.01 | 0.93 |
| Employment status * | −11.95 | 11.24 | 1.13 | 0.29 |
| No |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, M.-H.; Hsiao, P.-R.; Hsu, H.-C. Comparing the Effectiveness of Multicomponent Sleep-Promoting Interventions on the Sleep Quality of Menopausal Women: A Quasi-Experimental Study. Healthcare 2022, 10, 559. https://doi.org/10.3390/healthcare10030559
Lin M-H, Hsiao P-R, Hsu H-C. Comparing the Effectiveness of Multicomponent Sleep-Promoting Interventions on the Sleep Quality of Menopausal Women: A Quasi-Experimental Study. Healthcare. 2022; 10(3):559. https://doi.org/10.3390/healthcare10030559
Chicago/Turabian StyleLin, Mei-Hsiang, Ping-Ru Hsiao, and Hsiu-Chin Hsu. 2022. "Comparing the Effectiveness of Multicomponent Sleep-Promoting Interventions on the Sleep Quality of Menopausal Women: A Quasi-Experimental Study" Healthcare 10, no. 3: 559. https://doi.org/10.3390/healthcare10030559
APA StyleLin, M.-H., Hsiao, P.-R., & Hsu, H.-C. (2022). Comparing the Effectiveness of Multicomponent Sleep-Promoting Interventions on the Sleep Quality of Menopausal Women: A Quasi-Experimental Study. Healthcare, 10(3), 559. https://doi.org/10.3390/healthcare10030559






