Evaluating the Impact of Medication Risk Mitigation Services in Medically Complex Older Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design, Data Source, and Approvals
2.2. Intervention Description: TRHC’s MRM Services
2.3. Subjects and Outcomes
- Medical costs. We evaluated the total combined facility (e.g., hospital) and physician (e.g., outpatient services, office visit) expenditures as well as each type of expenditure individually (i.e., hospital, physician). Costs were defined as the total amount that was adjudicated each year (i.e., 2018 and 2019) in US dollars in the claims data. Facility and physician costs were defined from the claim details field in the data. Facility and physician charges were encoded as UB92 and HCFA, respectively. Thus, the total costs were the sum of UB92 and HCFA.
- Fraction of participants with ≥1 reported ADE. ADEs were defined as any A- or B-level International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10) code as defined previously by Hohl et al. [29].
- Fraction of participants with ≥1 fall. Falls were defined using the following W-group ICD-10 codes: 01, 03–11; 17–19 as well as R29.6.
- Number of ED visits and hospital admissions. Both were identified by line-item claims.
2.4. Analysis
- Set HCC bins with a set of boundaries.
- Calculate weight for each participant. Let xi represent the number of participants in the i-th bin for the MRM cohort. Let yi represent the number of participants in the same bin for the control cohort. Therefore, xi/yi represents the weight to apply to all participants in the i-th bin to make the control cohort equivalent to the MRM cohort.
- For each bin, add a padding parameter—0.001—to avoid bins with zero participants and provide a smoothing effect.
- Add a normalization step to ensure that the sum of the control cohort weights equals the control cohort sample size.
3. Results
3.1. Cohort Description
3.2. Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bouwmeester, C. The PACE program: Home-based long-term care. Consult. Pharm. 2012, 27, 24–30. [Google Scholar] [CrossRef] [PubMed]
- PACE by the Numbers. Available online: https://www.npaonline.org/sites/default/files/PDFs/5033_pace_infographic_update_july2021.pdf (accessed on 13 December 2021).
- Sloane, P.D.; Oudenhoven, M.D.; Broyles, I.; McNabney, M. Challenges to cost-effective care of older adults with multiple chronic conditions: Perspectives of Program of All-Inclusive Care for the Elderly medical directors. J. Am. Geriatr. Soc. 2014, 62, 564–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, J.H.; McInnis, T.; Hirsch, J.D. Cost of prescription drug-related morbidity and mortality. Ann. Pharm. 2018, 52, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Parameswaran Nair, N.; Chalmers, L.; Peterson, G.M.; Bereznicki, B.J.; Castelino, R.L.; Bereznicki, L.R. Hospitalization in older patients due to adverse drug reactions -the need for a prediction tool. Clin. Interv. Aging 2016, 11, 497–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavan, A.H.; Gallagher, P. Predicting risk of adverse drug reactions in older adults. Ther. Adv. Drug Saf. 2016, 7, 11–22. [Google Scholar] [CrossRef]
- Bootman, J.L.; Harrison, D.L.; Cox, E. The health care cost of drug-related morbidity and mortality in nursing facilities. Arch. Intern. Med. 1997, 157, 2089–2096. [Google Scholar] [CrossRef]
- Beijer, H.J.; de Blaey, C.J. Hospitalisations caused by adverse drug reactions (ADR): A meta-analysis of observational studies. Pharm. World Sci. 2002, 24, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.; Nicklason, F.; Vial, J.H. Adverse drug events as a cause of hospital admission in the elderly. Intern. Med. J. 2001, 31, 199–205. [Google Scholar] [CrossRef]
- Bankes, D.L.; Jin, H.; Finnel, S.; Michaud, V.; Knowlton, C.H.; Turgeon, J.; Stein, A. Association of a novel medication risk score with adverse drug events and other pertinent outcomes among participants of the Programs of All-Inclusive Care for the Elderly. Pharmacy 2020, 8, 87. [Google Scholar] [CrossRef] [PubMed]
- Bankes, D.L.; Amin, N.S.; Bardolia, C.; Awadalla, M.S.; Knowlton, C.H.; Bain, K.T. Medication-related problems encountered in the Program of All-Inclusive Care for the Elderly: An observational study. J. Am. Pharm. Assoc. 2020, 60, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Bain, K.T.; Schwartz, E.J.; Knowlton, O.V.; Knowlton, C.H.; Turgeon, J. Implementation of a pharmacist-led pharmacogenomics service for the Program of All-Inclusive Care for the Elderly (PHARM-GENOME-PACE). J. Am. Pharm Assoc. 2018, 58, 281–289.e1. [Google Scholar] [CrossRef] [PubMed]
- Bankes, D.L.; Schamp, R.O.; Knowlton, C.H.; Bain, K.T. Prescriber-initiated engagement of pharmacists for information and intervention in Programs of All-Inclusive Care for the Elderly. Pharmacy 2020, 8, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cicali, B.; Michaud, V.; Knowlton, C.H.; Turgeon, J. Application of a novel medication-related risk stratification strategy to a self-funded employer population. Benefits Q. 2018, 34, 49–55. [Google Scholar]
- Turgeon, J.; Michaud, V.; Cicali, B. Population-Based Medication Risk Stratification and Personalized Medication Risk Score. WO2019089725, 5 September 2019. [Google Scholar]
- Ratigan, A.R.; Michaud, V.; Turgeon, J.; Bikmetov, R.; Gaona Villarreal, G.; Anderson, H.D.; Pulver, G.; Pace, W.D. Longitudinal association of a medication risk score with mortality among ambulatory patients acquired through electronic health record data. J. Patient Saf. 2020, 17, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Michaud, V.; Smith, M.K.; Bikmetov, R.; Dow, P.; Johnson, J.; Stein, A.; Finnel, S.; Jin, H.; Turgeon, J. Association of the MedWise Risk Score with health care outcomes. Am. J. Manag Care 2021, 27 (Suppl. S16), S280–S291. [Google Scholar] [PubMed]
- Knowlton, C.H. Medication risk mitigation matrix: A pharmaceutical care opportunity for precision medication. J. Am. Pharm Assoc. 2015, 55, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Tranchina, K.; Turgeon, J.; Bingham, J. Integrating a novel medication risk score and use of an advanced clinical decision support system into a pharmacist- and nurse-coordinated transition of care program to mitigate drug interactions. Clin. Case Rep. J. 2021, 2, 1–5. [Google Scholar]
- Matos, A.; Bankes, D.L.; Bain, K.T.; Ballinghoff, T.; Turgeon, J. Opioids, polypharmacy, and drug interactions: A technological paradigm shift is needed to ameliorate the ongoing opioid epidemic. Pharmacy 2020, 8, 154. [Google Scholar] [CrossRef]
- Bardolia, C.; Michaud, V.; Turgeon, J.; Amin, N.S. Deprescribing dual therapy in benign prostatic hyperplasia: A patient case. Clin. Case Rep. 2020, 10, 1–4. [Google Scholar]
- Ballinghoff, T.; Bain, K.; Matos, A.; Bardolia, C.; Turgeon, J.; Amin, N.S. Opioid response in an individual with altered cytochrome P450 2D6 activity: Implications of a pharmacogenomics case. Clin. Case Rep. J. 2020, 1, 1–4. [Google Scholar]
- Bain, K.T.; McGain, D.; Cicali, E.J.; Knowlton, C.H.; Michaud, V.; Turgeon, J. Precision medication: An illustrative case series guiding the clinical application of multi-drug interactions and pharmacogenomics. Clin. Case Rep. 2020, 8, 305–312. [Google Scholar] [CrossRef] [Green Version]
- Shah, R.R.; Smith, R.L. Addressing phenoconversion: The Achilles’ heel of personalized medicine. Br. J. Clin. Pharmacol. 2015, 79, 222–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deodhar, M.; Dow, P.; Al Rihani, S.B.; Turgeon, J.; Michaud, V. An illustrative case of phenoconversion due to multi-drug interactions. Clin. Case Rep. J. 2020, 1, 1–6. [Google Scholar]
- Bain, K.T.; Matos, A.; Knowlton, C.H.; McGain, D. Genetic variants and interactions from a pharmacist-led pharmacogenomics service for PACE. Pharmacogenomics 2019, 20, 709–718. [Google Scholar] [CrossRef]
- Board of Pharmacy Specialties: Geriatric Pharmacy. Available online: https://www.bpsweb.org/bps-specialties/geriatric-pharmacy/ (accessed on 26 August 2021).
- Pharmacy Technician Certification Board. Available online: https://www.ptcb.org/ (accessed on 26 August 2021).
- Hohl, C.M.; Karpov, A.; Reddekopp, L.; Stausberg, J. ICD-10 codes used to identify adverse drug events in administrative data: A systematic review. J. Am. Med. Inform. Assoc. 2013, 21, 547–557. [Google Scholar] [PubMed]
- Kane, L.T.; Fang, T.; Galetta, M.S.; Goyal, D.K.C.; Nicholson, K.J.; Kepler, C.K.; Vaccaro, A.R.; Schroeder, G.D. Propensity score matching: A statistical method. Clin. Spine Surg. 2020, 33, 120–122. [Google Scholar] [CrossRef] [PubMed]
- Kautter, J.; Ingber, M.; Pope, G.C. Medicare risk adjustment for the frail elderly. Health Care Financ. Rev. 2008, 30, 83–93. [Google Scholar]
- Yeatts, J.P.; Sangvai, D. HCC coding, risk adjustment, and physician income: What you need to know. Fam. Pract. Manag. 2016, 23, 24–27. [Google Scholar]
- Mosley, D.G.; Peterson, E.; Martin, D.C. Do hierarchical condition category model scores predict hospitalization risk in newly enrolled Medicare Advantage participants as well as probability of repeated admission scores? J. Am. Geriatr. Soc. 2009, 57, 2306–2310. [Google Scholar] [PubMed]
- Haas, L.R.; Takahashi, P.Y.; Shah, N.D.; Stroebel, R.J.; Bernard, M.E.; Finnie, D.M.; Naessens, J.M. Risk-stratification methods for identifying patients for care coordination. Am. J. Manag Care 2013, 19, 725–732. [Google Scholar] [PubMed]
- Committee on Geographic Variation in Health Care Spending and Promotion of High-Value Care; Board on Health Care Spending; Institute of Medicine. Variation in Health Care Spending: Target. Decision Making, Not Geography, 1st ed.; National Academy of Sciences: Washington, DC, USA, 2013. [Google Scholar]
- Kautter, J.; Pope, G.C. CMS frailty adjustment model. Health Care Financ. Rev. 2004, 26, 1–19. [Google Scholar]
- Foley, R.N.; Collins, A.J. End-stage renal disease in the United States: An update from the United States Renal Data System. J. Am. Soc. Nephrol. JASN 2007, 18, 2644–2648. [Google Scholar] [CrossRef] [Green Version]
- Kish, L. Survey Sampling; John Wiley & Sons: New York, NY, USA, 1965. [Google Scholar]
- Keehan, S.P.; Stone, D.A.; Poisal, J.A.; Cuckler, G.A.; Sisko, A.M.; Smith, S.D.; Madison, A.J.; Wolfe, C.J.; Lizonitz, J.M. National health expenditure projections, 2016–2025: Price increases, aging push sector to 20 percent of economy. Health Aff. 2017, 36, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Part D Enhanced Medication Therapy. Available online: https://innovation.cms.gov/initiatives/enhancedmtm/ (accessed on 13 December 2021).
- Stein, A.; Finnel, S.; Bankes, D.; Jin, H.; Awadalla, M.S.; Johnson, J.; Turgeon, J. Health outcomes from an innovative enhanced medication therapy management model. Am. J. Manag. Care 2021, 27 (Suppl. S16), S300–S308. [Google Scholar] [PubMed]
- Bankes, D.; Pizzolato, K.; Finnel, S.; Awadalla, M.S.; Stein, A.; Johnson, J.; Turgeon, J. Medication-related problems identified by pharmacists in an enhanced medication therapy management model. Am. J. Manag. Care 2021, 27 (Suppl. S16), S292–S299. [Google Scholar]
- Hough, A.; Vartan, C.M.; Groppi, J.A.; Reyes, S.; Beckey, N.P. Evaluation of clinical pharmacy interventions in a Veterans Affairs medical center primary care clinic. Am. J. Health Syst. Pharm. 2013, 70, 1168–1172. [Google Scholar] [CrossRef] [PubMed]
- Iuga, A.O.; McGuire, M.J. Adherence and health care costs. Risk Manag. Healthc. Policy 2014, 7, 35–44. [Google Scholar] [PubMed] [Green Version]
- Bain, K.T.; Knowlton, C.H.; Matos, A. Cost avoidance related to a pharmacist-led pharmacogenomics service for the Program of All-inclusive Care for the Elderly. Pharmacogenomics 2020, 21, 651–661. [Google Scholar] [CrossRef]
- McCarrell, J. PACE: An interdisciplinary community-based practice opportunity for pharmacists. Sr. Care Pharm. 2019, 34, 439–443. [Google Scholar] [CrossRef] [PubMed]
- PACE in the States. Available online: https://www.npaonline.org/sites/default/files/PDFs/PACE%20in%20the%20States%20June%202021.pdf (accessed on 13 December 2021).
- Vouri, S.M.; Tiemeier, A. The ins and outs of pharmacy services at a program of all-inclusive care for the elderly. Consult. Pharm. 2012, 27, 803–807. [Google Scholar] [CrossRef] [PubMed]
- Bouwmeester, C.; Kraft, J.; Bungay, K.M. Optimizing inhaler use by pharmacist-provided education to community-dwelling elderly. Respir. Med. 2015, 109, 1363–1368. [Google Scholar] [CrossRef] [Green Version]
- Covington, L.P.; McCarrell, J.; Hoerster, N.S. Prevalence of anticholinergic medication use in the Program of All-Inclusive Care for the Elderly. Consult. Pharm. 2016, 31, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Hazell, L.; Shakir, S.A. Under-reporting of adverse drug reactions: A systematic review. Drug Saf. 2006, 29, 385–396. [Google Scholar] [CrossRef] [PubMed]
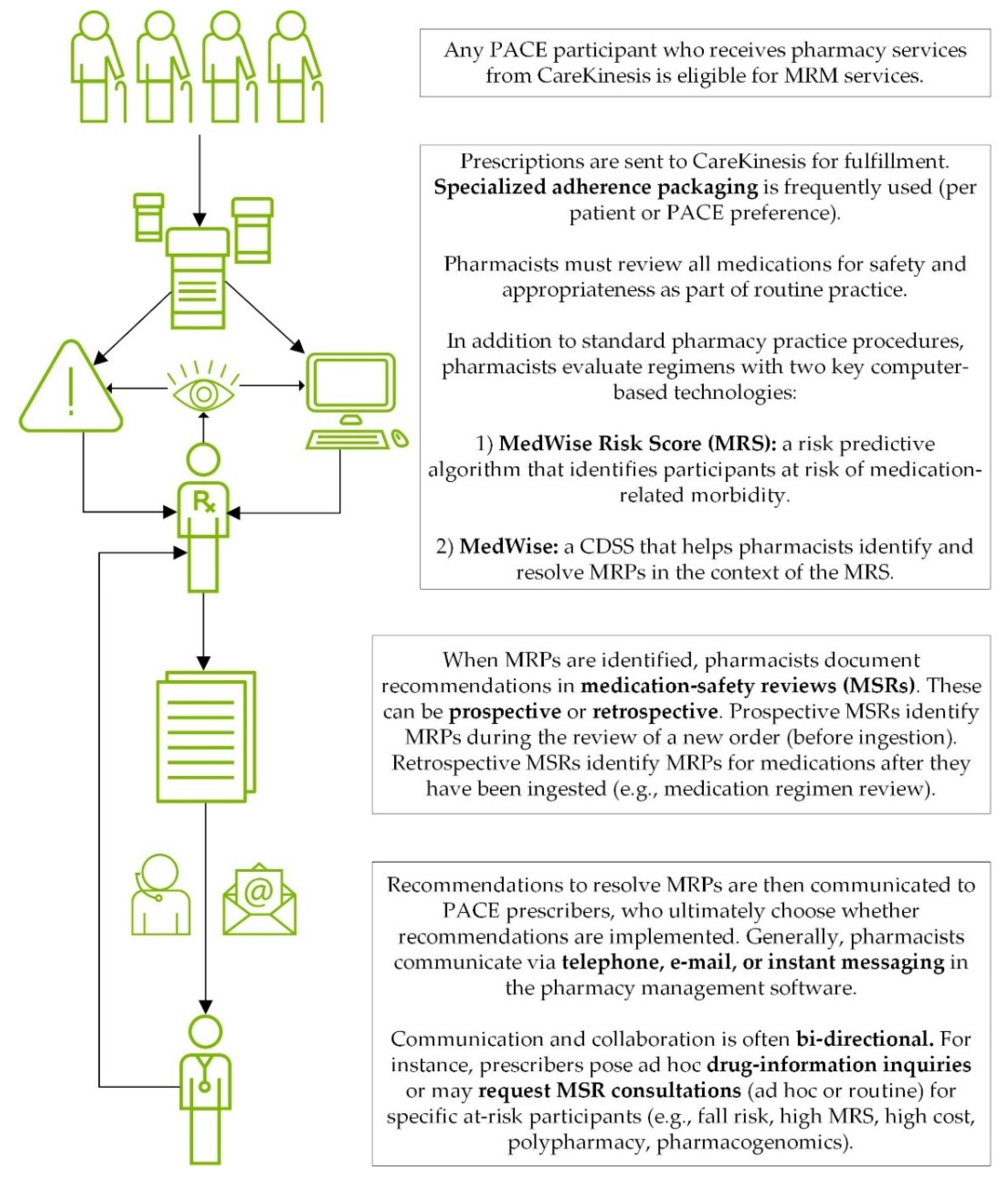
| MRM Component | Detailed Description |
|---|---|
| MedWise risk score (MRS) |
|
| MedWise |
|
| Pharmacogenomics services (PGx) | |
| Drug information support |
|
| Comprehensive adherence support |
|
| Staff competency |
|
| Medication safety review (MSR) |
|
| MRM | Control 1 | |
|---|---|---|
| ≥1 ADE in 2018 but not in 2019 | # participants | # participants |
| No year-over-year change in ADEs | # participants | # participants |
| No ADE in 2018 but ≥1 in 2019 | # participants | # participants |
| MRM + Control | MRM | Control | p-Value 1 | |
|---|---|---|---|---|
| Participants, n (%) | 2545 (100) | 1582 (62.2) | 963 (37.8) | N/A |
| Male, n (%) | 834 (32.8) | 537 (33.9) | 297 (30.8) | 0.11 |
| Age, mean (95% CI) | 77.0 (76.6, 77.3) | 76.7 (76.2, 77.2) | 77.4 (76.8, 78.1) | 0.09 |
| HCC score, mean (95% CI) | 2.64 (2.59, 2.69) | 2.68 (2.62, 2.74) | 2.58 (2.50, 2.65) | 0.042 |
| Conditions, n (%) | ||||
| Hypertension (I10) | 1460 (57.4) | 973 (61.5) | 487 (50.6) | <0.001 |
| Diabetes, type II (E11) | 1137 (44.7) | 776 (49.1) | 361 (37.5) | <0.001 |
| Dyslipidemia (E78) | 1046 (41.1) | 659 (41.7) | 387 (40.2) | 0.23 |
| Dementia (F03) | 506 (19.9) | 329 (20.8) | 177 (18.4) | 0.14 |
| COPD (J44) | 490 (19.3) | 293 (18.5) | 197 (20.5) | 0.23 |
| Major depressive disorder (F33) | 436 (17.1) | 300 (19.0) | 136 (14.1) | 0.002 |
| Heart failure (I50) | 144 (5.7) | 81 (5.1) | 63 (6.5) | 0.13 |
| Location of PACE, n (%) | ||||
| Northeast 2 | 859 (33.8) | 177 (11.2) | 682 (70.8) | <0.001 |
| South 3 | 623 (24.5) | 457 (28.9) | 166 (17.2) | |
| Midwest 4 | 294 (11.6) | 179 (11.3) | 115 (11.9) | |
| West 5 | 769 (30.2) | 769 (48.6) | 0 (0.0) |
| Group | 2018, Mean (95% CI) | 2019, Mean (95% CI) | Year-over-Year Change 2 (95% CI) | % Change (95% CI) | Weighted Mean Difference 3, Absolute | p-Value 4 |
|---|---|---|---|---|---|---|
| Mean total medical expenditures per participant: combined facility and physician (US Dollars) | ||||||
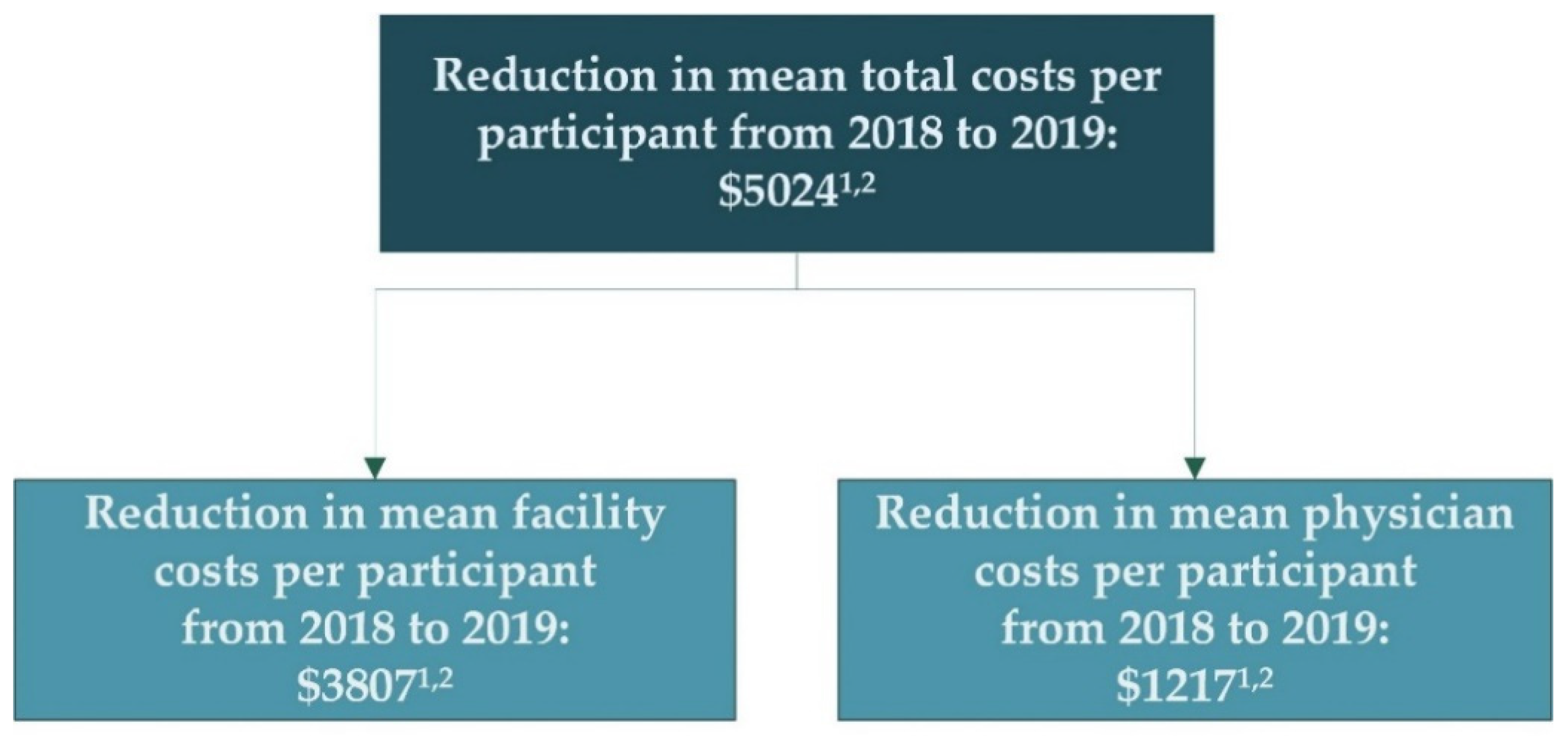
| MRM | USD 22,841 (USD 21,465, USD 24,218) | USD 27,228 (USD 25,664, USD 28,792) | USD 4386 (USD 3040, USD 5732) | 19.2% (13.3%, 25.1%) | USD 5024 | t: <0.001 W: <0.001 |
| Control | USD 25,418 (USD 23,781, USD 27,055) | USD 34,829 (USD 32,873, USD 36,784) | USD 9410 (USD 7737, USD 11,084) | 37.0% (30.4%, 43.6%) | ||
| Mean physician expenditures per participant (US Dollars): | ||||||
| MRM | USD 11,932 (USD 11,295, USD 12,570) | USD 13,800 (USD 13,064, USD 14,536) | USD 1868 (USD 1399, USD 2336) | 15.7% (11.7%, 19.6%) | USD 1217 | t: <0.001 W: <0.001 |
| Control | USD 10,727 (USD 10,061, USD 11,394) | USD 13,811 (USD 13,003, USD 14,621) | USD 3085 (USD 2493, USD 3676) | 28.8% (23.2%, 34.3%) | ||
| Mean facility expenditures per participant (US Dollars) | ||||||
| MRM | USD 10,909 (USD 9791, USD 12,027) | USD 13,428 (USD 12,165, USD 14,691) | USD 2519 (USD 1386, USD 3651) | 23.1% (12.7%, 33.5%) | USD 3807 | t: <0.001 W: <0.001 |
| Control | USD 14,691 (USD 13,195, USD 16,187) | USD 21,017 (USD 19,088, USD 22,945) | USD 6326 (USD 4757, USD 7894) | 41.3% (32.4%, 53.7%) | ||
| Group | 2018 (95% CI) | 2019 (95% CI) 1 | Year-over-Year Change, Absolute 2 (95% CI) | Year-over-Year Change, % (95% CI) | Weighted Difference 3, Absolute | p-Value 4 |
|---|---|---|---|---|---|---|
| ADEs (fraction of participants with at least 1 ADE per year): | ||||||
| MRM | 0.068 (0.056, 0.081) | 0.069 (0.056, 0.081) | 0.001 (−0.015, 0.016) | 0.9% (−21.4%, 23.2%) | 0.023 | χ2: 0.17 |
| Control | 0.055 (0.040, 0.071) | 0.079 (0.060, 0.097) | 0.023 (0.003, 0.043) | 42.2% (5.7%, 78.7%) | ||
| Falls (fraction of participants with at least 1 fall per year) | ||||||
| MRM | 0.11 (0.09, 0.12) | 0.12 (0.10, 0.14) | 0.013 (−0.007, 0.034) | 12.4% (−6.8%, 31.9%) | 0.016 | χ2: 0.65 |
| Control | 0.11 (0.09, 0.13) | 0.14 (0.12, 0.16) | 0.029 (0.000, 0.058) | 25.9% (0.1%, 51.7%) | ||
| Emergency department visits (mean number of visits per participant per year) | ||||||
| MRM | 1.5 (1.4, 1.7) | 1.6 (1.4, 1.7) | 0.04 (−0.12, 0.19) | 2.4% (−7.9%, 12.6%) | 0.14 | t: 0.20 W: 0.27 |
| Control | 1.9 (1.7, 2.2) | 2.1 (1.8, 2.4) | 0.17 (−0.06, 0.47) | 9.1% (−6.2%, 24.4%) | ||
| Hospital admissions (mean number of admissions per participant per year) | ||||||
| MRM | 0.32 (0.28, 0.35) | 0.36 (0.32, 0.40) | 0.04 (−0.01, 0.09) | 12.7% (−1.6%, 27.1%) | 0.025 | t: 0.26 W: 0.17 |
| Control | 0.33 (0.28, 0.38) | 0.40 (0.34, 0.46) | 0.07 (0.001, 0.13) | 19.6% (0.2%, 39.0%) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, H.; Yang, S.; Bankes, D.; Finnel, S.; Turgeon, J.; Stein, A. Evaluating the Impact of Medication Risk Mitigation Services in Medically Complex Older Adults. Healthcare 2022, 10, 551. https://doi.org/10.3390/healthcare10030551
Jin H, Yang S, Bankes D, Finnel S, Turgeon J, Stein A. Evaluating the Impact of Medication Risk Mitigation Services in Medically Complex Older Adults. Healthcare. 2022; 10(3):551. https://doi.org/10.3390/healthcare10030551
Chicago/Turabian StyleJin, Hubert, Sue Yang, David Bankes, Stephanie Finnel, Jacques Turgeon, and Alan Stein. 2022. "Evaluating the Impact of Medication Risk Mitigation Services in Medically Complex Older Adults" Healthcare 10, no. 3: 551. https://doi.org/10.3390/healthcare10030551
APA StyleJin, H., Yang, S., Bankes, D., Finnel, S., Turgeon, J., & Stein, A. (2022). Evaluating the Impact of Medication Risk Mitigation Services in Medically Complex Older Adults. Healthcare, 10(3), 551. https://doi.org/10.3390/healthcare10030551







