Muscle Quality Assessment by Ultrasound Imaging of the Intrinsic Foot Muscles in Individuals with and without Plantar Fasciitis: A Case–Control Study
Abstract
:1. Introduction
2. Methods
2.1. Study Design
2.2. Participants
2.3. Procedure
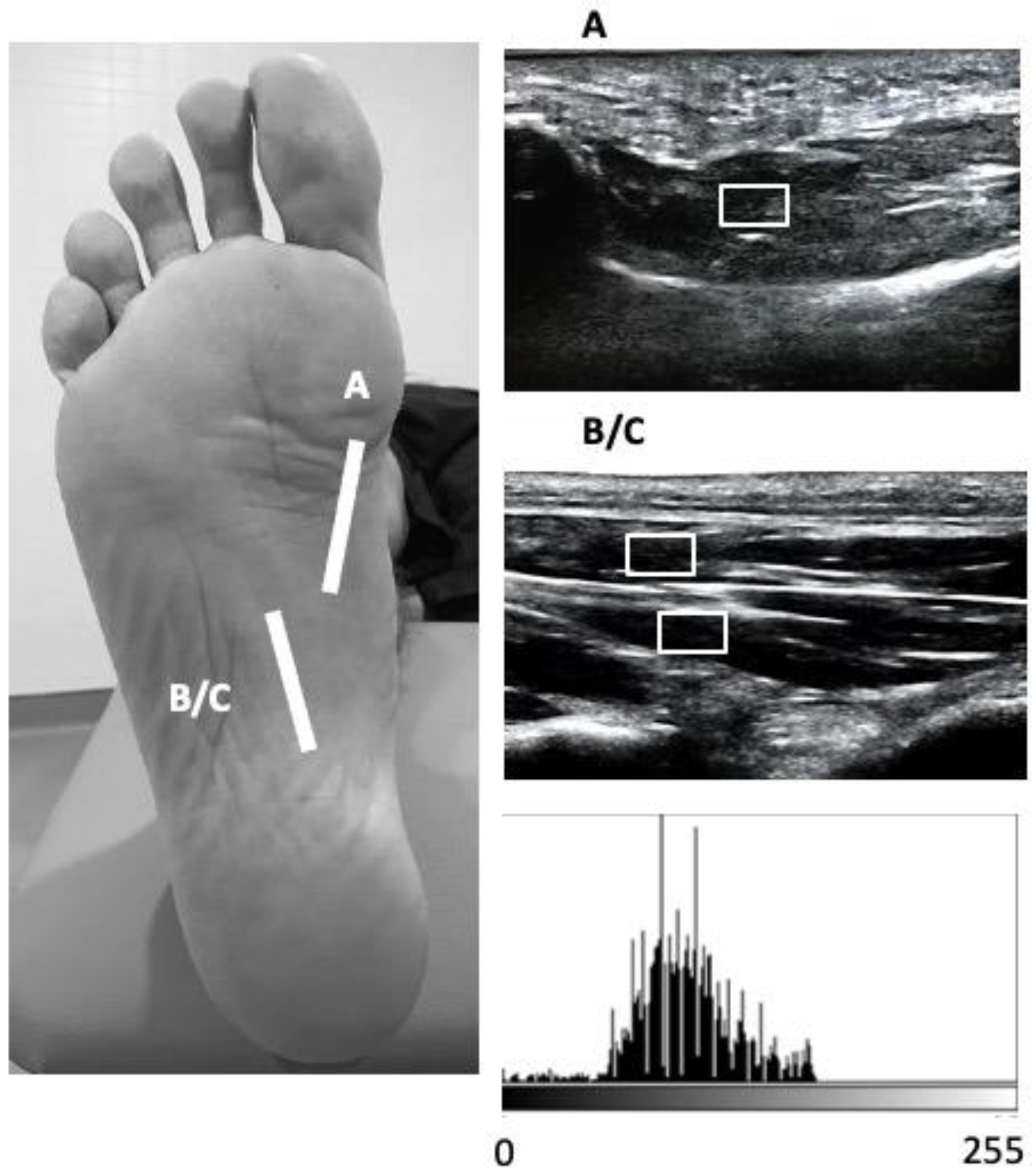
2.4. Outcome Measurements
2.5. Statistical Analysis
3. Results
4. Discussion
5. Clinical Applications
6. Methodological Considerations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Choudhary, R.; Kunal, K. Modifiable Risk Factors of Plantar Fasciitis in Non-Athletic Patients and Proposal of a New Objective Assessment System—RKISP. Rev. Bras. Ortop. 2021, 56, 368–371. [Google Scholar]
- Lopes, A.D.; Hespanhol, L.C., Jr.; Yeung, S.S.; Costa, L.O.P. What are the main running-related musculoskeletal injuries? A Systematic Review. Sports Med. 2012, 42, 891–905. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, A.J.; DiPreta, J.A.; Misener, D. Plantar heel pain. Med. Clin. N. Am. 2014, 98, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Irving, D.; Cook, J.; Menz, H. Factors associated with chronic plantar heel pain: A systematic review. J. Sci. Med. Sport 2006, 9, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Moyne-Bressand, S.; Dhieux, C.; Dousset, E.; Decherchi, P. Effectiveness of Foot Biomechanical Orthoses to Relieve Patients Suffering from Plantar Fasciitis: Is the Reduction of Pain Related to Change in Neural Strategy? Biomed Res. Int. 2018, 2018, 3594150. [Google Scholar] [CrossRef] [Green Version]
- Lemont, H.; Ammirati, K.M.; Usen, N. Plantar fasciitis: A degenerative process (fasciosis) without inflammation. J. Am. Podiatr. Med. Assoc. 2003, 93, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Becker, B.A.; Childress, M. Common Foot Problems: Over-the-Counter Treatments and Home Care. Am. Fam Physician 2018, 98, 298–303. [Google Scholar] [PubMed]
- Yang, W.; Han, Y.; Cao, X.; Pan, J.; Zeng, L.; Lin, J.; Liu, J. Platelet-rich plasma as a treatment for plantar fasciitis: A meta-analysis of randomized controlled trials. Medicine (Baltimore) 2017, 96, e8475. [Google Scholar] [CrossRef]
- Orchard, J. Plantar fasciitis. BMJ 2012, 345, e6603. [Google Scholar] [CrossRef] [PubMed]
- Rhim, H.C.; Kwon, J.; Park, J.; Borg-Stein, J.; Tenforde, A.S. A Systematic Review of Systematic Reviews on the Epidemiology, Evaluation, and Treatment of Plantar Fasciitis. Life 2021, 11, 1287. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, E.N.; Su, J. Plantar fasciitis: A concise review. Perm. J. 2014, 18, e105–e107. [Google Scholar] [CrossRef] [Green Version]
- Cotchett, M.; Rathleff, M.S.; Dilnot, M.; Landorf, K.B.; Morrissey, D.; Barton, C. Lived experience and attitudes of people with plantar heel pain: A qualitative exploration. J. Foot Ankle Res. 2020, 13, 12. [Google Scholar] [CrossRef] [Green Version]
- McKeon, P.O.; Hertel, J.; Bramble, D.; Davis, I. The foot core system: A new paradigm for understanding intrinsic foot muscle function. Br. J. Sports Med. 2015, 49, 290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, L.A.; Cresswell, A.G.; Racinais, S.; Whiteley, R.; Lichtwark, G. Intrinsic foot muscles have the capacity to control deformation of the longitudinal arch. J. R. Soc. Interface 2014, 11, 20131188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Zhang, Y.Z.; Gao, Y.; Luo, T.Y. Assessment the reliability of ultrasonography in the imaging of the plantar fascia: A comparative study. BMC Med. Imaging 2019, 19, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Romero-Morales, C.; Martín-Llantino, P.J.; Calvo-Lobo, C.; López-López, D.; Sánchez-Gómez, R.; De-La-Cruz-Torres, B.; Rodríguez-Sanz, D. Ultrasonography Features of the Plantar Fascia Complex in Patients with Chronic Non-Insertional Achilles Tendinopathy: A Case-Control Study. Sensors 2019, 19, 2052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calvo-Lobo, C.; Useros-Olmo, A.I.; Almazan-Polo, J.; Becerro-de-Bengoa-Vallejo, R.; Losa-Iglesias, M.E.; Palomo-Lopez, P.; Rodríguez-Sanz, D.; López-López, D. Rehabilitative ultrasound imaging of the bilateral intrinsic plantar muscles and fascia in post-stroke survivors with hemiparesis: A case-control study. Int. J. Med Sci. 2018, 15, 907–914. [Google Scholar] [CrossRef] [Green Version]
- Lobo, C.C.; Marin, A.G.; Sanz, D.R.; Lopez, D.L.; Lopez, P.P.; Morales, C.R.; Corbalán, I.S. Ultrasound evaluation of intrinsic plantar muscles and fascia in hallux valgus: A case-control study. Medicine (Baltimore) 2016, 95, e5243. [Google Scholar] [CrossRef] [PubMed]
- Angin, S.; Crofts, G.; Mickle, K.J.; Nester, C.J. Ultrasound evaluation of foot muscles and plantar fascia in pes planus. Gait Posture 2014, 40, 48–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franettovich Smith, M.M.; Hides, J.A.; Hodges, P.W.; Collins, N.J. Intrinsic foot muscle size can be measured reliably in weight bearing using ultrasound imaging. Gait Posture 2019, 68, 369–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molinari, F.; Caresio, C.; Acharya, U.R.; Mookiah, M.R.K.; Minetto, M.A. Advances in quantitative muscle ultrasonography using texture analysis of ultrasound images. Ultrasound Med. Biol. 2015, 41, 2520–2532. [Google Scholar] [CrossRef] [PubMed]
- Caresio, C.; Molinari, F.; Emanuel, G.; Minetto, M.A. Muscle echo intensity: Reliability and conditioning factors. Clin. Physiol. Funct. Imaging 2015, 35, 393–403. [Google Scholar] [CrossRef]
- Ríos-Díaz, J.; Del Baño-Aledo, M.E.; Tembl-Ferrairó, J.I.; Chumillas, M.J.; Vázquez-Costa, J.F.; Martínez-Payá, J.J. Quantitative neuromuscular ultrasound analysis as biomarkers in amyotrophic lateral sclerosis. Eur. Radiol. 2019, 29, 4266–4275. [Google Scholar] [CrossRef] [PubMed]
- De-La-Cruz-Torres, B.; Barrera-García-Martín, I.; Almazán-Polo, J.; Jaén-Crespo, G.; Romero-Morales, C. Ultrasound imaging evaluation of structural and textural features in asymptomatic achilles tendons in pre-professional dancers: A cross-sectional study. Phys. Ther. Sport 2020, 44, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Payá, J.J.; Ríos-Díaz, J.; Del Baño-Aledo, M.E.; Tembl-Ferrairó, J.I.; Vazquez-Costa, J.F.; Medina-Mirapeix, F. Quantitative Muscle Ultrasonography Using Textural Analysis in Amyotrophic Lateral Sclerosis. Ultrason Imaging 2017, 39, 357–368. [Google Scholar] [CrossRef]
- Mirón Mombiela, R.; Facal de Castro, F.; Moreno, P.; Borras, C. Ultrasonic Echo Intensity as a New Noninvasive In Vivo Biomarker of Frailty. J. Am. Geriatr. Soc. 2017, 65, 2685–2690. [Google Scholar] [CrossRef] [PubMed]
- Stock, M.S.; Thompson, B.J. Echo intensity as an indicator of skeletal muscle quality: Applications, methodology, and future directions. Eur J. Appl Physiol. 2021, 121, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Canosa-Carro, L.; López-López, D.; García-Bermejo, P.; Navarro-Flores, E.; de Labra, C.; Romero-Morales, C. Ultrasonographic features of the intrinsic foot muscles in patients with and without plantar fasciitis: A novel case-control research study. Arch. Med. Sci. 2021. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef] [Green Version]
- Thong-On, S.; Bovonsunthonchai, S.; Vachalathiti, R.; Intiravoranont, W.; Suwannarat, S.; Smith, R. Effects of Strengthening and Stretching Exercises on the Temporospatial Gait Parameters in Patients With Plantar Fasciitis: A Randomized Controlled Trial. Ann. Rehabil. Med. 2019, 43, 662–676. [Google Scholar] [CrossRef] [PubMed]
- Renan-Ordine, R.; Alburquerque-Sendín, F.; De Souza, D.P.R.; Cleland, J.A.; Fernández-De-Las-Penas, C. Effectiveness of myofascial trigger point manual therapy combined with a self-stretching protocol for the management of plantar heel pain: A randomized controlled trial. J. Orthop. Sports Phys. Ther. 2011, 41, 43–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunning, J.; Butts, R.; Henry, N.; Mourad, F.; Brannon, A.; Rodriguez, H.; Young, I.; Arias-Buría, J.L.; Fernández-De-Las-Peñas, C. Electrical dry needling as an adjunct to exercise, manual therapy and ultrasound for plantar fasciitis: A multi-center randomized clinical trial. PLoS ONE 2018, 13, e0205405. [Google Scholar] [CrossRef] [PubMed]
- Mickle, K.J.; Angin, S.; Crofts, G.; Nester, C.J. Effects of Age on Strength and Morphology of Toe Flexor Muscles. J. Orthop. Sports Phys. Ther. 2016, 46, 1065–1070. [Google Scholar] [CrossRef] [PubMed]
- De-La-Cruz-Torres, B.; Navarro-Flores, E.; López-López, D.; Romero-Morales, C. Ultrasound Imaging Evaluation of Textural Features in Athletes with Soleus Pathology—A Novel Case-Control Study. Int. J. Environ. Res. Public Heal. 2021, 18, 1983. [Google Scholar] [CrossRef] [PubMed]
- Tosovic, D.; Ghebremedhin, E.; Glen, C.; Gorelick, M.; Brown, J.M. The architecture and contraction time of intrinsic foot muscles. J. Electromyogr. Kinesiol. 2012, 22, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Cheung, R.; Sze, L.; Mok, N.; Ng, G. Intrinsic foot muscle volume in experienced runners with and without chronic plantar fasciitis. J. Sci. Med. Sport 2016, 19, 713–715. [Google Scholar] [CrossRef]
- Silbernagel, K.G.; Crossley, K. A Proposed Return-to-Sport Program for Patients With Midportion Achilles Tendinopathy: Rationale and Implementation. J. Orthop. Sports Phys. Ther. 2015, 45, 876–886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huffer, D.; Hing, W.; Newton, R.; Clair, M. Strength training for plantar fasciitis and the intrinsic foot musculature: A systematic review. Phys. Ther. Sport 2017, 24, 44–52. [Google Scholar] [CrossRef] [Green Version]
- Sikdar, S.; Diao, G.; Turo, D.; Stanley, C.J.; Sharma, A.; Chambliss, A.; Laughrey, L.; Aralar, A.; Damiano, D.L. Quantification of Muscle Tissue Properties by Modeling the Statistics of Ultrasound Image Intensities Using a Mixture of Gamma Distributions in Children With and Without Cerebral Palsy. J. Ultrasound Med. 2018, 37, 2157–2169. [Google Scholar] [CrossRef] [Green Version]
- Smith, L.R.; Lee, K.S.; Ward, S.R.; Chambers, H.G.; Lieber, R.L. Hamstring contractures in children with spastic cerebral palsy result from a stiffer extracellular matrix and increased in vivo sarcomere length. J. Physiol. 2011, 589 Pt 10, 2625–2639. [Google Scholar] [CrossRef] [PubMed]
- Romero-Morales, C.; Martin-Llantino, P.J.; Calvo-Lobo, C.; Almazan-Polo, J.; Lopez-Lopez, D.; de la Cruz-Torres, B.; Palomo-López, P.; Rodríguez-Sanz, D. Intrinsic foot muscles morphological modifications in patients with Achilles tendinopathy: A novel case-control research study. Phys. Ther. Sport. 2019, 40, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Schütte, K.H.; Vanwanseele, B. Foot muscle morphology is related to center of pressure sway and control mechanisms during single-leg standing. Gait Posture 2017, 57, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Taş, S.; Çetin, A. An investigation of the relationship between plantar pressure distribution and the morphologic and mechanic properties of the intrinsic foot muscles and plantar fascia. Gait Posture 2019, 72, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, M.; Maeda, N.; Komiya, M.; Hirota, A.; Mizuta, R.; Kobayashi, T.; Kaneda, K.; Nishikawa, Y.; Urabe, Y. Contribution of Plantar Fascia and Intrinsic Foot Muscles in a Single-Leg Drop Landing and Repetitive Rebound Jumps: An Ultrasound-Based Study. Int. J. Environ. Res. Public Health 2021, 18, 4511. [Google Scholar] [CrossRef] [PubMed]
- Zaidman, C.M.; Wu, J.S.; Wilder, S.; Darras, B.; Rutkove, S. Minimal training is required to reliably perform quantitative ultrasound of muscle. Muscle Nerve 2014, 50, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Baellow, A.; Jaffri, A.H.; Hertel, J.; Higgins, M.J.; Rangecroft, C.M.; Hryvniak, D.J.; Saliba, S.A. Intrinsic foot muscle size and quality in a single leg weight bearing position across foot posture types in individuals with Patellofemoral Pain compared to healthy. Phys. Ther. Sport 2022, 54, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Almazán-Polo, J.; López-López, D.; Romero-Morales, C.; Rodríguez-Sanz, D.; Becerro-de-Bengoa-Vallejo, R.; Losa-Iglesias, M.E.; Bravo-Aguilar, M.; Calvo-Lobo, C. Quantitative Ultrasound Imaging Differences in Multifidus and Thoracolumbar Fasciae between Athletes with and without Chronic Lumbopelvic Pain: A Case-Control Study. J. Clin. Med. 2020, 9, 2647. [Google Scholar] [CrossRef]


| Data | Plantar Fasciitis (n = 32) | Controls (n = 32) | p-Value Cases vs. Controls |
|---|---|---|---|
| Age, y | 43.00 ± 11.00 | 31.00 ± 9.50 | 0.001 |
| Weight, kg | 76.00 ± 27.00 | 73.00 ± 25.50 | 0.028 |
| Height, m | 1.71 ± 6.3 | 1.70 ± 9.68 | 0.465 |
| BMI, kg/m2 | 28.02 ± 5.56 | 24.69 ± 5.45 | 0.031 |
| Measurement | Plantar Fasciitis (n = 32) Mean ± SD (95% CI) | Controls (n = 32) Mean ± SD (95% CI) | p-Value |
|---|---|---|---|
| FHB EI | 41.30 ± 13.35 (36.4–46.1) | 44.37 ± 9.77 (40.8–47.9) | 0.297 |
| FHB EV | 41.19 ± 13.02 (36.4–45.8) | 44.77 ± 10.14 (41.11–48.4) | 0.225 |
| FDB EI | 52.02 ± 20.41 (44.6–59.3) | 54.71 ± 12.26 (50.2–59.1) | 0.527 |
| FDB EV | 21.35 ± 8.67 (18.2–24.4) | 23.63 ± 6.33 (21.3–25.9) | 0.236 |
| QP EI | 43.14 ± 19.37 (36.1–50.1) | 50.94 ± 18.45 (44.2–57.5) | 0.105 |
| QP EV | 22.94 ± 9.98 (19.3–26.5) | 22.48 ± 6.66 (20.0–24.8) | 0.829 |
| AHB EI | 52.06 ± 18.50 (45.3–58.7) | 58.50 ± 13.07 (53.7–63.2) | 0.113 |
| AHB EV | 52.12 ± 18.47 (45.4–58.7) | 59.00 ± 13.37 (54.1–63.8) | 0.093 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canosa-Carro, L.; López-López, D.; de Labra, C.; Díaz-Meco-Conde, R.; de-la-Cruz-Torres, B.; Romero-Morales, C. Muscle Quality Assessment by Ultrasound Imaging of the Intrinsic Foot Muscles in Individuals with and without Plantar Fasciitis: A Case–Control Study. Healthcare 2022, 10, 526. https://doi.org/10.3390/healthcare10030526
Canosa-Carro L, López-López D, de Labra C, Díaz-Meco-Conde R, de-la-Cruz-Torres B, Romero-Morales C. Muscle Quality Assessment by Ultrasound Imaging of the Intrinsic Foot Muscles in Individuals with and without Plantar Fasciitis: A Case–Control Study. Healthcare. 2022; 10(3):526. https://doi.org/10.3390/healthcare10030526
Chicago/Turabian StyleCanosa-Carro, Lorena, Daniel López-López, Carmen de Labra, Raquel Díaz-Meco-Conde, Blanca de-la-Cruz-Torres, and Carlos Romero-Morales. 2022. "Muscle Quality Assessment by Ultrasound Imaging of the Intrinsic Foot Muscles in Individuals with and without Plantar Fasciitis: A Case–Control Study" Healthcare 10, no. 3: 526. https://doi.org/10.3390/healthcare10030526
APA StyleCanosa-Carro, L., López-López, D., de Labra, C., Díaz-Meco-Conde, R., de-la-Cruz-Torres, B., & Romero-Morales, C. (2022). Muscle Quality Assessment by Ultrasound Imaging of the Intrinsic Foot Muscles in Individuals with and without Plantar Fasciitis: A Case–Control Study. Healthcare, 10(3), 526. https://doi.org/10.3390/healthcare10030526









