Combination of Multidisciplinary Therapies Successfully Treated Refractory Ventricular Arrhythmia in a STEMI Patient: Case Report and Literature Review
Abstract
:1. Introduction
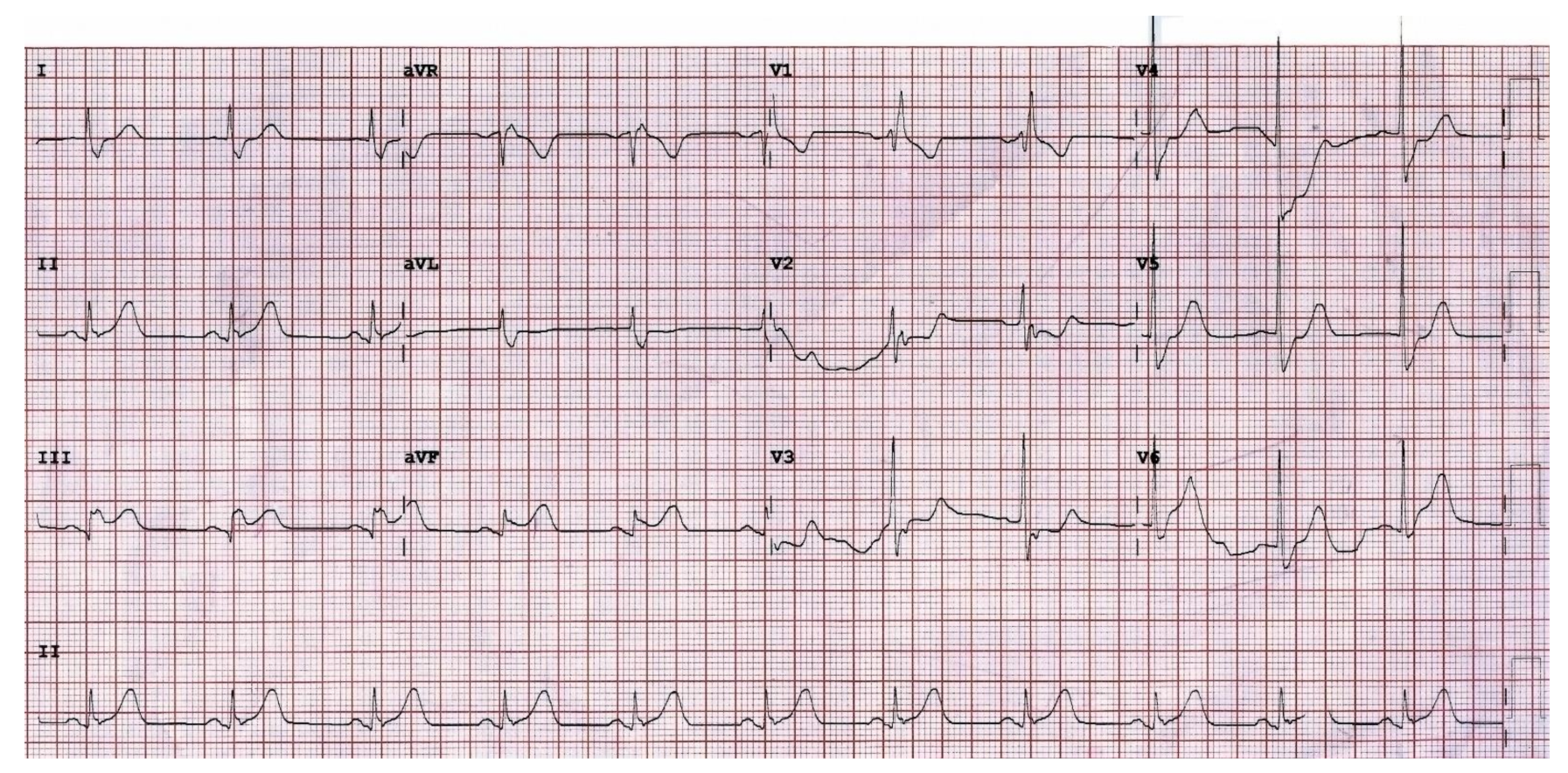
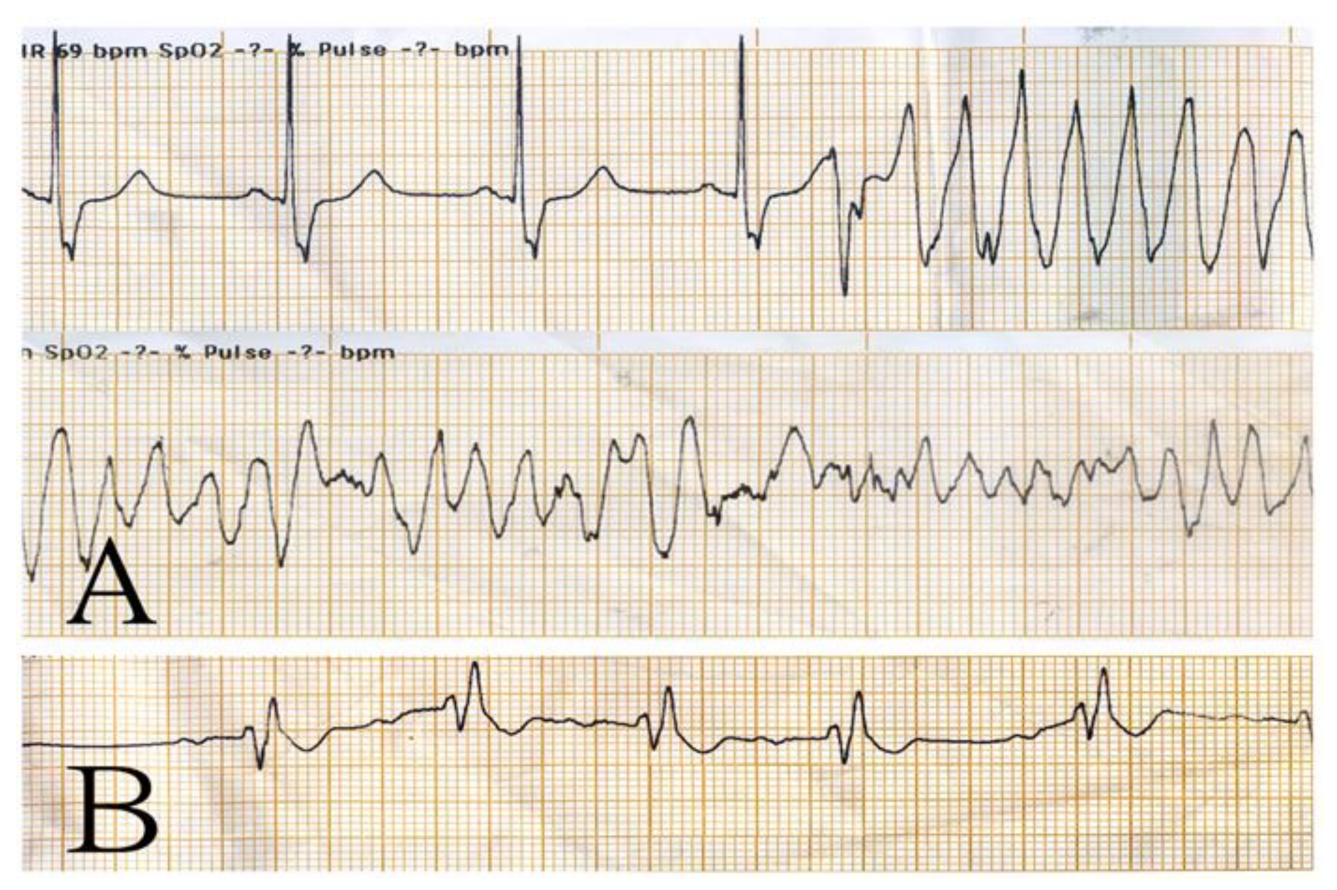
2. Case Presentation
3. Discussion
3.1. Definition and Incidence of Refractory VF/pVT
3.2. Treatment Categories of Refractory VF/pVT
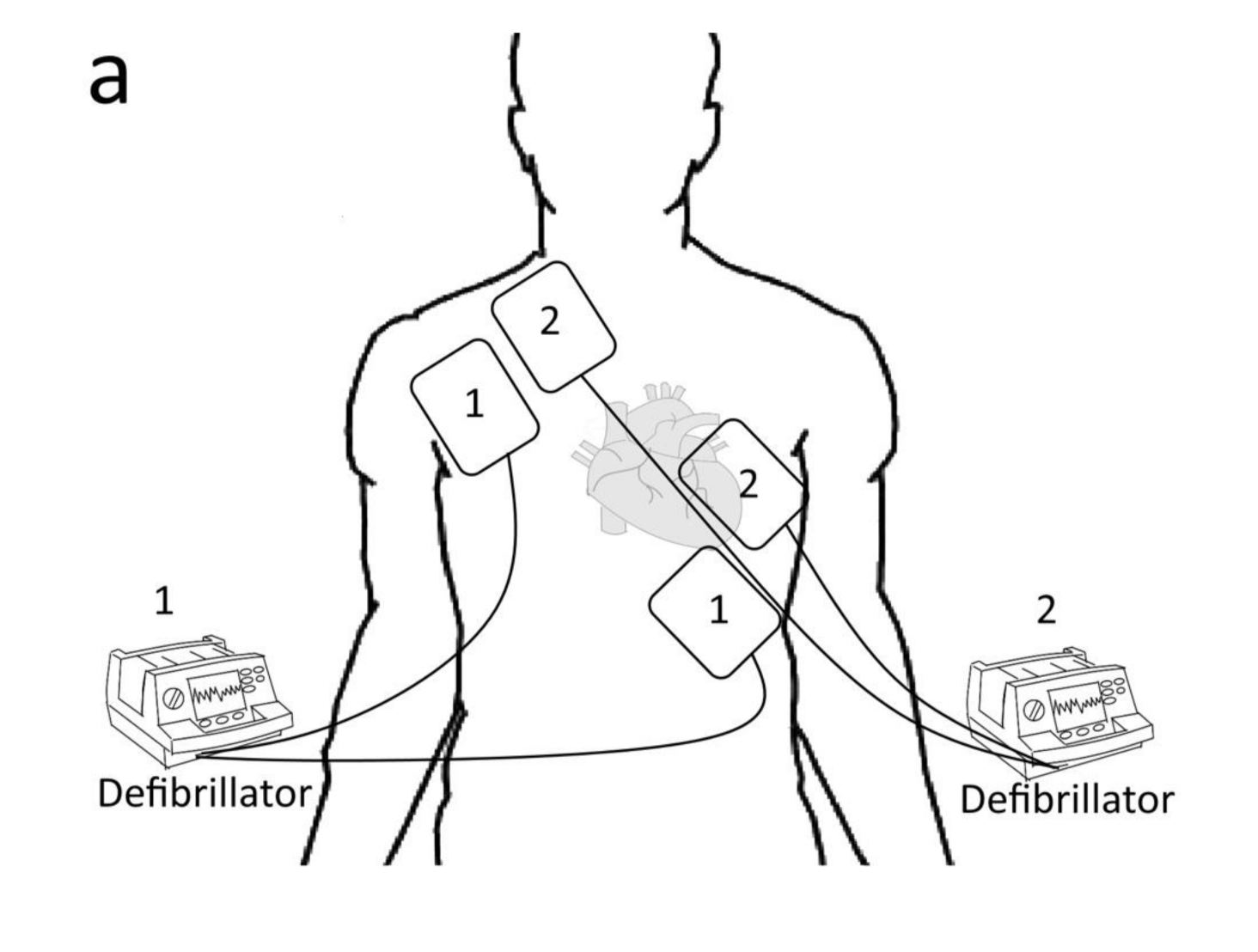
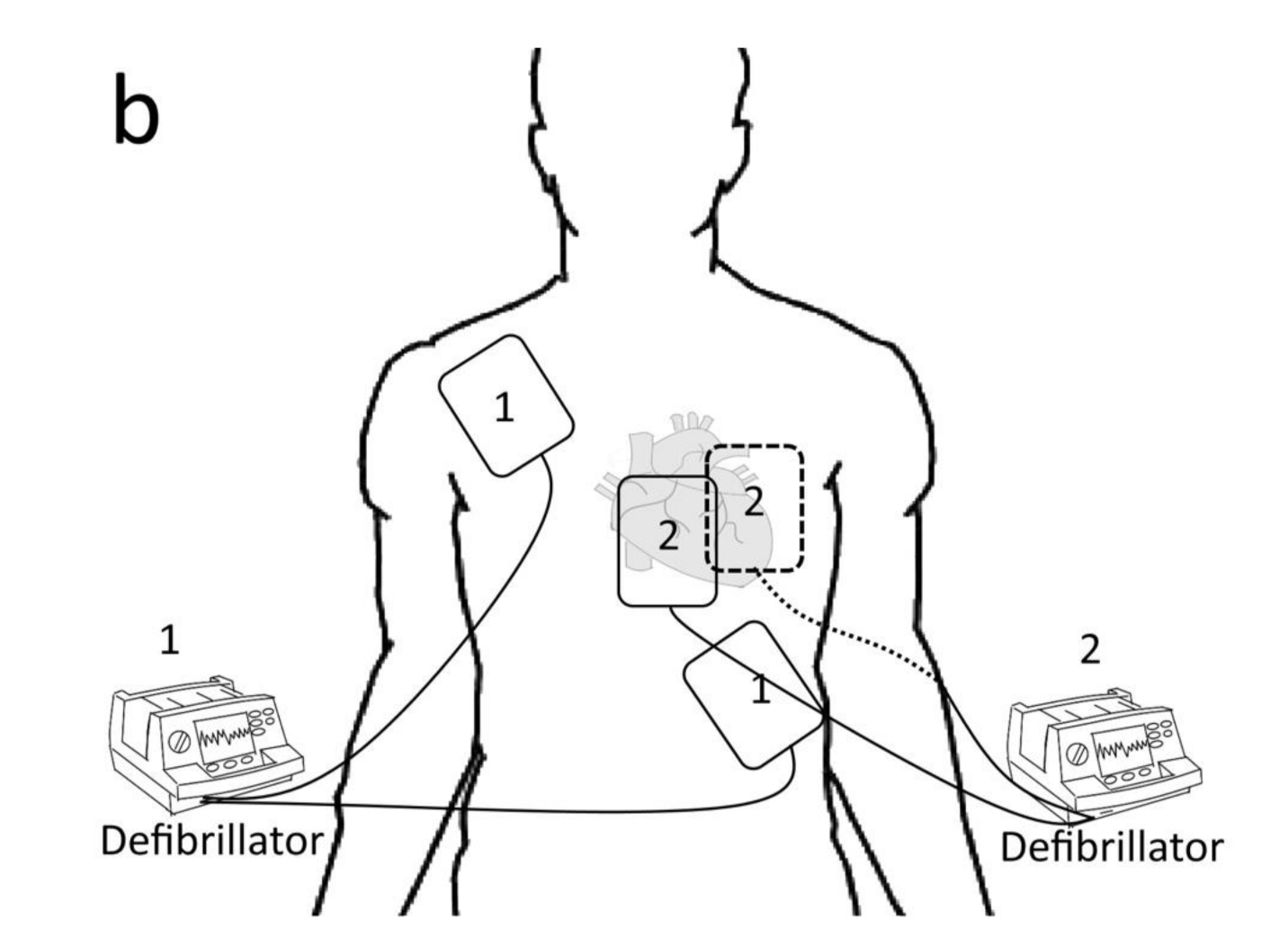
3.2.1. Electrical Defibrillation
3.2.2. Mechanical Support
3.2.3. Medical Treatments
3.2.4. Other Treatments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bell, S.M.; Lam, D.H.; Kearney, K.; Hira, R.S. Management of refractory ventricular fibrillation (prehospital and emergency department). Cardiol. Clin. 2018, 36, 395–408. [Google Scholar] [CrossRef] [PubMed]
- Panchal, A.R.; Berg, K.M.; Kudenchuk, P.J.; Del Rios, M.; Hirsch, K.G.; Link, M.S.; Kurz, M.C.; Chan, P.S.; Cabanas, J.G.; Morley, P.T.; et al. 2018 American heart association focused update on advanced cardiovascular life support use of antiarrhythmic drugs during and immediately after cardiac arrest: An update to the American heart association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2018, 138, e740–e749. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, M.; Dyer, S.; Peksa, G.D. Beta-blockade for the treatment of cardiac arrest due to ventricular fibrillation or pulseless ventricular tachycardia: A systematic review and meta-analysis. Resuscitation 2020, 146, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Miraglia, D.; Miguel, L.A.; Alonso, W. The evolving role of novel treatment techniques in the management of patients with refractory VF/pVT out-of-hospital cardiac arrest. Am. J. Emerg. Med. 2019, 38, 648–654. [Google Scholar] [CrossRef] [PubMed]
- The Lancet. Out-of-hospital cardiac arrest: A unique medical emergency. Lancet 2018, 391, 911. [Google Scholar] [CrossRef]
- Miraglia, D.; Miguel, L.A.; Alonso, W.; Ayala, J.E. Double sequential defibrillation for out-of-hospital refractory ventricular fibrillation: A scoping review. Am. J. Emerg. Med. 2019, 38, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Al-Khatib, S.M.; Stevenson, W.G.; Ackerman, M.J.; Bryant, W.J.; Callans, D.J.; Curtis, A.B.; Deal, B.J.; Dickfeld, T.; Field, M.E.; Fonarow, G.C.; et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: Executive summary. Circulation 2018, 138, e210–e271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yannopoulos, D.; Bartos, J.; Raveendran, G.; Walser, E.; Connett, J.; Murray, T.A.; Collins, G.; Zhang, L.; Kalra, R.; Kosmopoulos, M.; et al. Advanced reperfusion strategies for patients with out-of- hospital cardiac arrest and refractory ventricular fibrillation (ARREST): A phase 2, single centre, open-label, randomised controlled trial. Lancet 2020, 396, 1807–1816. [Google Scholar] [CrossRef]
- Bundgaard, J.S.; Jacobsen, P.K.; Grand, J.; Lindholm, M.G.; Hassager, C.; Pehrson, S.; Kjaergaard, J.; Bundgaard, H. Deep sedation as temporary bridge to definitive treatment of ventricular arrhythmia storm. Eur. Heart J. Acute Cardiovasc. Care 2020, 9, 657–664. [Google Scholar] [CrossRef]
- Yannopoulos, D.; Bartos, J.A.; Aufderheide, T.P.; Callaway, C.W.; Deo, R.; Garcia, S.; Halperin, H.R.; Kern, K.B.; Kudenchuk, P.J.; Neumar, R.W.; et al. The evolving role of the cardiac catheterization laboratory in the management of patients with out-of-hospital cardiac arrest: A scientific statement from the American heart association. Circulation 2019, 139, e530–e552. [Google Scholar] [CrossRef] [PubMed]
- Trayanova, N. Defibrillation of the heart: Insights into mechanisms from modelling studies. Exp. Physiol. 2006, 91, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Merlin, M.A.; Tagore, A.; Bauter, R.; Arshad, F.H. A case series of double sequence defibrillation. Prehosp. Emerg. Care 2016, 20, 550–553. [Google Scholar] [CrossRef] [PubMed]
- Cheskes, S.; Wudwud, A.; Turner, L.; McLeod, S.; Summers, J.; Morrison, L.J.; Verbeek, P.R. The impact of double sequential external defibrillation on termination of refractory ventricular fibrillation during out-of-hospital cardiac arrest. Resuscitation 2019, 139, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Deakin, C.D.; Morley, P.; Soar, J.; Drennan, I.R. Double (dual) sequential defibrillation for refractory ventricular fibrillation cardiac arrest: A systematic review. Resuscitation 2020, 155, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Yannopoulos, D.; Bartos, J.A.; Martin, C.; Raveendran, G.; Missov, E.; Conterato, M.; Frascone, R.J.; Trembley, A.; Sipprell, K.; John, R.; et al. Minnesota resuscitation consortium’s advanced perfusion and reperfusion cardiac life support strategy for out-of-hospital refractory ventricular fibrillation. J. Am. Heart Assoc. 2016, 5, e003732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Priori, S.G.; Blomström-Lundqvist, C.; Mazzanti, A.; Blom, N.A. 2015 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur. Heart J. 2015, 36, 2793–2867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Category | Modality | Possible Mechanisms | Reference |
|---|---|---|---|
| Electrical management | 1. Conventional treatment | Successful defibrillation affects most of the heart, resulting in too-little remaining heart muscle continuing the arrhythmia. | [7] |
| 2. DSD |
| [4] | |
| Mechanical support | 1. CPR | Restores the partial flow of oxygenated blood to the brain and heart. | [7] |
| 2. ECMO | Normalizes perfusion reliably and provides cardiopulmonary support, to facilitate identification and treatment of the most common cause of refractory arrest (i.e., ACS). | [8] | |
| Drugs | 1. Amiodarone | Class III antiarrhythmic drug: blocks potassium rectifier currents that are responsible for the repolarization of the heart during phase 3 of the cardiac action potential. | [2] |
| 2. Lidocaine | Class Ib antiarrhythmic drug: blocks fast sodium channels responsible for the rapid phase-0 depolarization of the cardiac action potential in non-nodal tissue. | [2] | |
| 3. Epinephrine | Stimulates alpha-adrenergic receptors, resulting in peripheral vasoconstriction, to increase coronary perfusion. | [1] | |
| 4. Beta-blocker | Counteracts the negative beta-adrenergic effects of epinephrine to decrease the sensitivity of the myocardium to arrhythmias. | [3] | |
| 5. Deep Sedation (Propofol) |
| [9] | |
| Miscellaneous | 1. PCI | Restores myocardial blood flow. | [10] |
| 2. SGB | Blocks the stellate ganglion to attenuate noradrenaline signalling (efferent sympathetic outflow) to the heart. | [4] |
| Parameters | Results | Normal Values |
|---|---|---|
| White blood cell count (/µL) | 10,480 | 4800–10,800 |
| Haemoglobin (g/dL) | 13.1 | 14–18 |
| Platelet count (/µL) | 260,000 | 130,000–400,000 |
| Mean corpuscular volume (fL) | 90.7 | 80–94 |
| BUN (mg/dL) | 25.5 | 6–24 |
| Creatinine (mg/dL) | 1.63 | 0.5–1.4 |
| Sodium (mEq/L) | 138.9 | 137–145 |
| Potassium (mEq/L) | 3.9 | 3.1–5.3 |
| GOT (mEq/L) | 21.7 | 10–30 |
| GPT (mg/dL) | 18.2 | 2–32 |
| CPK (U/L) | 160 | 13–167 |
| CRP (mg/dL) | 1.04 | <0.5 |
| Hs-Troponin-I (pg/mL) | <10 | <34.2 |
| Category | Modality | Possible Mechanisms | Reference |
|---|---|---|---|
| Electrical management | 1. Conventional treatment | Successful defibrillation affects most of the heart, resulting in too-little remaining heart muscle continuing the arrhythmia. | [7] |
| 2. DSD |
1. Higher energy to overcome the increasing defibrillatory threshold. 2. The first shock lowers the defibrillation threshold, thus increasing the second shock’s success. 3. Alternate vectors of defibrillation provided by the second set of pads may be more likely to stop the myocytes fibrillating. | [4] | |
| Mechanical support | 1. CPR | Restores the partial flow of oxygenated blood to the brain and heart. | [7] |
| 2. ECMO | Normalizes perfusion reliably and provides cardiopulmonary support, to facilitate identification and treatment of the most common cause of refractory arrest (i.e., ACS). | [8] | |
| Drugs | 1. Amiodarone | Class III antiarrhythmic drug: blocks potassium rectifier currents that are responsible for the repolarization of the heart during phase 3 of the cardiac action potential. | [2] |
| 2. Lidocaine | Class Ib antiarrhythmic drug: blocks fast sodium channels responsible for the rapid phase-0 depolarization of the cardiac action potential in non-nodal tissue. | [2] | |
| 3. Epinephrine | Stimulates alpha-adrenergic receptors, resulting in peripheral vasoconstriction, to increase coronary perfusion. | [1] | |
| 4. Beta-blocker | Counteracts the negative beta-adrenergic effects of epinephrine to decrease the sensitivity of the myocardium to arrhythmias. | [3] | |
| 5. Deep Sedation (Propofol) |
1. Reduces sympathetic activity and enhance vagal tone. 2. Direct effect on the cardiomyocyte through changes in protein kinase C translocation to different targets in the cell. | [9] | |
| Miscellaneous | 1. PCI | Restores myocardial blood flow. | [10] |
| 2. SGB | Blocks the stellate ganglion to attenuate noradrenaline signalling (efferent sympathetic outflow) to the heart. | [4] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, N.-S.; Lin, Y.-Y.; Kao, Y.-H.; Chuu, C.-P.; Wu, K.-A.; Chan, J.-S.; Hsiao, P.-J. Combination of Multidisciplinary Therapies Successfully Treated Refractory Ventricular Arrhythmia in a STEMI Patient: Case Report and Literature Review. Healthcare 2022, 10, 507. https://doi.org/10.3390/healthcare10030507
Lin N-S, Lin Y-Y, Kao Y-H, Chuu C-P, Wu K-A, Chan J-S, Hsiao P-J. Combination of Multidisciplinary Therapies Successfully Treated Refractory Ventricular Arrhythmia in a STEMI Patient: Case Report and Literature Review. Healthcare. 2022; 10(3):507. https://doi.org/10.3390/healthcare10030507
Chicago/Turabian StyleLin, Nung-Sheng, Yen-Yue Lin, Yung-Hsi Kao, Chih-Pin Chuu, Kuo-An Wu, Jenq-Shyong Chan, and Po-Jen Hsiao. 2022. "Combination of Multidisciplinary Therapies Successfully Treated Refractory Ventricular Arrhythmia in a STEMI Patient: Case Report and Literature Review" Healthcare 10, no. 3: 507. https://doi.org/10.3390/healthcare10030507
APA StyleLin, N.-S., Lin, Y.-Y., Kao, Y.-H., Chuu, C.-P., Wu, K.-A., Chan, J.-S., & Hsiao, P.-J. (2022). Combination of Multidisciplinary Therapies Successfully Treated Refractory Ventricular Arrhythmia in a STEMI Patient: Case Report and Literature Review. Healthcare, 10(3), 507. https://doi.org/10.3390/healthcare10030507







