Neck and Shoulder Morbidity in Patients with Oral Cancer and Clinically Negative Node Neck Status: A Comparison between the Elective Neck Dissection and Sentinel Lymph Node Biopsy Strategies
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Setting and Participants
2.2. Study Procedure
2.2.1. Shoulder Morbidity
2.2.2. Neck Morbidity
2.2.3. Shoulder and Neck Morbidity
2.2.4. Shoulder and Neck Active Range of Motion
2.2.5. Quality of Life
2.3. Sample Size
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Warnakulasuriya, S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009, 45, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Wells, M.; Swartzman, S.; Lang, H.; Cunningham, M.; Taylor, L.; Thomson, J.; Philp, J.; McCowan, C. Predictors of quality of life in head and neck cancer survivors up to 5 years after end of treatment: A cross-sectional survey. Support Care Cancer 2016, 24, 2463–2472. [Google Scholar] [CrossRef] [PubMed]
- Eickmeyer, S.M.; Walczak, C.K.; Myers, K.B.; Lindstrom, D.R.; Layde, P.; Campbell, B.H. Quality of life, shoulder range of motion, and spinal accessory nerve status in 5-year survivors of head and neck cancer. PM R J. Inj. Funct. Rehabil. 2014, 6, 1073–1080. [Google Scholar] [CrossRef]
- van Hinte, G.; Wetzels, J.G.H.; Merkx, M.A.W.; de Haan, A.F.J.; Koole, R.; Speksnijder, C.M. Factors influencing neck and shoulder function after oral oncology treatment: A five-year prospective cohort study in 113 patients. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2019, 27, 2553–2560. [Google Scholar] [CrossRef] [PubMed]
- Wetzels, J.W.; Merkx, M.A.; de Haan, A.F.; Koole, R.; Speksnijder, C.M. Maximum mouth opening and trismus in 143 patients treated for oral cancer: A 1-year prospective study. Head Neck 2014, 36, 1754–1762. [Google Scholar] [CrossRef]
- Gane, E.M.; McPhail, S.M.; Hatton, A.L.; Panizza, B.J.; O’Leary, S.P. The relationship between physical impairments, quality of life and disability of the neck and upper limb in patients following neck dissection. J. Cancer Surviv. Res. Pract. 2018, 12, 619–631. [Google Scholar] [CrossRef]
- Capozzi, L.C.; Nishimura, K.C.; McNeely, M.L.; Lau, H.; Culos-Reed, S.N. The impact of physical activity on health-related fitness and quality of life for patients with head and neck cancer: A systematic review. Br. J. Sport. Med. 2016, 50, 325–338. [Google Scholar] [CrossRef]
- Bossi, P.; Di Pede, P.; Guglielmo, M.; Granata, R.; Alfieri, S.; Iacovelli, N.A.; Orlandi, E.; Guzzo, M.; Bianchi, R.; Ferella, L.; et al. Prevalence of Fatigue in Head and Neck Cancer Survivors. Ann. Otol. Rhinol. Laryngol. 2019, 128, 413–419. [Google Scholar] [CrossRef]
- Oskam, I.M.; Leeuw, I.M.V.-D.; Aaronson, N.K.; Witte, B.I.; de Bree, R.; Doornaert, P.; Langendijk, J.A.; Leemans, C.R. Prospective evaluation of health-related quality of life in long-term oral and oropharyngeal cancer survivors and the perceived need for supportive care. Oral Oncol. 2013, 49, 443–448. [Google Scholar] [CrossRef]
- Gane, E.M.; McPhail, S.M.; Hatton, A.L.; Panizza, B.J.; O’Leary, S.P. Neck and Shoulder Motor Function following Neck Dissection: A Comparison with Healthy Control Subjects. Otolaryngol. Head Neck Surg. Off. J. Am. Acad. Otolaryngol.-Head Neck Surg. 2019, 160, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, S.J.; Yang, X.; Peng, H. Diagnostic Efficacy of Sentinel Lymph Node Biopsy in Early Oral Squamous Cell Carcinoma: A Meta-Analysis of 66 Studies. PLoS ONE 2017, 12, e0170322. [Google Scholar] [CrossRef] [PubMed]
- Robbins, K.T.; Clayman, G.; Levine, P.A.; Medina, J.; Sessions, R.; Shaha, A.; Som, P.; Wolf, G.T.; The Committee for Head and Neck Surgery and Oncology, American Academy of Otolaryngology–Head and Neck Surgery. Neck dissection classification update: Revisions proposed by the American Head and Neck Society and the American Academy of Otolaryngology-Head and Neck Surgery. Arch. Otolaryngol. Head Neck Surg. 2002, 128, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Garrel, R.; Poissonnet, G.; Moyà Plana, A.; Fakhry, N.; Dolivet, G.; Lallemant, B.; Sarini, J.; Vergez, S.; Guelfucci, B.; Choussy, O.; et al. Equivalence Randomized Trial to Compare Treatment on the Basis of Sentinel Node Biopsy versus Neck Node Dissection in Operable T1-T2N0 Oral and Oropharyngeal Cancer. J. Clin. Oncol. 2020, 38, Jco2001661. [Google Scholar] [CrossRef] [PubMed]
- Moya-Plana, A.; Aupérin, A.; Guerlain, J.; Gorphe, P.; Casiraghi, O.; Mamelle, G.; Melkane, A.; Lumbroso, J.; Janot, F.; Temam, S. Sentinel node biopsy in early oral squamous cell carcinomas: Long-term follow-up and nodal failure analysis. Oral Oncol. 2018, 82, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Cramer, J.D.; Sridharan, S.; Ferris, R.L.; Duvvuri, U.; Samant, S. Sentinel Lymph Node Biopsy Versus Elective Neck Dissection for Stage I to II Oral Cavity Cancer. Laryngoscope 2019, 129, 162–169. [Google Scholar] [CrossRef]
- (IKNL) NCCO; Dutch Head and Neck Society (NWHHT). National Guideline Oral Cavity- and Oropharyngeal Carcinoma (Mondholte-en Orofarynxcarcinoom), version 1.4; IKNL: Utrecht, The Netherlands, 2004. [Google Scholar]
- Murer, K.; Huber, G.F.; Haile, S.R.; Stoeckli, S.J. Comparison of morbidity between sentinel node biopsy and elective neck dissection for treatment of the n0 neck in patients with oral squamous cell carcinoma. Head Neck 2011, 33, 1260–1264. [Google Scholar] [CrossRef] [PubMed]
- Govers, T.M.; Schreuder, W.; Klop, W.; Grutters, J.; Rovers, M.; Merkx, M.A.; Takes, R.P. Quality of life after different procedures for regional control in oral cancer patients: Cross-sectional survey. Clin. Otolaryngol. 2016, 41, 228–233. [Google Scholar] [CrossRef]
- van Hinte, G.; Tolunay, S.; Weijs, W.L.J.; Merkx, M.A.W.; Leijendekkers, R.A.; Nijhuis-van der Sanden, M.W.G.; Takes, R.; Speksnijder, C.M.; Withagen, K.P.A.; De Bree, R.; et al. Effect of elective neck dissection versus sentinel lymph node biopsy on shoulder morbidity and health-related quality of life in patients with oral cavity cancer: A longitudinal comparative cohort study. Oral Oncology. 2021, 122, 105510. [Google Scholar] [CrossRef]
- Flach, G.B.; Leeuw, I.M.V.-D.; Witte, B.I.; Klop, W.M.C.; van Es, R.J.; Schepman, K.-P.; de Bree, R. Patients’ perspective on the impact of sentinel node biopsy in oral cancer treatment. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 122, 279–286. [Google Scholar] [CrossRef]
- Crocetta, F.M.; Botti, C.; Pernice, C.; Murri, D.; Castellucci, A.; Menichetti, M.; Costantini, M.; Venturelli, F.; Bassi, M.C.; Ghidini, A. Sentinel node biopsy versus elective neck dissection in early-stage oral cancer: A systematic review. Eur. Arch. Otorhinolaryngol. 2020, 277, 3247–3260. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Prev. Med. 2007, 45, 247–251. [Google Scholar] [CrossRef] [PubMed]
- van der Heijden, G.J.; Leffers, P.; Bouter, L.M. Shoulder disability questionnaire design and responsiveness of a functional status measure. J. Clin. Epidemiol. 2000, 53, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Roach, K.E.; Budiman-Mak, E.; Songsiridej, N.; Lertratanakul, Y. Development of a shoulder pain and disability index. Arthritis Care Res. 1991, 4, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Jorritsma, W.; de Vries, G.E.; Dijkstra, P.U.; Geertzen, J.H.; Reneman, M.F. Neck Pain and Disability Scale and Neck Disability Index: Validity of Dutch language versions. Eur. Spine J. 2012, 21, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.J.; Chepeha, J.C.; Teknos, T.N.; Bradford, C.R.; Sharma, P.K.; Terrell, J.E.; Hogikyan, N.D.; Wolf, G.T.; Chepeha, D.B. Development and validation of the neck dissection impairment index: A quality of life measure. Arch. Otolaryngol. Head Neck Surg. 2002, 128, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Stuiver, M.M.; Tusscher, M.R.T.; Van Opzeeland, A.; Brendeke, W.; Lindeboom, R.; Dijkstra, P.U.; Aaronson, N.K. Psychometric properties of 3 patient-reported outcome measures for the assessment of shoulder disability after neck dissection. Head Neck 2016, 38, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.P.; Gore, D.R.; Gardner, G.M.; Mollinger, L.A. Shoulder motion and muscle strength of normal men and women in two age groups. Clin. Orthop. Relat. Res. 1985, 192, 268–273. [Google Scholar] [CrossRef]
- Whitcroft, K.L.; Massouh, L.; Amirfeyz, R.; Bannister, G. Comparison of methods of measuring active cervical range of motion. Spine 2010, 35, E976–E980. [Google Scholar] [CrossRef]
- Fayers, P.; Bottomley, A. Quality of life research within the EORTC-the EORTC QLQ-C30. European Organisation for Research and Treatment of Cancer. Eur. J. Cancer 2002, 38 (Suppl. S4), S125–S133. [Google Scholar] [CrossRef]
- Bjordal, K.; Hammerlid, E.; Ahlner-Elmqvist, M.; De Graeff, A.; Boysen, M.; Evensen, J.F.; Biorklund, A.; De Leeuw, J.R.J.; Fayers, P.M.; Jannert, M.; et al. Quality of life in head and neck cancer patients: Validation of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-H&N35. J. Clin. Oncol. 1999, 17, 1008–1019. [Google Scholar] [PubMed]
- van Hinte, G.; Leijendekkers, R.A.; Te Molder, B.; Jansen, L.; Bol, C.; Merkx, M.A.; Takes, R.; Nijhuis-van der Sanden, M.W.; Speksnijder, C.M. Reproducibility of measurements on physical performance in head and neck cancer survivors; measurements on maximum mouth opening, shoulder and neck function, upper and lower body strength, level of physical mobility, and walking ability. PLoS ONE 2020, 15, e0233271. [Google Scholar] [CrossRef] [PubMed]
- Kelley, M.J.; Kane, T.E.; Leggin, B.G. Spinal accessory nerve palsy: Associated signs and symptoms. J. Orthop. Sport. Phys. Ther. 2008, 38, 78–86. [Google Scholar] [CrossRef] [PubMed]
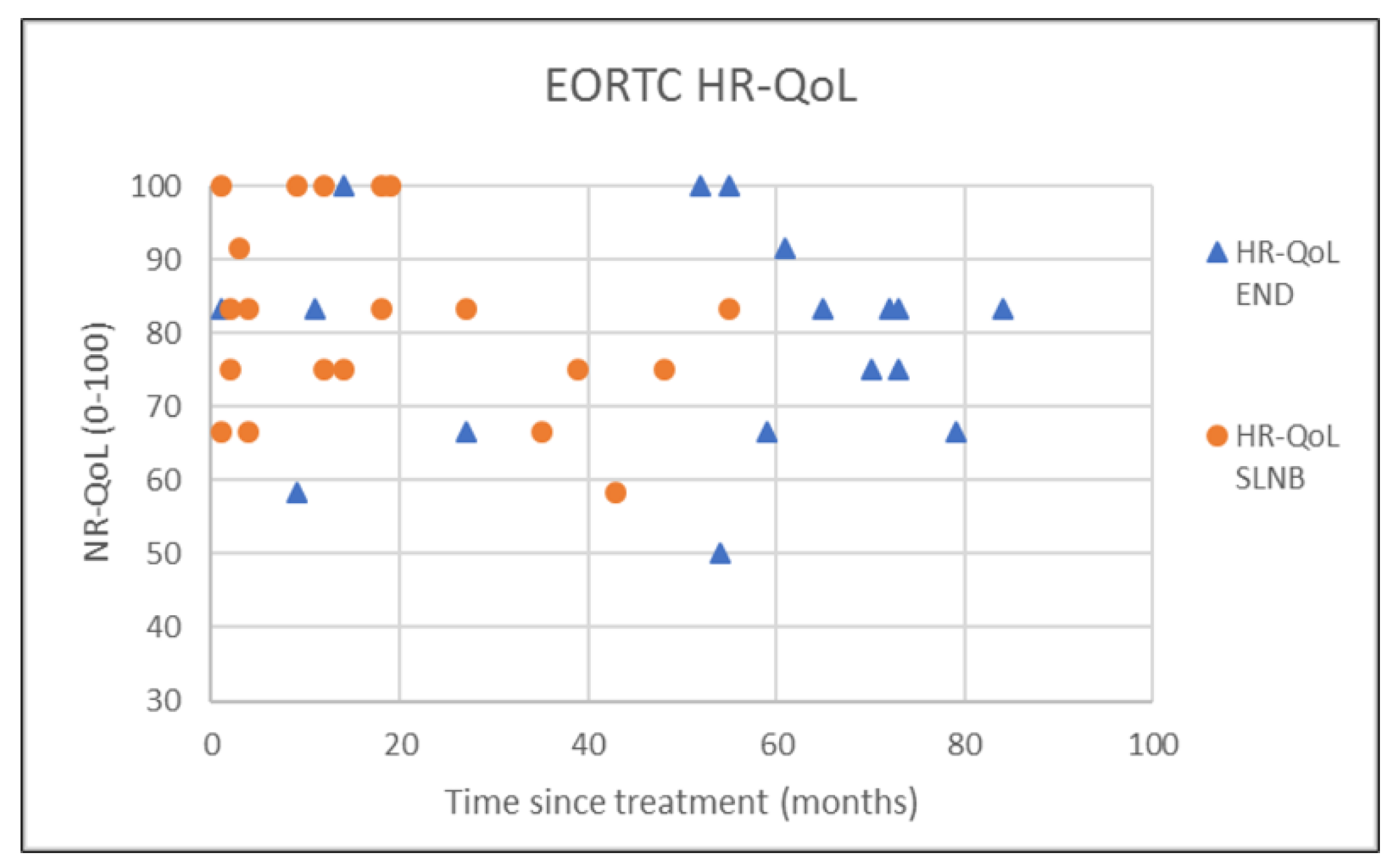
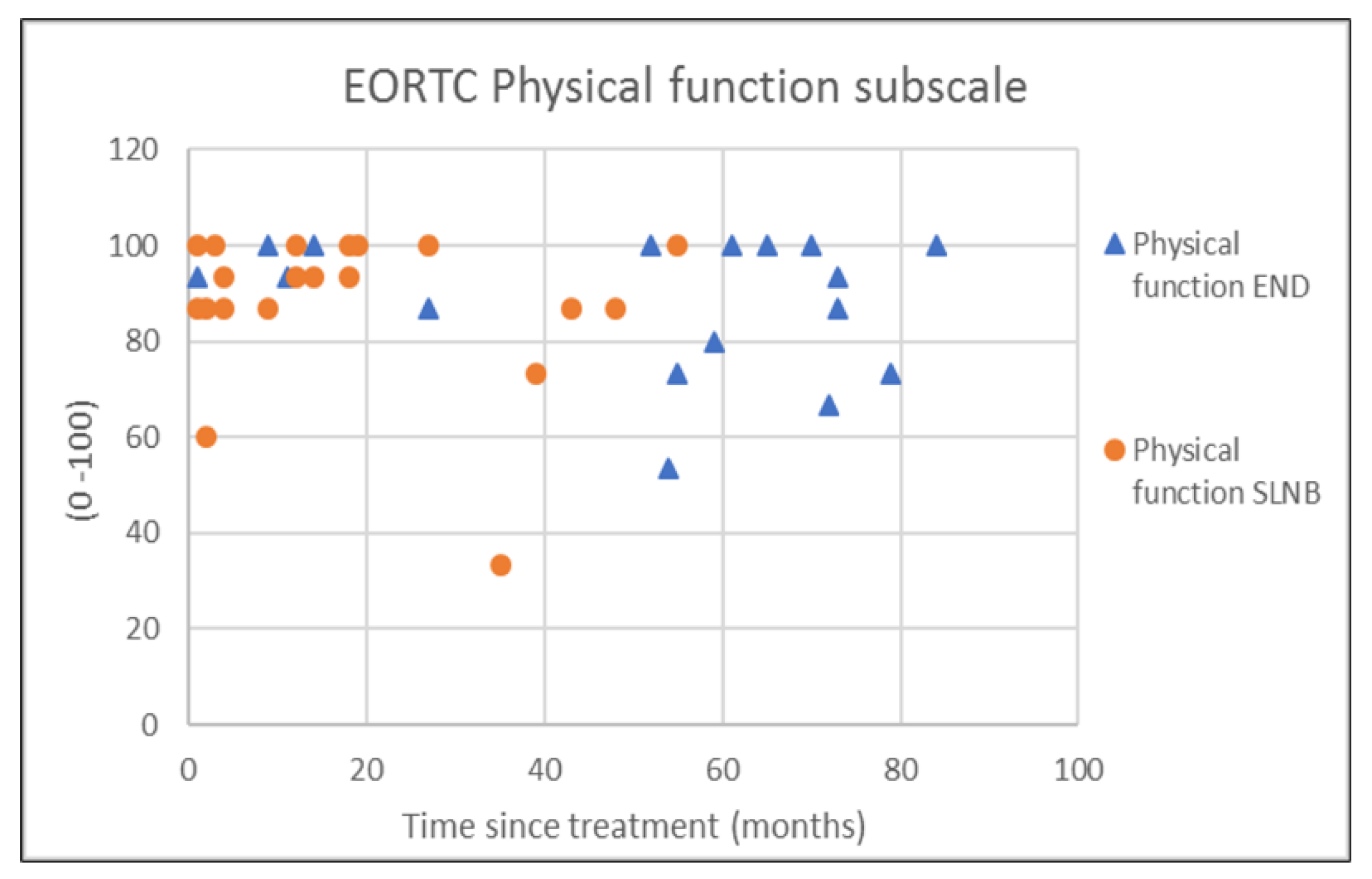
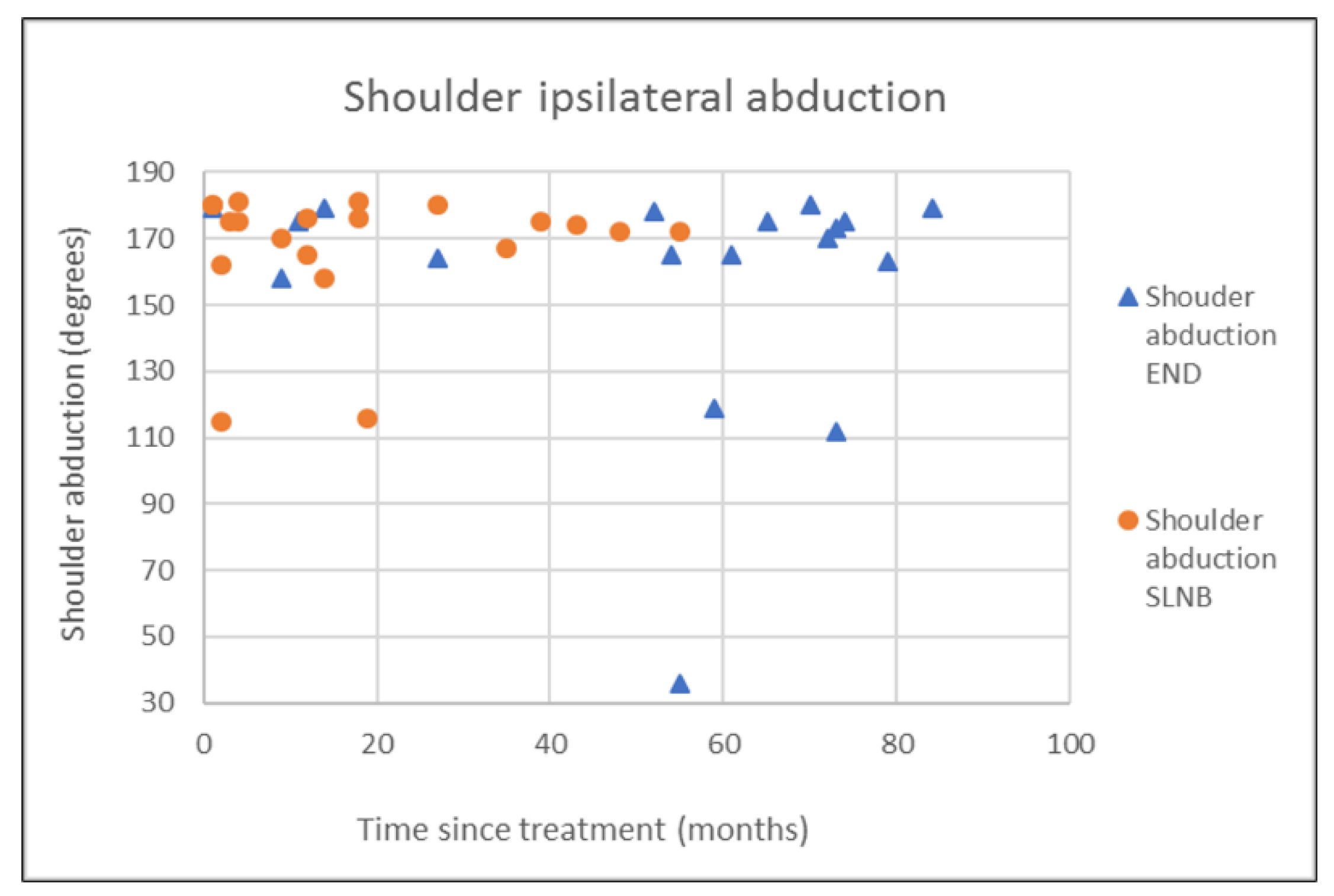
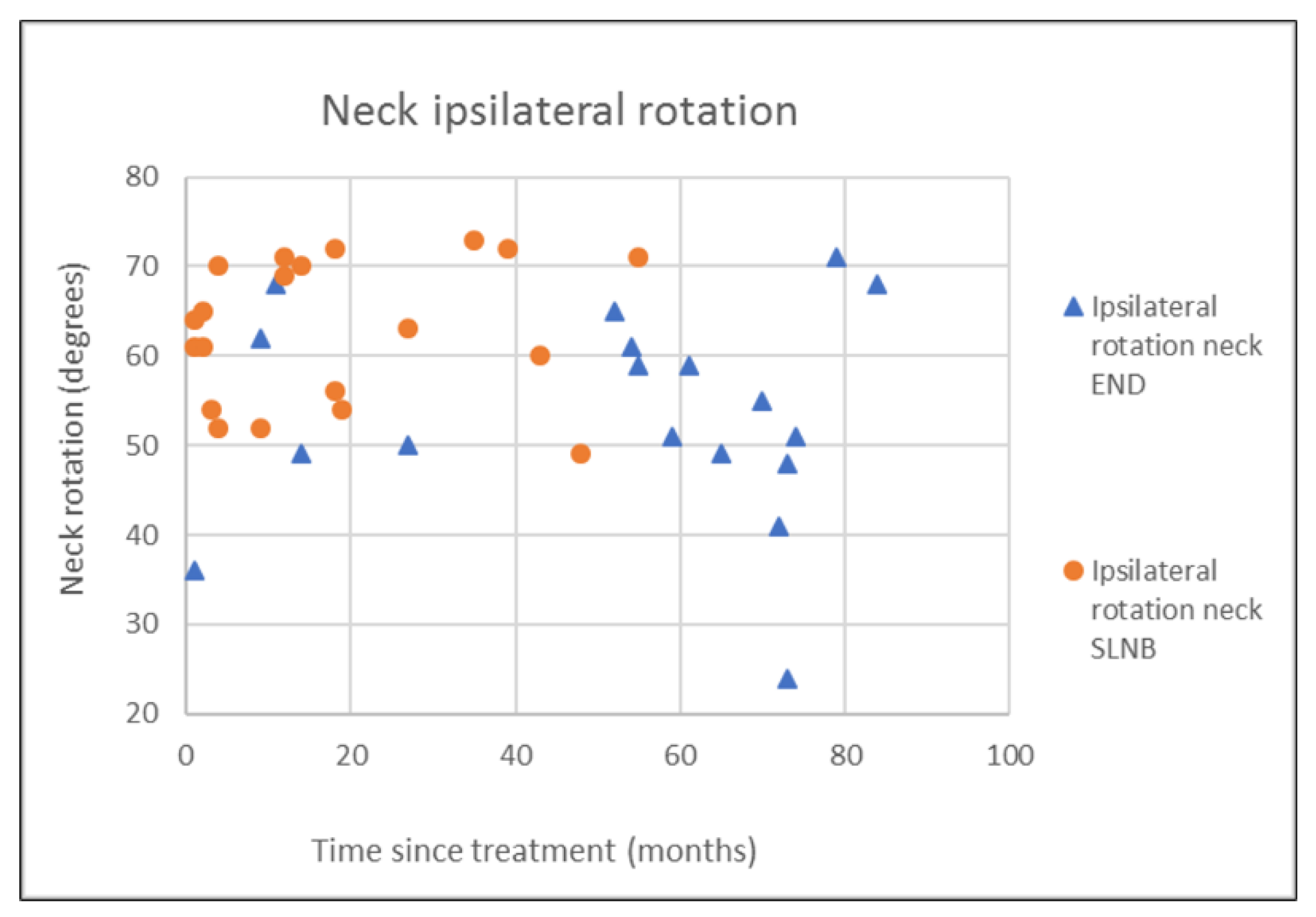
| END (N = 18) | SLNB (N = 20) | p-Value | |
|---|---|---|---|
| Sex (n, %) | 0.299 ˠ | ||
| Male | 12 (66.7) | 10 (50.0) | |
| Female | 6 (33.3) | 10 (50.0) | |
| Age in years (median, IQR) | 72.0 (11.0) | 63.5 (16.5) | 0.001 †** |
| BMI (median, IQR) | 24.8 (6.4) | 26.4 (8.0) | 0.907 ° |
| Time since treatment in months (median, IQR) | 60.0 (49.3) | 13.0 (29.8) | 0.000 †*** |
| Treated side (n, %) | 0.107 ˠ | ||
| Left | 11 (61.1) | 7 (35.0) | |
| Right | 7 (38.9) | 13 (65.0) | |
| TNM-stage (n, %) | 0.552 ˠ | ||
| cT1 | 10 (55.6) | 13 (65.0) | |
| cT2 | 8 (44.4) | 7 (35.0) | |
| Tumor location (n, %) | 0.032 ˠ* | ||
| Mandible | 4 (22.0) | 2 (10.0) | |
| Tongue/flour of mouth | 10 (55.6) | 18 (90.0) | |
| Buccal mucosa | 4 (22.0) | 0 (0.0) | |
| Pack years (median, IQR) | 6.7 (38.8) | 1.1 (37.5) | 0.613 † |
| Alcohol use in units daily (median, IQR) | 1.0 (1.6) | 0.0 (1.6) | 0.346 † |
| Post-operative physiotherapy (n, %) | 0.024 ˠ* | ||
| Yes | 6 (33.3) | 1 (5.0) | |
| No | 12 (66.7) | 19 (95.0) | |
| Numbers of physiotherapy treatment (median, IQR) | 0.0 (27.5) | 0.0 (0.0) | 0.022 †* |
| END (N = 18) | SLNB (N = 20) | ||||
|---|---|---|---|---|---|
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | p-Value | |
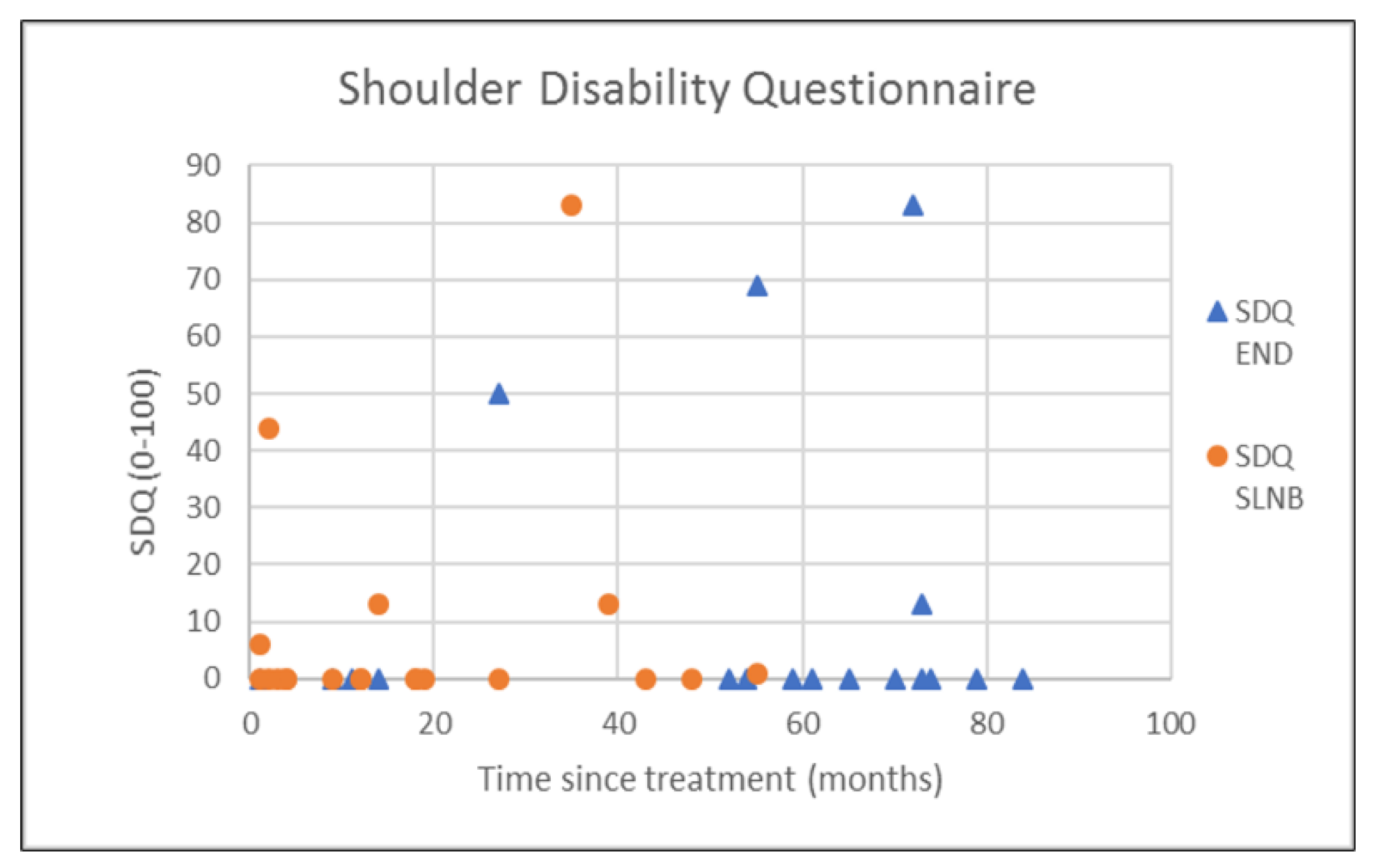
| SDQ | 11.9 (26.3) | 0.0 (3.25) | 8.0 (20.4) | 0.0 (4.75) | 0.828 † |
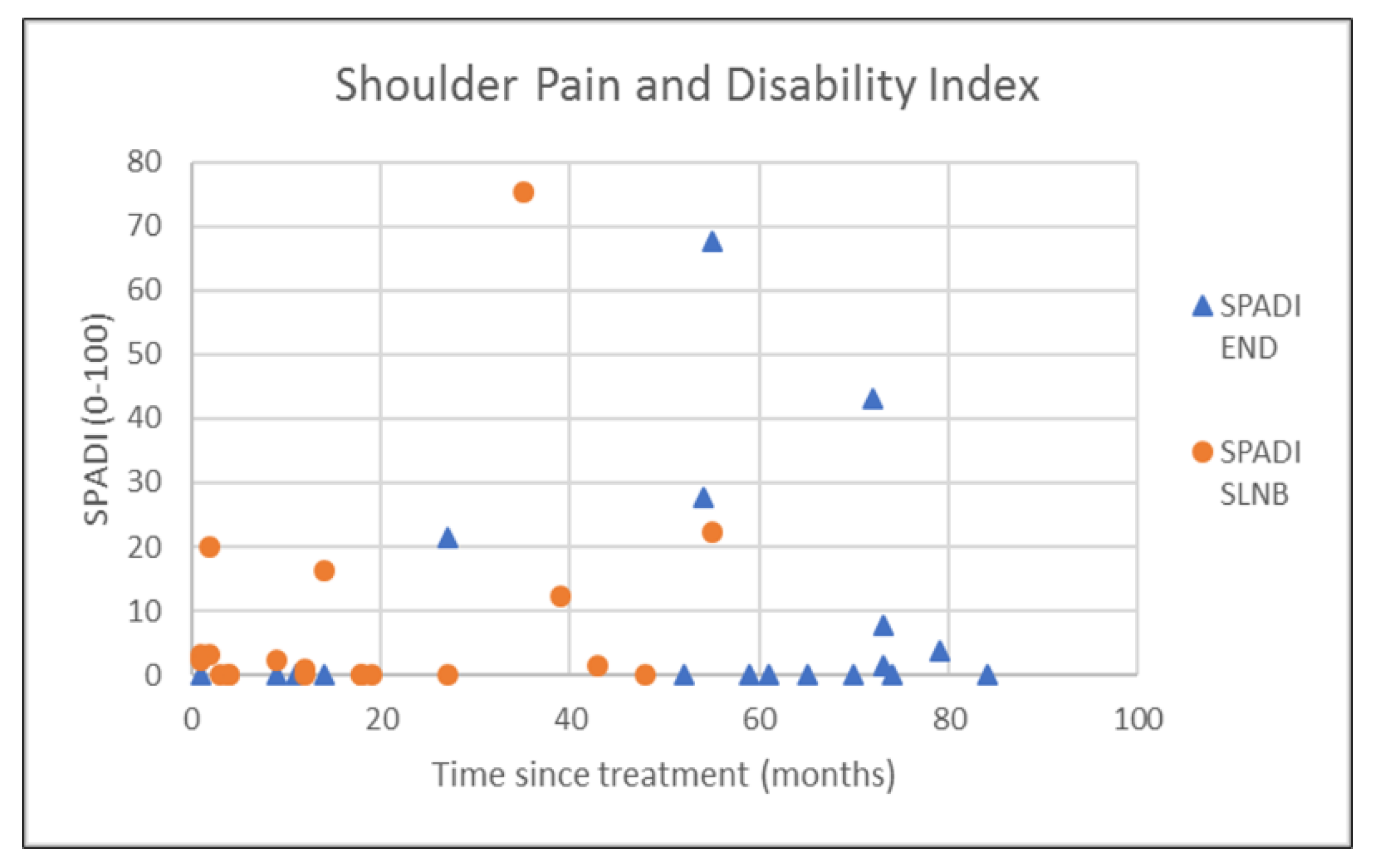
| SPADI | 9.6 (18.9) | 0.0 (11.2) | 8.0 (17.4) | 1.2 (10.0) | 0.635 † |
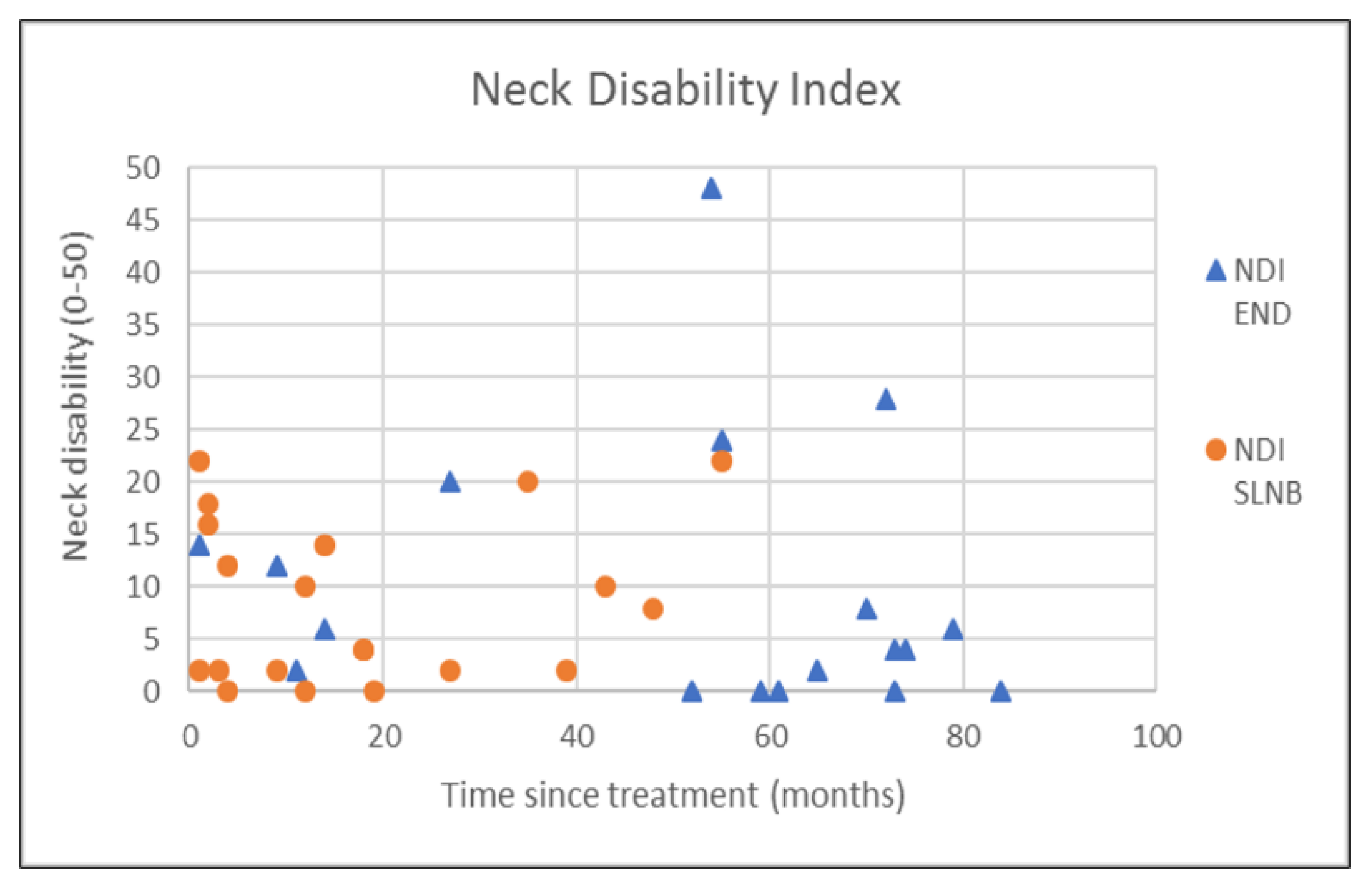
| NDI | 9.9 (12.9) | 5.0 (15.5) | 8.5 (7.8) | 6.0 (13.5) | 0.825 † |
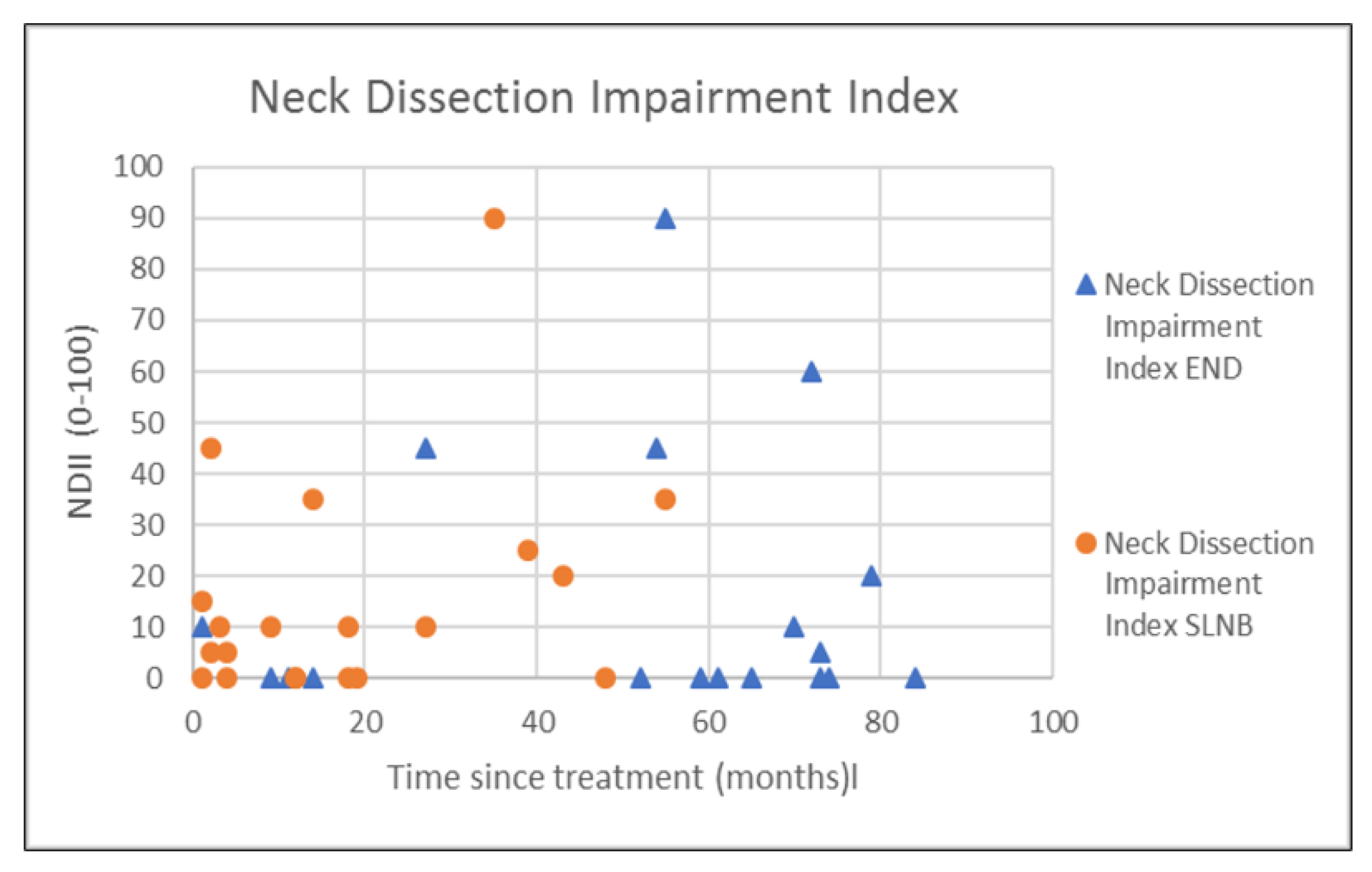
| NDII | 7.9 (13.2) | 0.0 (13.1) | 7.9 (11.1) | 5.0 (11.9) | 0.442 † |
| AROM shoulder | |||||
| External rotation ipsi | 62.1 (14.8) | 64.0 (19.8) | 63.5 (15.6) | 63.5 (19.5) | 0.788 ° |
| Abduction ipsi | 158.1 (36.0) | 171.5 (16.5) | 167.5 (18.9) | 174.5 (13.5) | 0.237 † |
| Forward flexion ipsi | 168.3 (17.4) | 174.0 (11.7) | 174.6 (7.1) | 175.0 (7.5) | 0.176 † |
| AROM neck | |||||
| Rotation ipsi | 53.7 (12.0) | 53.0 (14.0) | 63.0 (7.9) | 63.5 (16.3) | 0.008 °** |
| Rotation contra | 55.8 (11.0) | 59.0 (11.7) | 62.5 (6.7) | 62.0 (7.5) | 0.029 °* |
| Flexion | 49.7 (12.0) | 50.0 (14.5) | 56.5 (11.6) | 52.5 (15.0) | 0.086 ° |
| Extension | 53.1 (10.4) | 52.0 (18.6) | 56.8 (14.1) | 59.0 (24.7) | 0.362 ° |
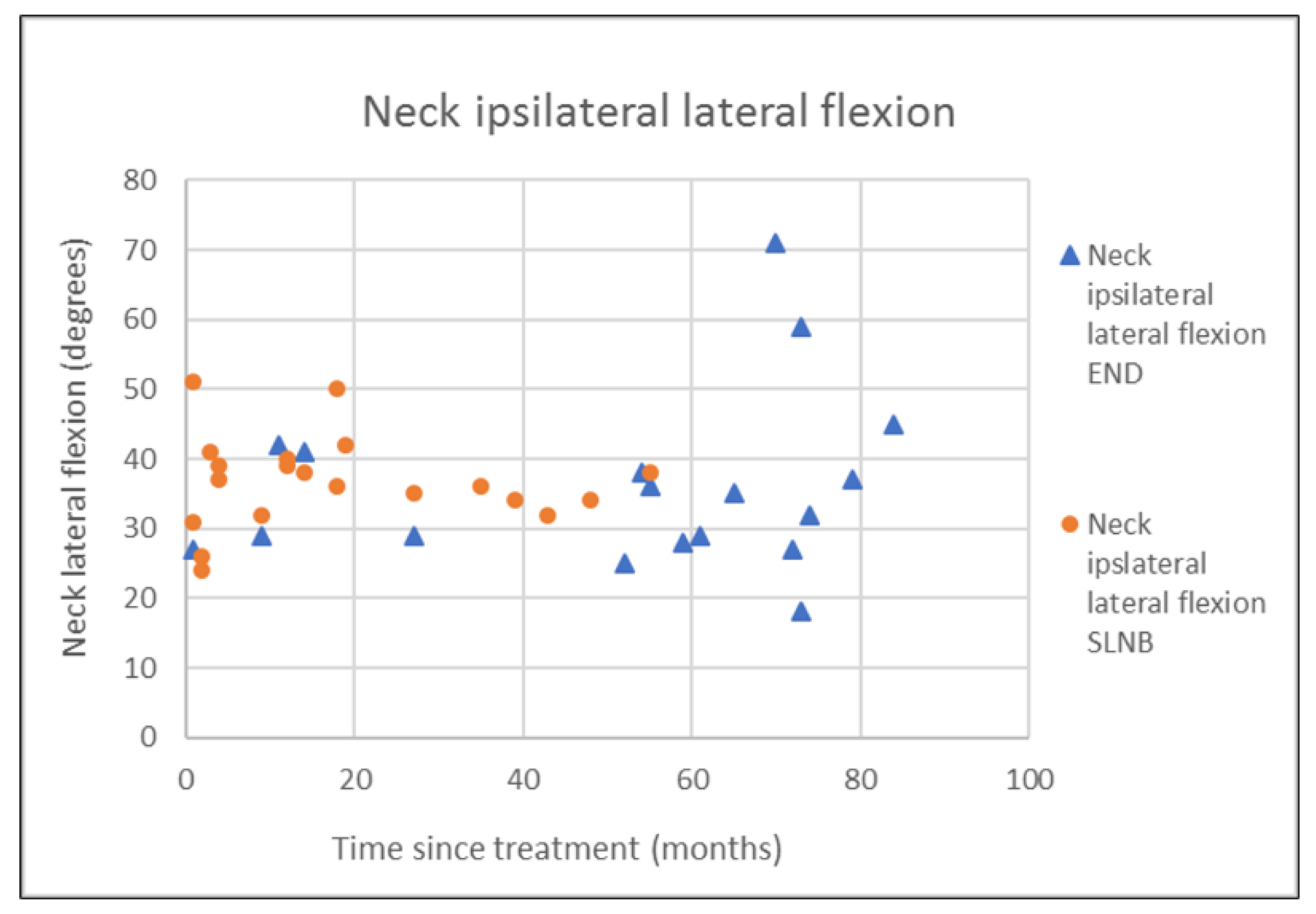
| Lateral flexion ipsi | 36.0 (12.7) | 33.5 (13.5) | 36.8 (6.6) | 36.5 (7.3) | 0.349 † |
| Lateral flexion contra | 36.0 (13.5) | 35.5 (12.5) | 38.4 (5.5) | 38.0 (6.7) | 0.468 ° |
| EORTC-QLQ-C30 ¥ | |||||
| Global Quality of Life | 79.4 (14.5) | 83.3 (20.8) | 82.1 (13.0) | 83.3 (22.9) | 0.729 † |
| Physical functioning | 88.2 (14.2) | 93.3 (23.3) | 88.0 (16.4) | 93.3 (13.3) | 0.964 † |
| Role functioning | 81.4 (28.2) | 100.0 (33.3) | 85.8 (26.6) | 100.0 (29.2) | 0.598 † |
| Emotional functioning | 84.3 (20.8) | 91.7 (29.2) | 82.5 (19.5) | 87.5 (31.3) | 0.707 † |
| Cognitive functioning | 83.3 (25.0) | 100.0 (16.7) | 89.2 (9.8) | 83.3 (16.7) | 0.845 † |
| Social functioning | 89.2 (17.7) | 100.0 (16.7) | 89.2 (17.3) | 100.0 (16.7) | 0.916 † |
| EORTC-QLQ-HN35 | |||||
| Oral Pain | 15.7 (21.6) | 0.0 (29.2) | 15.0 (17.0) | 8.3 (25.0) | 0.675 † |
| Swallowing problems | 12.0 (22.0) | 0.0 (16.7) | 8.7 (12.5) | 0.0 (20.8) | 0.988 † |
| Senses problems | 10.2 (19.1) | 0.0 (8.3) | 11.7 (14.4) | 8.3 (16.7) | 0.426 † |
| Speech problems | 14.2 (23.7) | 0.0 (33.3) | 13.9 (13.4) | 11.1 (22.2) | 0.317 † |
| Trouble with social eating | 19.4 (30.4) | 0.0 (25.0) | 9.2 (11.8) | 0.0 (22.9) | 0.534 † |
| Trouble with social contact | 6.3 (9.8) | 0.0 (13.3) | 3.3 (8.2) | 0.0 (0.0) | 0.313 † |
| Less sexuality | 8.3 (20.0) | 0.0 (0.0) | 15.8 (22.6) | 0.0 (33.3) | 0.276 † |
| Teeth problems | 19.3 (15.4) | 0.0 (33.3) | 11.7 (24.8) | 0.0 (25.0) | 0.942 † |
| Trouble with opening mouth | 14.8 (23.5) | 0.0 (33.3) | 13.3 (27.4) | 0.0 (25.0) | 0.718 † |
| Dry mouth | 33.3 (32.3) | 33.3 (66.7) | 23.3 (24.4) | 33.3 (33.0) | 0.409 † |
| Sticky saliva | 12.3 (23.3) | 0.0 (33.3) | 10.0 (15.7) | 0.0 (33.3) | 0.965 † |
| Coughing | 16.7 (23.6) | 0.0 (33.3) | 20.0 (29.4) | 0.0 (33.0) | 0.874 † |
| Feeling ill | 5.6 (12.8) | 0.0 (0.0) | 15.0 (22.9) | 0.0 (33.0) | 0.303 † |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Hinte, G.; Withagen, K.P.A.; de Bree, R.; Speksnijder, C.M. Neck and Shoulder Morbidity in Patients with Oral Cancer and Clinically Negative Node Neck Status: A Comparison between the Elective Neck Dissection and Sentinel Lymph Node Biopsy Strategies. Healthcare 2022, 10, 2555. https://doi.org/10.3390/healthcare10122555
van Hinte G, Withagen KPA, de Bree R, Speksnijder CM. Neck and Shoulder Morbidity in Patients with Oral Cancer and Clinically Negative Node Neck Status: A Comparison between the Elective Neck Dissection and Sentinel Lymph Node Biopsy Strategies. Healthcare. 2022; 10(12):2555. https://doi.org/10.3390/healthcare10122555
Chicago/Turabian Stylevan Hinte, Gerben, Koen P. A. Withagen, Remco de Bree, and Caroline M. Speksnijder. 2022. "Neck and Shoulder Morbidity in Patients with Oral Cancer and Clinically Negative Node Neck Status: A Comparison between the Elective Neck Dissection and Sentinel Lymph Node Biopsy Strategies" Healthcare 10, no. 12: 2555. https://doi.org/10.3390/healthcare10122555
APA Stylevan Hinte, G., Withagen, K. P. A., de Bree, R., & Speksnijder, C. M. (2022). Neck and Shoulder Morbidity in Patients with Oral Cancer and Clinically Negative Node Neck Status: A Comparison between the Elective Neck Dissection and Sentinel Lymph Node Biopsy Strategies. Healthcare, 10(12), 2555. https://doi.org/10.3390/healthcare10122555






