Local Tranexamic Acid for Preventing Hemorrhage in Anticoagulated Patients Undergoing Dental and Minor Oral Procedures: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
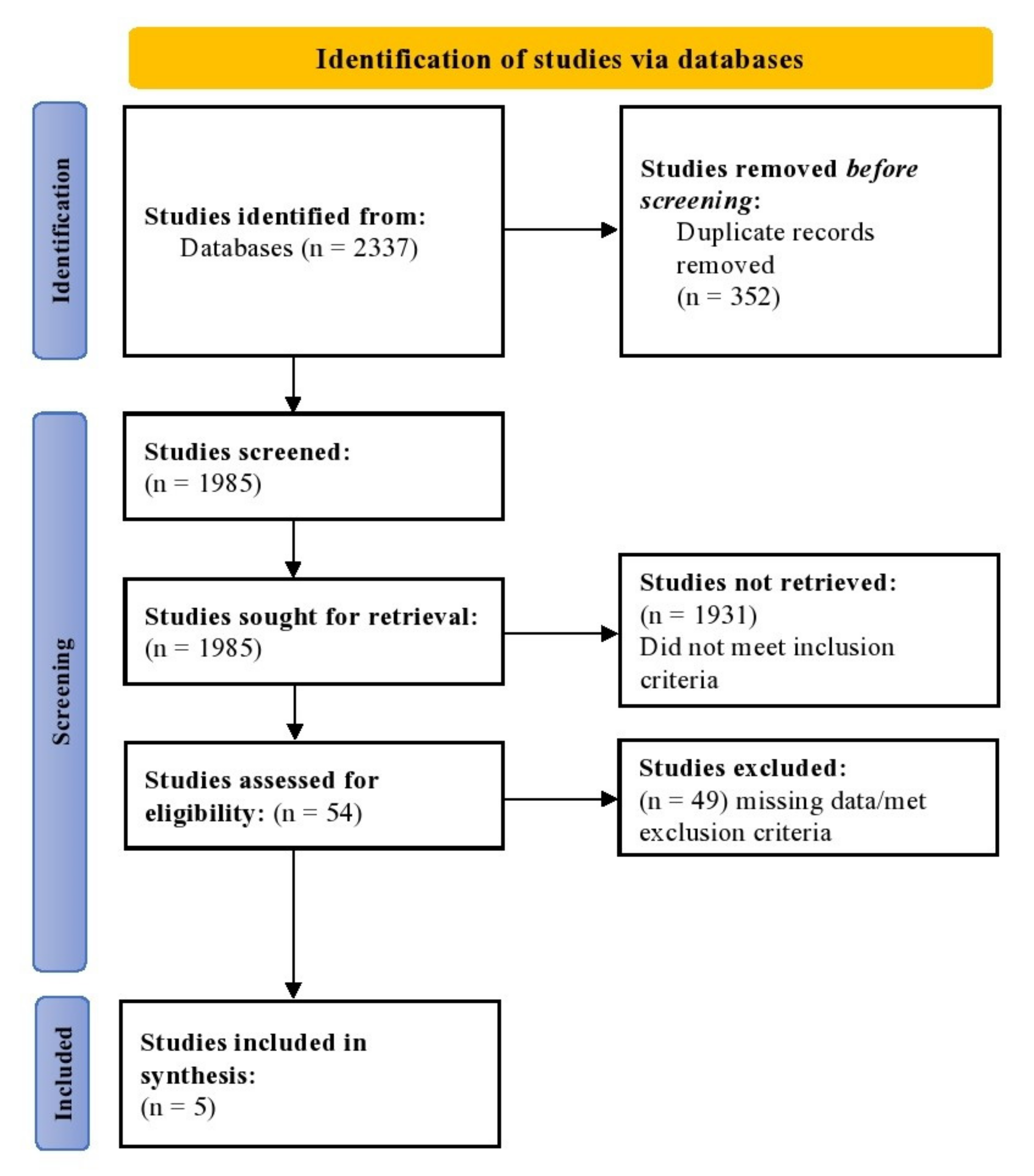
2.1. Search Strategy and Study Selection
2.2. Outcomes
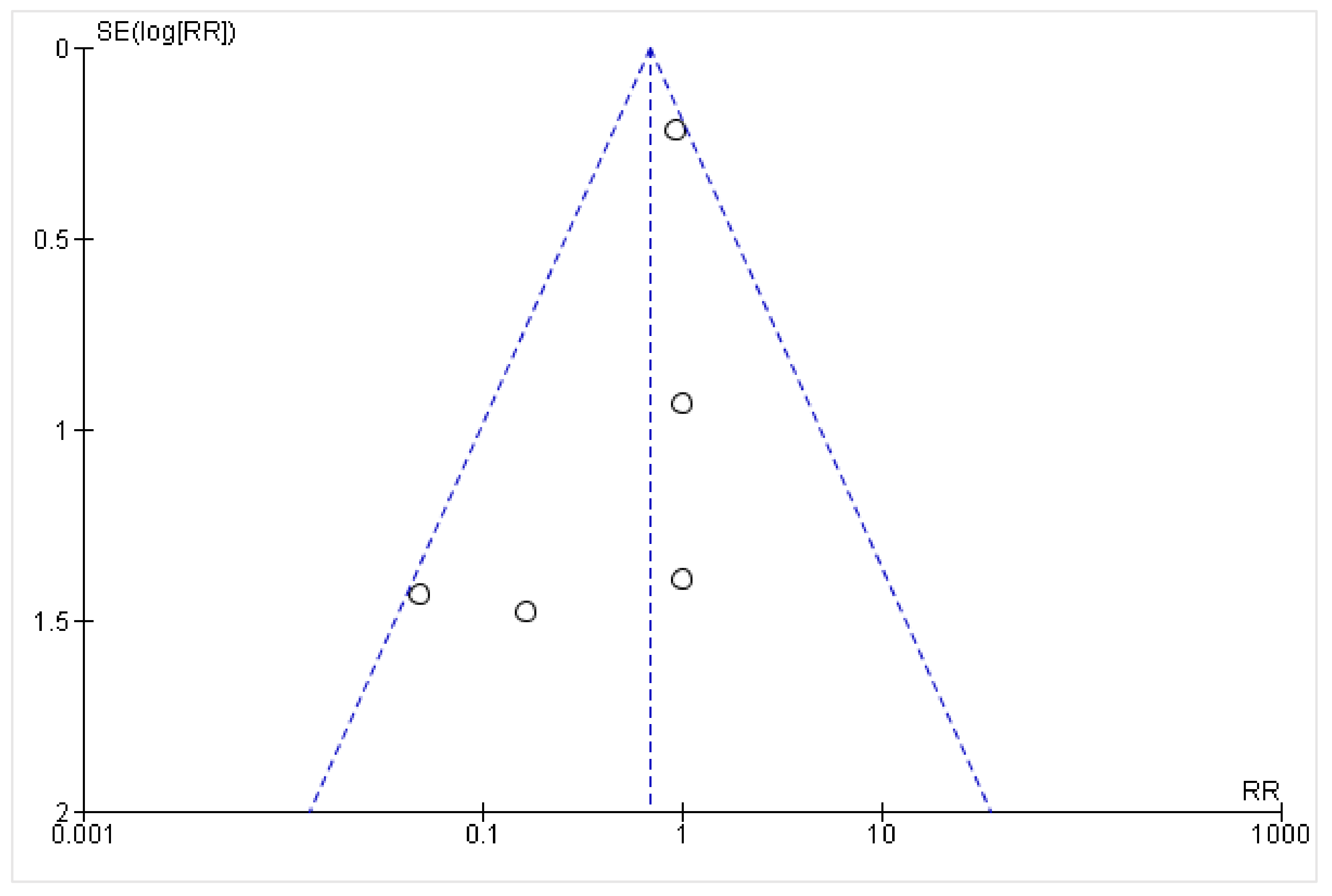
2.3. Data Analysis
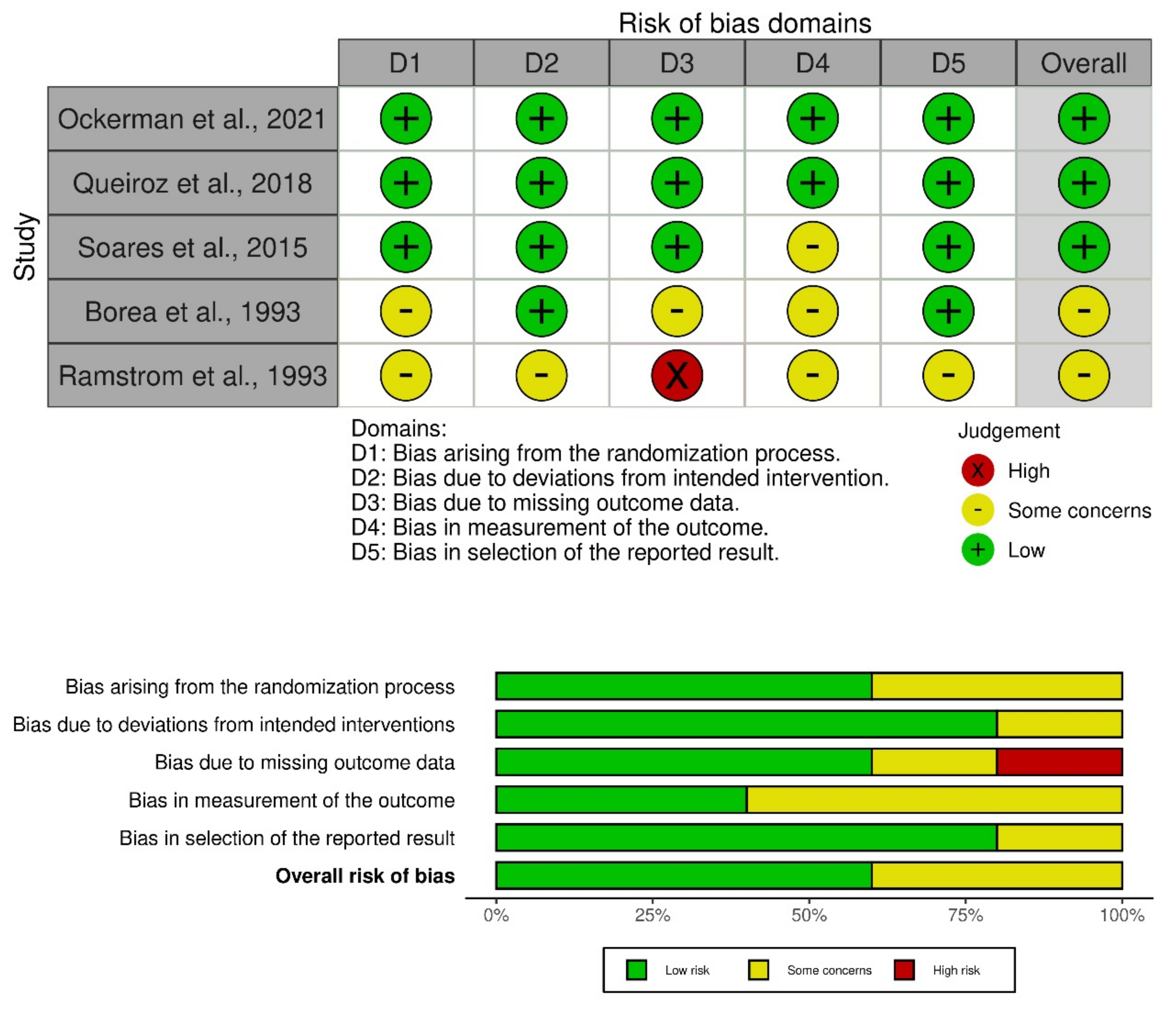
2.4. Risk of Bias Assessment
2.5. Role of Funding and Ethical Approval
3. Results
4. Discussion
4.1. Key Pharmacological Interactions of Tranexamic Acid
4.2. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Task Force Members; Lip, G.Y.H.; Windecker, S.; Huber, K.; Kirchhof, P.; Marin, F.; Ten Berg, J.M.; Haeusler, K.G.; Boriani, G.; Capodanno, D.; et al. Management of Antithrombotic Therapy in Atrial Fibrillation Patients Presenting with Acute Coronary Syndrome and/or Undergoing Percutaneous Coronary or Valve Interventions: A Joint Consensus Document of the European Society of Cardiology Working Group On. Eur. Heart J. 2014, 35, 3155–3179. [Google Scholar]
- Kumbhani, D.J.; Cannon, C.P.; Beavers, C.J.; Bhatt, D.L.; Cuker, A.; Gluckman, T.J.; Marine, J.E.; Mehran, R.; Messe, S.R.; Patel, N.S.; et al. 2020 ACC Expert Consensus Decision Pathway for Anticoagulant and Antiplatelet Therapy in Patients with Atrial Fibrillation or Venous Thromboembolism Undergoing Percutaneous Coronary Intervention or with Atherosclerotic Cardiovascular Disease: A Report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2021, 77, 629–658. [Google Scholar]
- Chan, N.; Sobieraj-Teague, M.; Eikelboom, J.W. Direct Oral Anticoagulants: Evidence and Unresolved Issues. Lancet 2020, 396, 1767–1776. [Google Scholar] [CrossRef] [PubMed]
- Caturano, A.; Galiero, R.; Pafundi, P.C. Atrial Fibrillation and Stroke. A Review on the Use of Vitamin K Antagonists and Novel Oral Anticoagulants. Medicina 2019, 55, 617. [Google Scholar] [CrossRef] [PubMed]
- Mingarro-de-León, A.; Chaveli-López, B.; Gavaldá-Esteve, C. Dental Management of Patients Receiving Anticoagulant and/or Antiplatelet Treatment. J. Clin. Exp. Dent. 2014, 6, e155–e161. [Google Scholar] [CrossRef] [PubMed]
- Wahl, M.J. Dental Surgery in Anticoagulated Patients. Arch. Intern. Med. 1998, 158, 1610–1616. [Google Scholar] [CrossRef]
- Lombardi, N.; Varoni, E.M.; Sorrentino, D.; Lodi, G. International Normalized Ratio (INR) Values in Patients Receiving Oral Vitamin K Antagonists and Undergoing Oral Surgery: A Clinical Audit. Spec. Care Dent. 2020, 40, 374–381. [Google Scholar] [CrossRef]
- Martinez, M.; Tsakiris, D.A. Management of Antithrombotic Agents in Oral Surgery. Dent. J. 2015, 3, 93–101. [Google Scholar] [CrossRef]
- Engelen, E.T.; Schutgens, R.E.G.; Mauser-Bunschoten, E.P.; van Es, R.J.J.; van Galen, K.P.M. Antifibrinolytic Therapy for Preventing Oral Bleeding in People on Anticoagulants Undergoing Minor Oral Surgery or Dental Extractions. Cochrane Database Syst. Rev. 2018, 7, 16–19. [Google Scholar] [CrossRef]
- Chisci, G. Sutureless Technique in Third Molar Surgery: An Overview. J. Craniofac. Surg. 2013, 24, 2210–2211. [Google Scholar] [CrossRef]
- Chisci, G.; Parrini, S.; Capuano, A. The Use of Suture-Less Technique Following Third Molar Surgery. Int. J. Oral Maxillofac. Surg. 2013, 42, 150–151. [Google Scholar] [CrossRef]
- Chisci, G.; Capuano, A.; Parrini, S. Alveolar Osteitis and Third Molar Pathologies. J. Oral Maxillofac. Surg. 2018, 76, 235–236. [Google Scholar] [CrossRef]
- Yang, J.-H.; Chen, H.-M.; Kuo, Y.-S.; Chiang, C.-P. Management of Patients Taking Antithrombotic Drugs before Dental Surgery. J. Dent. Sci. 2020, 15, 222. [Google Scholar] [CrossRef]
- Tomaselli, G.F.; Mahaffey, K.W.; Cuker, A.; Dobesh, P.P.; Doherty, J.U.; Eikelboom, J.W.; Florido, R.; Hucker, W.; Mehran, R.; Messé, S.R.; et al. 2017 ACC Expert Consensus Decision Pathway on Management of Bleeding in Patients on Oral Anticoagulants: A Report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J. Am. Coll. Cardiol. 2017, 70, 3042–3067. [Google Scholar] [CrossRef]
- Lusk, K.A.; Snoga, J.L.; Benitez, R.M.; Sarbacker, G.B. Management of Direct-Acting Oral Anticoagulants Surrounding Dental Procedures with Low-to-Moderate Risk of Bleeding. J. Pharm. Pract. 2018, 31, 202–207. [Google Scholar] [CrossRef]
- Chahine, J.; Khoudary, M.N.; Nasr, S. Anticoagulation Use Prior to Common Dental Procedures: A Systematic Review. Cardiol. Res. Pract. 2019, 2019, 9308631. [Google Scholar] [CrossRef]
- Bajkin, B.V.; Wahl, M.J.; Miller, C.S. Dental Implant Surgery and Risk of Bleeding in Patients on Antithrombotic Medications: A Review of the Literature. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2020, 130, 522–532. [Google Scholar] [CrossRef]
- De Andrade, N.K.; Motta, R.H.L.; Bergamaschi, C.d.C.; Oliveira, L.B.; Guimarães, C.C.; Araujo, J.d.O.; Lopes, L.C. Bleeding Risk in Patients Using Oral Anticoagulants Undergoing Surgical Procedures in Dentistry: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2019, 10, 866. [Google Scholar] [CrossRef]
- Ullah, K.; Mukhtar, H.; Khalid, U.; Sarfraz, Z.; Sarfraz, A. Is Antifibrinolytic Therapy Effective for Preventing Hemorrhage in Patients with Hemophilia Undergoing Dental Extractions? A Systematic Review and Meta-Analysis. Clin. Appl. Thromb. 2022, 28, 10760296221114862. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. PLoS Med. 2021, 18, 89. [Google Scholar] [CrossRef]
- Cochrane. RoB 2: A Revised Cochrane Risk-of-Bias Tool for Randomized Trials. Available online: https://methods.cochrane.org/bias/resources/rob-2-revised-cochrane-risk-bias-tool-randomized-trials (accessed on 7 August 2022).
- Ockerman, A.; Miclotte, I.; Vanhaverbeke, M.; Vanassche, T.; Belmans, A.; Vanhove, J.; Meyns, J.; Nadjmi, N.; Van Hemelen, G.; Winderickx, P.; et al. Tranexamic Acid and Bleeding in Patients Treated with Non-Vitamin K Oral Anticoagulants Undergoing Dental Extraction: The EXTRACT-NOAC Randomized Clinical Trial. PLoS Med. 2021, 18, e1003601. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, S.I.M.L.; Silvestre, V.D.; Soares, R.M.; Campos, G.B.P.; Germano, A.R.; da Silva, J.S.P. Tranexamic Acid as a Local Hemostasis Method after Dental Extraction in Patients on Warfarin: A Randomized Controlled Clinical Study. Clin. Oral Investig. 2018, 22, 2281–2289. [Google Scholar] [CrossRef] [PubMed]
- Borea, G.; Montebugnoli, L.; Capuzzi, P.; Magelli, C. Tranexamic Acid as a Mouthwash in Anticoagulant-Treated Patients Undergoing Oral Surgery: An Alternative Method to Discontinuing Anticoagulant Therapy. Oral Surg. Oral Med. Oral Pathol. 1993, 75, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Ramström, G.; Sindet-Pedersen, S.; Hall, G.; Blombäck, M.; Älander, U. Prevention of Postsurgical Bleeding in Oral Surgery Using Tranexamic Acid without Dose Modification of Oral Anticoagulants. J. Oral Maxillofac. Surg. 1993, 51, 1211–1216. [Google Scholar] [CrossRef] [PubMed]
- Soares, E.C.S.; Costa, F.W.G.; Bezerra, T.P.; Nogueira, C.B.P.; de Barros Silva, P.G.; Batista, S.H.B.; Sousa, F.B.; Sá Roriz Fonteles, C. Postoperative Hemostatic Efficacy of Gauze Soaked in Tranexamic Acid, Fibrin Sponge, and Dry Gauze Compression Following Dental Extractions in Anticoagulated Patients with Cardiovascular Disease: A Prospective, Randomized Study. Oral Maxillofac. Surg. 2015, 19, 209–216. [Google Scholar] [CrossRef]
- Torn, M.; Rosendaal, F.R. Oral Anticoagulation in Surgical Procedures: Risks and Recommendations. Br. J. Haematol. 2003, 123, 676–682. [Google Scholar] [CrossRef]
- Hong, C.; Napenas, J.J.; Brennan, M.; Furney, S.; Lockhart, P. Risk of Postoperative Bleeding after Dental Procedures in Patients on Warfarin: A Retrospective Study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, 464–468. [Google Scholar] [CrossRef]
- Levy, J.H.; Koster, A.; Quinones, Q.J.; Milling, T.J.; Key, N.S. Antifibrinolytic Therapy and Perioperative Considerations. Anesthesiology 2018, 128, 657–670. [Google Scholar] [CrossRef]
- Owattanapanich, D.; Ungprasert, P.; Owattanapanich, W. Efficacy of Local Tranexamic Acid Treatment for Prevention of Bleeding after Dental Procedures: A Systematic Review and Meta-Analysis. J. Dent. Sci. 2019, 14, 21–26. [Google Scholar] [CrossRef]
- Ziffer, A.M.; Scopp, I.W.; Beck, J.; Baum, J.; Berger, A.R. Profound Bleeding after Dental Extractions during Dicumarol Therapy. N. Engl. J. Med. 1957, 256, 351–353. [Google Scholar] [CrossRef]
- Ockerman, A.; Vanassche, T.; Garip, M.; Vandenbriele, C.; Engelen, M.M.; Martens, J.; Politis, C.; Jacobs, R.; Verhamme, P. Tranexamic Acid for the Prevention and Treatment of Bleeding in Surgery, Trauma and Bleeding Disorders: A Narrative Review. Thromb. J. 2021, 19, 54. [Google Scholar] [CrossRef]
- Zirk, M.; Zinser, M.; Buller, J.; Bilinsky, V.; Dreiseidler, T.; Zöller, J.E.; Kreppel, M. Supportive Topical Tranexamic Acid Application for Hemostasis in Oral Bleeding Events—Retrospective Cohort Study of 542 Patients. J. Cranio-Maxillofac. Surg. 2018, 46, 932–936. [Google Scholar] [CrossRef]
- Yang, S.; Shi, Q.; Liu, J.; Li, J.; Xu, J. Should Oral Anticoagulant Therapy Be Continued during Dental Extraction? A Meta-Analysis. BMC Oral Health 2016, 16, 81. [Google Scholar] [CrossRef]
- Perry, D.J.; Noakes, T.J.C.; Helliwell, P.S. Guidelines for the Management of Patients on Oral Anticoagulants Requiring Dental Surgery. Br. Dent. J. 2007, 203, 389–393. [Google Scholar] [CrossRef]
- van Galen, K.P.M.; Engelen, E.T.; Mauser-Bunschoten, E.P.; van Es, R.J.J.; Schutgens, R.E.G. Antifibrinolytic Therapy for Preventing Oral Bleeding in Patients with Haemophilia or Von Willebrand Disease Undergoing Minor Oral Surgery or Dental Extractions. Cochrane Database Syst. Rev. 2019, 4, 3–7. [Google Scholar] [CrossRef]
- Juurlink, D.N. Drug Interactions with Warfarin: What Clinicians Need to Know. CMAJ 2007, 177, 369–371. [Google Scholar] [CrossRef]
- Schwarb, H.; Tsakiris, D.A. New Direct Oral Anticoagulants (DOAC) and Their Use Today. Dent. J. 2016, 4, 5. [Google Scholar] [CrossRef]
- Healey, J.S.; Eikelboom, J.; Yang, S.; Themeles, E.; Connolly, S.J.; Yusuf, S.; Douketis, J.; Wallentin, L.; Oldgren, J.; Heidbuchel, H.; et al. Periprocedural Bleeding and Thromboembolic Events with Dabigatran Compared with Warfarin: Results from the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) Randomized Trial. Circulation 2012, 126, 343–348. [Google Scholar] [CrossRef]
- Spyropoulos, A.C.; Al-Badri, A.; Sherwood, M.W.; Douketis, J.D. Periprocedural Management of Patients Receiving a Vitamin K Antagonist or a Direct Oral Anticoagulant Requiring an Elective Procedure or Surgery. J. Thromb. Haemost. 2016, 14, 875–885. [Google Scholar] [CrossRef]
- Yagyuu, T.; Kawakami, M.; Ueyama, Y.; Imada, M.; Kurihara, M.; Matsusue, Y.; Imai, Y.; Yamamoto, K.; Kirita, T. Risks of Postextraction Bleeding after Receiving Direct Oral Anticoagulants or Warfarin: A Retrospective Cohort Study. BMJ Open 2017, 7, e015952. [Google Scholar] [CrossRef]
- Hua, W.; Huang, Z.; Huang, Z. Bleeding Outcomes After Dental Extraction in Patients Under Direct-Acting Oral Anticoagulants vs. Vitamin K Antagonists: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2021, 12, 702057. [Google Scholar] [CrossRef] [PubMed]
- Mauprivez, C.; Khonsari, R.H.; Razouk, O.; Goudot, P.; Lesclous, P.; Descroix, V. Management of Dental Extraction in Patients Undergoing Anticoagulant Oral Direct Treatment: A Pilot Study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 122, e146–e155. [Google Scholar] [CrossRef] [PubMed]
- Caliskan, M.; Tükel, H.-C.; Benlidayi, E.; Deniz, A. Is It Necessary to Alter Anticoagulation Therapy for Tooth Extraction in Patients Taking Direct Oral Anticoagulants? Med. Oral Patol. Oral Cir. Bucal 2017, 22, e767. [Google Scholar] [CrossRef] [PubMed]
- Dubois, V.; Dincq, A.-S.; Douxfils, J.; Ickx, B.; Samama, C.-M.; Dogne, J.-M.; Gourdin, M.; Chatelain, B.; Mullier, F.; Lessire, S. Perioperative Management of Patients on Direct Oral Anticoagulants. Thromb. J. 2017, 15, 14. [Google Scholar] [CrossRef] [PubMed]
- Ockerman, A.; Miclotte, I.; Vanhaverbeke, M.; Verhamme, P.; Poortmans, L.-L.; Vanassche, T.; Politis, C.; Jacobs, R. Local Haemostatic Measures after Tooth Removal in Patients on Antithrombotic Therapy: A Systematic Review. Clin. Oral Investig. 2019, 23, 1695–1708. [Google Scholar] [CrossRef]
- Ambrogio, R.I.; Levine, M.H. Tranexamic Acid as a Hemostatic Adjunct in Dentistry. Compendium 2018, 39, 392–401. [Google Scholar]
- Brown, R.S. Antifibrinolytic Drugs (Aminocaproic Acid and Tranexamic Acid): Treatment Perspectives for Dental Surgery. Curr. Oral Health Rep. 2015, 2, 143–147. [Google Scholar] [CrossRef]
- Sindet-Pedersen, S.; Stenbjerg, S. Effect of Local Antifibrinolytic Treatment with Tranexamic Acid in Hemophiliacs Undergoing Oral Surgery. J. Oral Maxillofac. Surg. 1986, 44, 703–707. [Google Scholar] [CrossRef]
- McCormack, P.L. Tranexamic Acid. Drugs 2012, 72, 585–617. [Google Scholar] [CrossRef]
- Sayani, S.; Iqbal, O.; Hoppensteadt, D.; Fareed, J. Drug Interactions of Newer Oral Anticoagulants Dabigatran, Rivaroxaban, and Apixaban with Routinely Used Nonanticoagulant/Antiplatelet Drugs. Blood 2014, 124, 4267. [Google Scholar] [CrossRef]
- CRASH-2 Collaborators. The Importance of Early Treatment with Tranexamic Acid in Bleeding Trauma Patients: An Exploratory Analysis of the CRASH-2 Randomised Controlled Trial. Lancet 2011, 377, 1096–1101. [Google Scholar] [CrossRef]

| Mean Age (Years) | ||
|---|---|---|
| Author, Year | Intervention Group | Placebo Group |
| Ockerman et al., 2021 [22] | 74.8 | 72.7 |
| Queiroz et al., 2018 [23] | 50.5 | 41.4 |
| Borea et al., 1993 [24] | 62.7 | 61.1 |
| Ramstrom et al., 1993 [25] | 69.8 | 67.1 |
| Soares et al., 2015 [26] | 51 | 51.1 |
| Gender (male/female), n (%) | ||
| Ockerman et al., 2021 [22] | Male = 81/106 (76.4%); Female = 25/106 (23.6%) | Male = 64/112 (57.1%); Female = 48/112 (42.9%) |
| Queiroz et al., 2018 [23] | Male = 8/17 (47.1%); Female = 9/17 (52.9%) | Male = 6/20 (30%); Female = 14/20 (70%) |
| Borea et al., 1993 [24] | Male = 5/15 (33.3%); Female = 10/15 (66.7%) | Male = 7/15 (46.7%); Female = 8/15 (53.3%) |
| Ramstrom et al., 1993 [25] | Male = 25/44 (56.8%); Female = 19/44 (43.2%) | Male = 28/45 (62.2%); Female = 17/45 (37.8%) |
| Soares et al., 2015 [26] | Male = 18/28 (64.3%); Female = 10/28 (35.7%) | Male = 18/28 (64.3%); Female = 10/28 (35.7%) |
| Dosage and route of administration of local tranexamic acid | ||
| Ockerman et al., 2021 [22] | 10% tranexamic acid solution mouthwash or placebo solution once before dental extraction and for 1 min 3 times a day for 3 days after dental extraction | |
| Queiroz et al., 2018 [23] | Two methods of local hemostasis for a minimum of 5 min once and repeated for each group if postoperative bleeding occurs later: (1) IG = irrigation and compression with gauze soaked in 5% tranexamic acid solution and suture, (2) CG = socket irrigation with saline gauze compression only and suture | |
| Borea et al., 1993 [24] | 5% tranexamic acid solution mouthwash (10 mL) or placebo solution after the dental procedure for 2 min 4 times a day for 7 days | |
| Ramstrom et al., 1993 [25] | Surgical irrigation with 10 mL of 4.8% tranexamic acid solution before suturing followed by 4.8% tranexamic acid solution mouthwash after the dental procedure for 2 min 4 times a day for 7 days, and application of a gauze pad soaked in the solution applied to the bleeding site for 20 min under biting pressure if persistent postoperative bleeding | |
| Soares et al., 2015 [26] | Three methods of local hemostasis for 8 min once each and repeated for each group if postoperative bleeding occurred later: IG = gauze pad soaked in either 4.8% tranexamic acid solution or fibrin sponge applied to the surgical alveolus for 8 min under biting pressure; CG = dry gauze compression performed under biting pressure on the surgical alveolus for 8 min | |
| Indication for anticoagulation in patients | ||
| Ockerman et al., 2021 [22] | AF = 88 (83%); VTE = 11 (10.4%) | AF = 88 (78.6%); VTE = 14 (12.5%) |
| Queiroz et al., 2018 [23] | CV = 48.6%; DVT = 40.5%; CVA = 10.8% | |
| Borea et al., 1993 [24] | All (N = 30; 100%) patients had a CV and were on anticoagulation | |
| Ramstrom et al., 1993 [25] | Prosthetic CV = 3 (6.5%); VTE = 16 (34.8%); CVD = 6 (13%) | Prosthetic CV = 3 (6.4%); VTE = 13 (27.7%); CVD = 9 (19.6%) |
| Soares et al., 2015 | MV prolapse = 47.4%; Prosthetic CV = 23.7%; VTE = 21.1%; PE = 5.2% | |
| Anticoagulation agent used and window before the last dose | ||
| Ockerman et al., 2021 [22] | Direct oral anticoagulants (rivaroxaban, apixaban, edoxaban, or dabigatran) with an 18–24-h window prior to the dental procedure | |
| Queiroz et al., 2018 [23] | Warfarin was not suspended prior to the dental procedure | |
| Borea et al., 1993 [24] | Warfarin was not suspended prior to the dental procedure | Warfarin dose reduced prior to the dental procedure |
| Ramstrom et al., 1993 [25] | Coumarin drugs (warfarin, dicumarol, or phenprocoumon) not suspended prior to the dental procedure | |
| Soares et al., 2015 [26] | Warfarin was not suspended prior to the dental procedure | |
| Number of patients with postoperative bleeds | ||
| Ockerman et al., 2021 [22] | 28/106 (26.4%) | 32/112 (28.6%) |
| Queiroz et al., 2018 [23] | 0/17 (0%) | 3/20 (15%) |
| Borea et al., 1993 [24] | 2/15 (13.3%) | 2/15 (13.3%) |
| Ramstrom et al., 1993 [25] | 0/44 (0%) | 10/45 (22.2%) |
| Soares et al., 2015 [26] | 1/28 (3.6%) | 1/28 (3.6%) |
| Adverse events due to stopping anticoagulation for dental extraction | ||
| Ockerman et al., 2021 [22] | 0/106 (0%) | 1/112 (0.9%) |
| Queiroz et al., 2018 [23] | 3/37 (8.1%) were discontinued in both groups | |
| Borea et al., 1993 [24] | NR | NR |
| Ramstrom et al., 1993 [25] | 0/44 (0%) | 0/45 (0%) |
| Soares et al., 2015 [26] | 0/28 (0%) | 0/28 (0%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaib, A.; Shaheryar, M.; Shakil, M.; Sarfraz, A.; Sarfraz, Z.; Cherrez-Ojeda, I. Local Tranexamic Acid for Preventing Hemorrhage in Anticoagulated Patients Undergoing Dental and Minor Oral Procedures: A Systematic Review and Meta-Analysis. Healthcare 2022, 10, 2523. https://doi.org/10.3390/healthcare10122523
Zaib A, Shaheryar M, Shakil M, Sarfraz A, Sarfraz Z, Cherrez-Ojeda I. Local Tranexamic Acid for Preventing Hemorrhage in Anticoagulated Patients Undergoing Dental and Minor Oral Procedures: A Systematic Review and Meta-Analysis. Healthcare. 2022; 10(12):2523. https://doi.org/10.3390/healthcare10122523
Chicago/Turabian StyleZaib, Asma, Muhammad Shaheryar, Muhammad Shakil, Azza Sarfraz, Zouina Sarfraz, and Ivan Cherrez-Ojeda. 2022. "Local Tranexamic Acid for Preventing Hemorrhage in Anticoagulated Patients Undergoing Dental and Minor Oral Procedures: A Systematic Review and Meta-Analysis" Healthcare 10, no. 12: 2523. https://doi.org/10.3390/healthcare10122523
APA StyleZaib, A., Shaheryar, M., Shakil, M., Sarfraz, A., Sarfraz, Z., & Cherrez-Ojeda, I. (2022). Local Tranexamic Acid for Preventing Hemorrhage in Anticoagulated Patients Undergoing Dental and Minor Oral Procedures: A Systematic Review and Meta-Analysis. Healthcare, 10(12), 2523. https://doi.org/10.3390/healthcare10122523







