Subsiding of Periodontitis in the Permanent Dentition in Individuals with Papillon-Lefèvre Syndrome through Specific Periodontal Treatment: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Literature Search Strategy
2.3. Screening and Selection
2.3.1. Assessment of Heterogeneity
2.3.2. Quality Assessment
2.4. Data Extraction
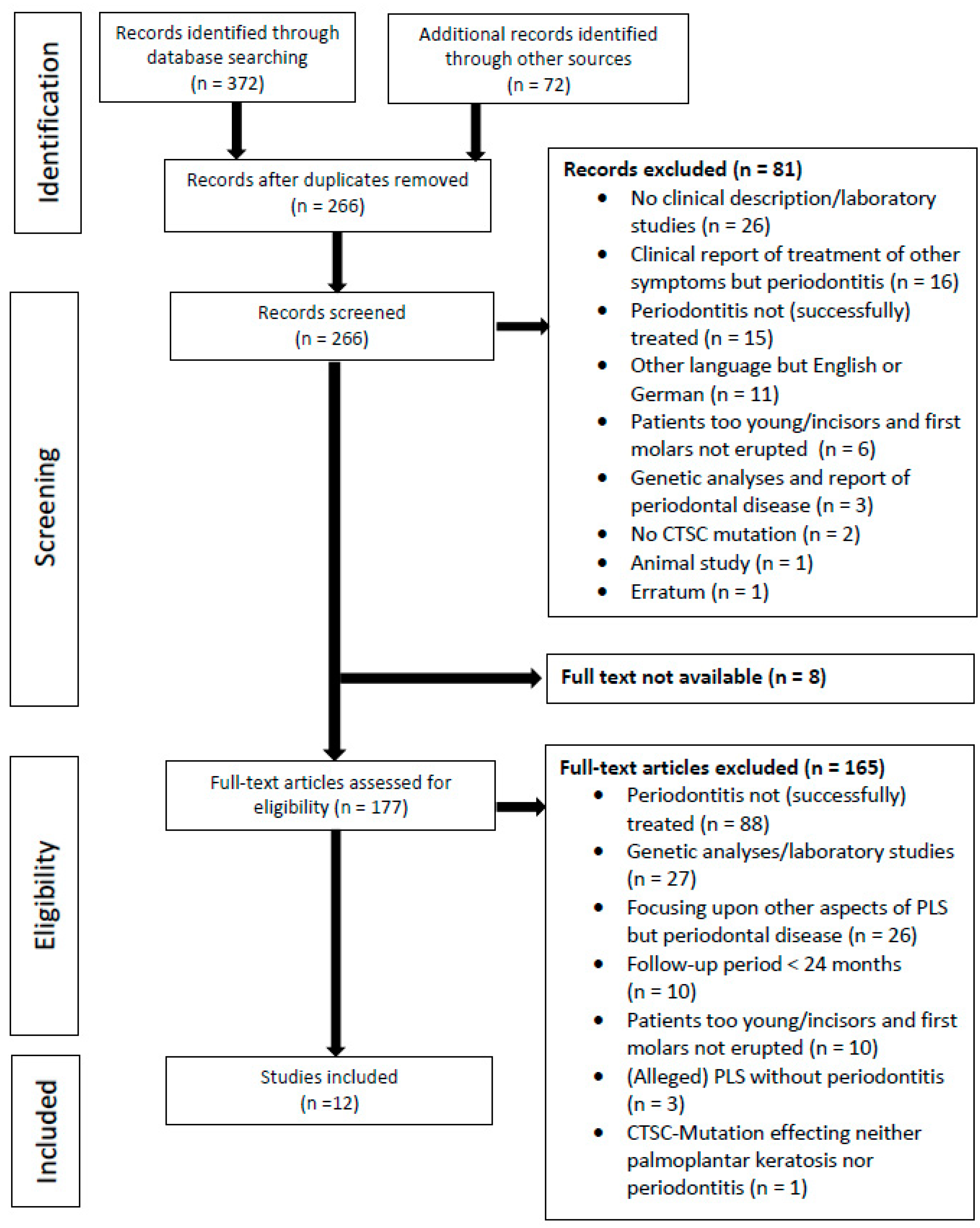
3. Results
3.1. Study Selection
3.2. Subjects, Diagnosis, and Therapy
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bloch-Zupan, A.; Sedano, H.O.; Scully, C. Anomalies of Teeth Eruption and/or Resorption. In Dento/Oro/Craniofacial Anomalies and Genetics, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 170–174. [Google Scholar]
- Preus, H.; Gjermo, P. Clinical management of prepubertal periodontitis in 2 siblings with Papillon-Lefèvre syndrome. J. Clin. Periodontol. 1987, 14, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Haneke, E. The Papillon-Lefèvre syndrome: Keratosis palmoplantaris with periodontopathy. Report of a case and review of the cases in the literature. Hum Genet. 1979, 51, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. Clin. Periodontol. 2018, 45 (Suppl. 20), S162–S170. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G.; Lamont, R.J. Beyond the red complex and into more complexity: The polymicrobial synergy and dysbiosis (PSD) model of periodontal disease etiology. Mol. Oral Microbiol. 2012, 27, 409–419. [Google Scholar] [CrossRef]
- Hajishengallis, G. Immunomicrobial pathogenesis of periodontitis: Keystones, pathobionts, and host response. Trends Immunol. 2014, 35, 3–11. [Google Scholar] [CrossRef]
- Löe, H.; Anerud, A.; Boysen, H.; Morrison, E. Natural history of periodontal disease in man. Rapid, moderate and no loss of attachment in Sri Lankan laborers 14 to 46 years of age. J. Clin. Periodontol. 1986, 13, 431–445. [Google Scholar] [CrossRef]
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Beglundh, T.; Sculean, A.; Tonetti, M.S.; EFP Workshop Participants and Methodological Consultants. Treatment of stage I-III periodontitis-The EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2020, 47 (Suppl. 22), 4–60. [Google Scholar] [CrossRef]
- Janjua, S.A.; Khachemoune, A. Papillon-Lefèvre syndrome: Case report and review of the literature. Dermatology 2004, 10, 13. [Google Scholar] [CrossRef]
- Hart, T.C.; Hart, P.S.; Bowden, D.W.; Michalec, M.D.; Callison, S.A.; Walker, S.J.; Zang, Y.; Firatli, E. Mutations of the cathepsin C gene are responsible for Papillon-Lefèvre syndrome. J. Med. Genet. 1999, 36, 881–887. [Google Scholar] [CrossRef]
- Eick, S.; Puklo, M.; Adamowicz, K.; Kantyka, T.; Hiemstra, P.; Stennicke, H.; Guentsch, A.; Schacher, B.; Eickholz, P.; Potempa, J. Lack of cathelicidin processing in Papillon-Lefèvre syndrome patients reveals essential role of LL-37 in periodontal homeostasis. Orphanet. J. Rare Dis. 2014, 9, 148. [Google Scholar] [CrossRef]
- Christersson, L.A.; Slots, J.; Rosling, B.G.; Genco, R.J. Microbiological and clinical effects of surgical treatment of localized juvenile periodontitis. J. Clin. Periodontol. 1985, 12, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Kornman, K.S.; Robertson, P.B. Clinical and microbiological evaluation of therapy for juvenile periodontitis. J. Periodontol. 1985, 56, 443–446. [Google Scholar] [CrossRef] [PubMed]
- Mombelli, A.; Gmür, R.; Gobbi, C.; Lang, N.P. Actinobacillus actinomycetemcomitans in adult periodontitis. I. Topographic distribution before and after treatment. J. Periodontol. 1994, 65, 820–826. [Google Scholar] [CrossRef]
- van Winkelhoff. A.J.; Rodenburg, J.P.; Goené, R.J.; Abbas, F.; Winkel, E.G.; de Graaff, J. Metronidazole plus amoxycillin in the treatment of Actinobacillus actinomycetemcomitans associated periodontitis. J. Clin. Periodontol. 1989, 16, 128–131. [Google Scholar] [CrossRef] [PubMed]
- Guay, D.; Beaulieu, C.; Percival, M.D. Therapeutic utility and medicinal chemistry of cathepsin C inhibitors. Curr. Top. Med. Chem. 2010, 10, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Toomes, C.; James, J.; Wood, A.J.; Wu, C.L.; McCormick, D.; Lench, N.; Hewitt, C.; Moynihan, L.; Roberts, E.; Woods, C.G.; et al. Loss-of-function mutations in the cathepsin C gene result in periodontal disease and palmoplantar keratosis. Nat. Genet. 1999, 23, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Nakano, A.; Nomura, K.; Nakano, H.; Ono, Y.; LaForgia, S.; Pulkkinen, L.; Hashimoto, I.; Uitto, J. Papillon-Lefèvre syndrome: Mutations and polymorphisms in the cathepsin C gene. J. Investig. Dermatol. 2001, 116, 339–343. [Google Scholar] [CrossRef]
- Czauderna, P.; Sznurkowska, K.; Korzon, M.; Roszkiewicz, A.; Stoba, C. Association of inflammatory pseudotumor of the liver and Papillon-Lefevre syndrome—Case report. Eur. J. Pediatr. Surg. 1999, 9, 343–346. [Google Scholar] [CrossRef]
- Van Dyke, T.E.; Taubman, M.A.; Ebersole, J.L.; Haffajee, A.D.; Socransky, S.S.; Smith, D.J.; Genko, R.J. The Papillon-Lefèvre syndrome: Neutrophil dysfunction with severe periodontal disease. Clin. Immunol. Immunopathol. 1984, 31, 419–429. [Google Scholar] [CrossRef]
- Borroni, G.; Pagani, A.; Carcaterra, A.; Pericoli, R.; Gabba, P.; Marconi, M. Immunological alterations in a case of Papillon-Lefèvre syndrome with recurrent cutaneous infections. Dermatologica 1985, 170, 27–30. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [PubMed]
- The Joanna Briggs Institute Critical Appraisal Tools for Use in JBI Systematic Reviews. Checklist for Case Reports. Available online: https://jbi.global/sites/default/files/201905/JBI_Critical_Appraisal-Checklist_for_Case_Reports2017_0.pdf (accessed on 15 July 2022).
- Soskolne, W.A.; Stabholz, A.; van Dyke, T.E.; Hart, T.C.; Meyle, J. Partial expression of the Papillon-Lefèvre syndrome in 2 unrelated families. J. Clin. Periodontol. 1999, 23, 764–769. [Google Scholar] [CrossRef] [PubMed]
- Fardal, O.; Drangsholt, E.; Olsen, I. Palmar plantar keratosis and unusual periodontal findings. Observations from a family of 4 members. J. Clin. Periodontol. 1998, 25, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Sugiura, K.; Takeichi, T.; Akiyama, M. The novel CTSC homozygous nonsense mutation p.Lys106X in a patient with Papillon-Lefèvre syndrome with all permanent teeth remaining at over 40 years of age. Br. J. Dermatol. 2016, 16, 948–950. [Google Scholar] [CrossRef]
- Allende, L.M.; García-Pérez, M.A.; Moreno, A.; Corell, A.; Carasol, M.; Martínez-Canut, P.; Arnaiz-Villena, A. Cathepsin C gene: First compound heterozygous patient with Papillon-Lefèvre syndrome and a novel symptomless mutation. Hum. Mutat. 2001, 17, 152–153. [Google Scholar] [CrossRef]
- Ishikawa, I.; Umeda, M.; Laosrisin, N. Clinical, bacteriological, and immunological examinations and the treatment process of two Papillon-Lefèvre syndrome patients. J. Periodontol. 1994, 65, 364–371. [Google Scholar] [CrossRef]
- Rüdiger, S.; Petersilka, G.; Flemmig, T.F. Combined systemic and local antimicrobial therapy of periodontal disease in Papillon-Lefèvre syndrome. A report of 4 cases. J. Clin. Periodontol. 1999, 2, 847–854. [Google Scholar]
- Kressin, S.; Herforth, A.; Preis, S.; Wahn, V.; Lenard, H.G. Papillon-Lefèvre syndrome--successful treatment with a combination of retinoid and concurrent systematic periodontal therapy: Case reports. Quintessence Int. 1995, 26, 795–803. [Google Scholar]
- Wiebe, C.B.; Häkkinen, L.; Putnins, E.E.; Walsh, P.; Larjava, H.S. Successful periodontal maintenance of a case with Papillon-Lefèvre syndrome: 12-year follow-up and review of the literature. J. Periodontol. 2001, 72, 824–830. [Google Scholar] [CrossRef]
- French, D.; Scott, H.; Overall, C.M. Papillon-Lefèvre syndrome associated early onset periodontitis: A review and case study. J. Can. Dent. Assoc. 1995, 61, 432–438. [Google Scholar]
- Nickles, K.; Schacher, B.; Schuster, G.; Valesky, E.; Eickholz, P. Evaluation of two siblings with Papillon-Lefèvre syndrome 5 years after treatment of periodontitis in primary and mixed dentition. J. Periodontol. 2011, 82, 1536–1547. [Google Scholar] [CrossRef] [PubMed]
- Schacher, B.; Baron, F.; Ludwig, B.; Valesky, E.; Noack, B.; Eickholz, P. Periodontal therapy in siblings with Papillon-Lefèvre syndrome and tinea capitis: A report of two cases. J. Clin. Periodontol. 2006, 33, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Nickles, K.; Schacher, B.; Ratka-Krüger, P.; Krebs, M.; Eickholz, P. Long-term results after treatment of periodontitis in patients with Papillon-Lefèvre syndrome: Success and failure. J. Clin. Periodontol. 2013, 40, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Lux, C.J.; Kugel, B.; Komposch, G.; Pohl, S.; Eickholz, P. Orthodontic treatment in a patient with Papillon-Lefèvre syndrome. J. Periodontol. 2005, 76, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Eickholz, P.; Kugel, B.; Pohl, S.; Näher, H.; Staehle, H.J. Combined mechanical and antibiotic periodontal therapy in a case of Papillon-Lefèvre syndrome. J. Periodontol. 2001, 72, 542–549. [Google Scholar] [CrossRef]
- Umlauft, J.; Schnabl, D.; Blunder, S.; Moosbrugger-Martinz, V.; Kapferer-Seebacher, I.; Zschocke, J.; Schmuth, M.; Gruber, R. Two patients with Papillon-Lefèvre syndrome without periodontal involvement of the permanent dentition. J. Dermatol. 2021, 48, 537–541. [Google Scholar] [CrossRef]
- Herrera, D.; Sanz, M.; Kebschull, M.; Jepsen, S.; Sculean, A.; Berglundh, T.; Papapanou, P.N.; Chapple, I.; Tonetti, M.S. EFP Workshop Participants and Methodological Consultant. Treatment of stage IV periodontitis: The EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2022, 49 (Suppl. 24), 4–71. [Google Scholar] [CrossRef]
- Nagy, N.; Vályi, P.; Csoma, Z.; Sulák, A.; Tripolszki, K.; Farkas, K.; Paschali, E.; Papp, F.; Tóth, L.; Fábos, B.; et al. CTSC and Papillon-Lefèvre syndrome: Detection of recurrent mutations in Hungarian patients, a review of published variants and database update. Mol. Genet. Genom. Med. 2014, 2, 217–228. [Google Scholar] [CrossRef]
- Selvaraju, V.; Markandaya, M.; Prasad, P.V.; Sathyan, P.; Sethuraman, G.; Srivastava, S.C.; Thakker, N.; Kumar, A. Mutation analysis of the cathepsin C gene in Indian families with Papillon-Lefèvre syndrome. BMC Med. Genet. 2003, 4, 5. [Google Scholar] [CrossRef]
- Atalaia, A.; Thompson, R.; Corvo, A.; Carmody, L.; Piscia, D.; Matalonga, L.; Macaya, A.; Lochmuller, A.; Fontaine, B.; Zurek, B.; et al. A guide to writing systematic reviews of rare disease treatments to generate FAIR-compliant datasets: Building a Treatabolome. Orphanet. J. Rare Dis. 2020, 15, 206. [Google Scholar] [CrossRef]
| Reference | ID | Age (y) | Gender | Periodontal Therapy | Adjunctive Antibiotic Treatment | Additional Therapeutic Interventions | Follow-Up (y) |
|---|---|---|---|---|---|---|---|
| Preus et al. (1987) [2] | 1 | 9 | male | supra- and subgingival debridement, OHI, 0.2% chlorhexidine mouthrinse extraction of all primary teeth | tetracycline 250 mg/d for 2–4 weeks intermittently in case of suspected gingival abnormality and 0.25 mg/day continuously for 2 y | none | 5 |
| Ishikawa et al. (1994) [28] | 2 | 9 | female | extraction of all primary teeth | none | full dentures | 5 |
| Ishikawa et al. (1994) [28] | 3 | 12 | female | supra- and subgingival debridement, OHI, flap surgery | minocycline 200 mg/d for 2 weeks erythromycin 1 g/d for 1 month ofloxacin 300 mg/day for 1 month | none | 5 |
| Rüdiger et al. (1999) [29] Kressin et al. (1995) [30] | 4 | 11 | female | supra- and subgingival debridement, OHI Meridol® mouth rinse extraction of two mandibular incisors 0.06% chlorhexidine jet irrigation | amoxicillin 750 mg/d plus clavulanic acid and metronidazole 375 mg/d for 1 week | removable partial denture | 6 |
| Wiebe et al. (2001) [31] French et al. (1995) [32] | 5 | 17 | male | supra- and subgingival debridement, OHI 0.12% chlorhexidine mouthrinse extraction of all primary teeth | 2 rounds of metronidazole | full dentures | 12 |
| Nickles et al. (2011) [33] Schacher et al. (2006) [34] | 6 | 10 | male | supra- and subgingival debridement, OHI extraction of severely affected primary teeth 1% chlorhexidine gel 0.12% chlorhexidine mouthrinse | amoxicillin 750 mg/d and metronidazole 750 mg/d for 1 week | none | 5 |
| Nickles et al. (2013) [35] Lux et al. (2005) [36] Eickholz et al. (2001) [37] | 7 | 24 | male | supra- and subgingival debridement, OHI 0.1% chlorhexidine mouthrinse e 1% chlorhexidine gel 30% chlorhexidine chips in 4 teeth | amoxicillin 750 mg/d and metronidazole 500 mg/d for 1 week | orthodontics | 17 |
| Nickles et al. (2013) [35] | 8 | 17 | male | supra- and subgingival debridement, OHI extraction of severely affected teeth | amoxicillin 750 mg/d plus clavulanic acid and metronidazole 600 mg/d for 1 week repeated in case of recurrence | orthodontics | 13 |
| Umlauft et al. (2021) [38] | 9 | 18 | male | supra- and subgingival debridement, OHI 0.2% chlorhexidine mouthrinse | amoxicillin and metronidazole | none | 15 |
| Reference | ID | Microbiological Testing Baseline | Antibiotics | Extraction of All Primary Teeth | Presence of A. actinomycetemcomitans after Therapy |
|---|---|---|---|---|---|
| Preus et al. (1987) [2] | 1 | not evaluated | Tetracycline | yes | not evaluated |
| Ishikawa et al. (1994) [28] | 2 | A. actinomycetemcomitans P. gingivalis P. intermedia F. nucleatum C. rectus E. corrodens | None | yes | negative after extraction of all primary teeth |
| Ishikawa et al. (1994) [28] | 3 | A. actinomycetemcomitans P. gingivalis P. intermedia F. nucleatum C. rectus E. corrodens | minocycline erythromycin ofloxacin | no | positive after minocycline positive after erythromycin negative after ofloxacine |
| Rüdiger et al. (1999) [29] Kressin et al. (1995) [30] | 4 | A. actinomycetemcomitans P. gingivalis | amoxicillin and metronidazole | no | negative after 3 y |
| Wiebe et al. (2001) [31], French et al. (1995) [32] | 5 | P. gingivalis T. forsythia | None | yes | not evaluated |
| Nickles et al. (2011) [33] Schacher et al. (2006) [34] | 6 | A. actinomycetemcomitans P. gingivalis T. forsythia T. denticola | amoxicillin and metronidazole | no | negative 1 and 5 y |
| Nickles et al. (2013) [35] Lux et al. (2005) [36] Eickholz et al. (2001) [37] | 7 | A. actinomycetemcomitans P. gingivalis P. intermedia F. nucleatum C. rectus E. corrodens | amoxicillin and metronidazole | no | negative after 7 months, 2, 3, and 4 y positive after 7 y negative after 8 and 12 y |
| Nickles et al. (2013) [35] | 8 | A. actinomycetemcomitans | amoxicillin/clavulanic acid and metronidazole initially and at recurrences after 3 and 8 y | no | positive after 3 and 8 y negative after 9 y |
| Umlauft et al. (2021) [38] | 9 | not evaluated | amoxicillin and metronidazole | no | not evaluated |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schnabl, D.; Thumm, F.M.; Kapferer-Seebacher, I.; Eickholz, P. Subsiding of Periodontitis in the Permanent Dentition in Individuals with Papillon-Lefèvre Syndrome through Specific Periodontal Treatment: A Systematic Review. Healthcare 2022, 10, 2505. https://doi.org/10.3390/healthcare10122505
Schnabl D, Thumm FM, Kapferer-Seebacher I, Eickholz P. Subsiding of Periodontitis in the Permanent Dentition in Individuals with Papillon-Lefèvre Syndrome through Specific Periodontal Treatment: A Systematic Review. Healthcare. 2022; 10(12):2505. https://doi.org/10.3390/healthcare10122505
Chicago/Turabian StyleSchnabl, Dagmar, Felix Maximilian Thumm, Ines Kapferer-Seebacher, and Peter Eickholz. 2022. "Subsiding of Periodontitis in the Permanent Dentition in Individuals with Papillon-Lefèvre Syndrome through Specific Periodontal Treatment: A Systematic Review" Healthcare 10, no. 12: 2505. https://doi.org/10.3390/healthcare10122505
APA StyleSchnabl, D., Thumm, F. M., Kapferer-Seebacher, I., & Eickholz, P. (2022). Subsiding of Periodontitis in the Permanent Dentition in Individuals with Papillon-Lefèvre Syndrome through Specific Periodontal Treatment: A Systematic Review. Healthcare, 10(12), 2505. https://doi.org/10.3390/healthcare10122505







