Systematic Review and Critical Evaluation of Quality of Clinical Practice Guidelines on Nutrition in Pregnancy
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol, Information Sources, Literature Search, and Data Extraction
2.2. Outcomes Measures
- Specific nutritional requirements;
- Description of balanced diet before and through pregnancy;
- Optimal weight gain;
- Prevention of food-borne diseases;
- Nutrition in peculiar sub-groups of women;
- Maternal outcomes, including anemia, caesarean section, and mortality;
- Perinatal outcomes, including SGA, perinatal mortality, preterm birth, and congenital anomalies.
2.3. Quality Appraisal of Guidelines and Risk of Bias
- Scope and purpose;
- Stakeholder involvement;
- Rigor of development;
- Clarity of presentation;
- Applicability;
- Editorial independence.
2.4. Statistical Analysis
3. Results
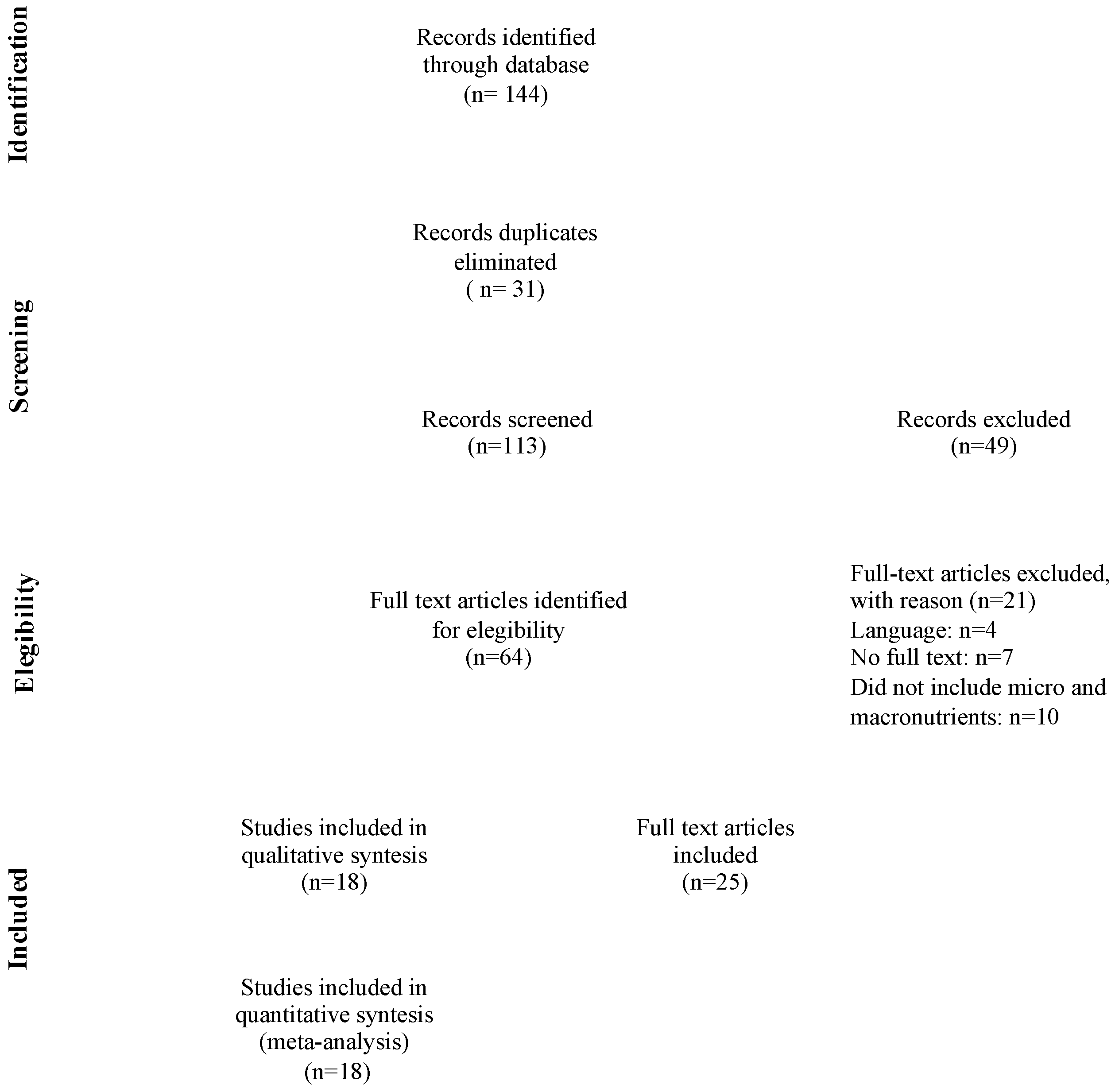
3.1. Study Selection and General Characteristics
3.2. Synthesis of the Results
4. Discussion
4.1. Main Findings
4.2. Strengths and Limitations; Comparison with Other Systematic Reviews
4.3. Clinical and Research Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Concina, F.; Pani, P.; Carletti, C.; Rosolen, V.; Knowles, A.; Parpinel, M.; Ronfani, L.; Mariuz, M.; Vecchi Brumatti, L.; Valent, F.; et al. Nutrient Intake during Pregnancy and Adherence to Dietary Recommendations: The Mediterranean PHIME Cohort. Nutrients 2021, 13, 1434. [Google Scholar] [CrossRef] [PubMed]
- Centro di Ricerca Alimenti e Nutrizione. Linee Guida Per Una Sana Alimentazione. 2018. Available online: https://www.salute.gov.it/imgs/C_17_pubblicazioni_2915_allegato.pdf (accessed on 12 October 2022).
- Goldstein, R.F.; Abell, S.K.; Anasinha, S.; Misso, M.; Boyle, J.A.; Black, M.H.; Li, N.; Hu, G.; Corrado, F.; Rode, L.; et al. Association of Gestational Weight Gain with Maternal and Infant Outcomes: A Systematic Review and Meta-analysis. JAMA 2017, 317, 2207–2225. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, R.F.; Abell, S.K.; Ranasinha, S.; Misso, M.L.; Boyle, J.A.; Harrison, C.L.; Black, M.H.; Li, N.; Hu, G.; Corrado, F.; et al. Gestational weight gain across continents and ethnicity: Systematic review and meta-analysis of maternal and infant outcomes in more than one million women. BMC Med. 2018, 16, 153. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Abe, S.K.; Kanda, M.; Narita, S.; Rahman, M.S.; Bilano, V.; Ota, E.; Gilmour, S.; Shibuya, K. Maternal body mass index and risk of birth and maternal health outcomes in low- and middle-income countries: A systematic review and meta-analysis. Obes. Rev. 2015, 16, 758–770. [Google Scholar] [CrossRef] [PubMed]
- Yong, H.Y.; Mohd Shariff, Z.; Appannah, G.; Rejali, Z.; Mohd Yusof, B.N.; Bindels, J.; Tee, Y.Y.S.; van der Beek, E.M. Rate of gestational weight gain trajectory is associated with adverse pregnancy outcomes. Public Health Nutr. 2020, 23, 3304–3314. [Google Scholar] [CrossRef] [PubMed]
- Lipworth, H.; Melamed, N.; Berger, H.; Geary, M.; McDonald, S.D.; Murray-Davis, B.; Murphy, K.E.; Redelmeier, D.A.; Yoon, E.W.; Barrett, J.F.R.; et al. Diabetes, Obesity, and Hypertension In Pregnancy Research Network Investigators. Maternal weight gain and pregnancy outcomes in twin gestations. Am. J. Obstet. Gynecol. 2021, 225, e1–e532. [Google Scholar] [CrossRef]
- Simon, A.; Pratt, M.; Hutton, B.; Skidmore, B.; Fakhraei, R.; Rybak, N.; Corsi, D.J.; Walker, M.; Velez, M.P.; Smith, G.N.; et al. Guidelines for the management of pregnant women with obesity: A systematic review. Obes. Rev. 2020, 21, e12972. [Google Scholar] [CrossRef]
- Grammatikopoulou, M.G.; Theodoridis, X.; Gkiouras, K.; Lampropoulou, M.; Petalidou, A.; Patelida, M.; Tsirou, E.; Papoutsakis, C.; Goulis, D.G. Methodological quality of clinical practice guidelines for nutrition and weight gain during pregnancy: A systematic review. Nutr. Rev. 2020, 78, 546–562. [Google Scholar] [CrossRef]
- Tsakiridis, I.; Kasapidou, E.; Dagklis, T.; Leonida, I.; Leonida, C.; Bakaloudi, D.R.; Chourdakis, M. Nutrition in Pregnancy: A Comparative Review of Major Guidelines. Obstet. Gynecol. Surv. 2020, 75, 692–702. [Google Scholar] [CrossRef]
- Mustafa, S.T.; Hofer, O.J.; Harding, J.E.; Wall, C.R.; Crowther, C.A. Dietary recommendations for women with gestational diabetes mellitus: A systematic review of clinical practice guidelines. Nutr. Rev. 2021, 79, 988–1021. [Google Scholar] [CrossRef]
- Harrison, C.L.; Teede, H.; Khan, N.; Lim, S.; Chauhan, A.; Drakeley, S.; Moran, L.; Boyle, J. Weight management across preconception, pregnancy, and postpartum: A systematic review and quality appraisal of international clinical practice guidelines. Obes. Rev. 2021, 22, e13310. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, T.; Li, D.; Bay, J.L. Weight Gain and Nutrition during Pregnancy: An Analysis of Clinical Practice Guidelines in the Asia-Pacific Region. Nutrients 2022, 14, 1288. [Google Scholar] [CrossRef] [PubMed]
- Cattani, A.; Teixeira, P.P.; Eckert, I.d.C.; Busnello, F.M.; Gabriel, F.C.; Stein, A.T.; Silva, F.M. Quality appraisal of clinical nutrition practice guidelines for critically ill adult patients: A systematic review using the AGREE II and AGREE-REX tools. Br. J. Nutr. 2022, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Noyahr, J.K.; Tatucu-Babet, O.A.; Chapple, L.S.; Barlow, C.J.; Chapman, M.J.; Deane, A.M.; Fetterplace, K.; Hodgson, C.L.; Winderlich, J.; Udy, A.A.; et al. Methodological Rigor and Transparency in Clinical Practice Guidelines for Nutrition Care in Critically Ill Adults: A Systematic Review Using the AGREE II and AGREE-REX Tools. Nutrients 2022, 14, 2603. [Google Scholar] [CrossRef]
- Johnston, A.; Kelly, S.E.; Hsieh, S.C.; Skidmore, B.; Wells, G.A. Systematic reviews of clinical practice guidelines: A methodological guide. J. Clin. Epidemiol. 2019, 108, 64–76. [Google Scholar] [CrossRef]
- Brouwers, M.C.; Kho, M.E.; Browman, G.P.; Burgers, J.S.; Cluzeau, F.; Feder, G.; Fervers, B.; Graham, I.D.; Grimshaw, J.; Hanna, S.E.; et al. AGREE Next Steps Consortium. AGREE II: Advancing guideline development, reporting and evaluation in health care. CMAJ 2010, 182, E839–E842. [Google Scholar] [CrossRef]
- Amer, Y.S.; Shaiba, L.A.; Hadid, A.; Anabrees, J.; Almehery, A.; Aassiri, M.; Alnemri, A.; Darwish, A.R.A.; Baqawi, B.; Aboshaiqah, A.; et al. Quality assessment of clinical practice guidelines for neonatal sepsis using the Appraisal of Guidelines for Research and Evaluation (AGREE) II Instrument: A systematic review of neonatal guidelines. Front. Pediatr. 2022, 16, 891572. [Google Scholar] [CrossRef]
- Istitute of Obstetricians and Gynaecologists, Royal College of Physicians of Irleand and Directorate of Clinical Strategy and Programmes, Health Service Executive. Clinical Practice Guideline: Nutrtition in Pregnancy. 2019. Available online: https://www.hse.ie/eng/about/who/acute-hospitals-division/woman-infants/clinical-guidelines/nutrition-during-pregnancy.pdf (accessed on 22 October 2022).
- Hanson, M.A.; Bardsley, A.; De-Regil, L.M.; Moore, S.E.; Oken, E.; Poston, L.; Ma, R.C.; McAuliffe, F.M.; Maleta, K.; Purandare, C.N.; et al. The International Federation of Gynecology and Obstetrics (FIGO) recommendations on adolescent, preconception, and maternal nutrition: “Think Nutrition First”. Int. J. Gynaecol. Obstet. 2015, 131 (Suppl. S4), S213–S253. [Google Scholar] [CrossRef]
- Marangoni, F.; Cetin, I.; Verduci, E.; Canzone, G.; Giovannini, M.; Scollo, P.; Corsello, G.; Poli, A. Maternal Diet and Nutrient Requirements in Pregnancy and Breastfeeding. An Italian Consensus Document. Nutrients 2016, 14, 629. [Google Scholar] [CrossRef]
- Ministry of Health, New Zeland. Food and Nutrition Guidelines for Healthy Pregnant and Breastfeeding Women. 2006. Available online: https://www.health.govt.nz/system/files/documents/publications/food-and-nutrition-guidelines-preg-and-bfeed.pdf (accessed on 12 October 2022).
- Alberta Health Services. Nutritional Guideline Pregnancy. 2019. Available online: https://www.albertahealthservices.ca/assets/info/nutrition/if-nfs-ng-pregnancy.pdf (accessed on 12 October 2022).
- Zimmer, M.; Sieroszewski, P.; Oszukowski, P.; Huras, H.; Fuchs, T.; Pawlosek, A. Polish Society of Gynecologists and Obstetricians recommendations on supplementation during pregnancy. Ginekol. Pol. 2020, 9, 644–653. [Google Scholar] [CrossRef]
- Fondazione Confalonieri Aragonese. Nutrizione in Gravidanza e Durante L’allattamento. 2018. Available online: https://www.sigo.it/wp-content/uploads/2018/06/LG_NutrizioneinGravidanza.pdf (accessed on 12 October 2022).
- National Institute for Health and Care Excellence. Maternal and Child Nutrition, Public Health Guideline. 2008. Available online: https://www.nice.org.uk/guidance/ph11/resources/maternal-and-child-nutrition-pdf-1996171502533 (accessed on 12 October 2022).
- Australian Government, Department of Health. Clinical Practice Guidelines, Pregnancy Care. 2020. Available online: https://www.health.gov.au/sites/default/files/documents/2021/02/pregnancy-care-guidelines-pregnancy-care-guidelines.pdf (accessed on 12 October 2022).
- WHO Antenatal Care Recommendations for A Positive Pregnancy Experience. Nutritional Interventions Update: Multiple Micronutrient Supplements during Pregnancy. 2020. Available online: https://apps.who.int/iris/bitstream/handle/10665/333561/9789240007789-eng.pdf?sequence=1&isAllowed=y (accessed on 12 October 2022).
- Minister of Health Canada. Prenatal Nutrition Guidelines for Health Professionals, Background on Canada’s Food Guide. 2009. Available online: https://www.canada.ca/content/dam/hc-sc/migration/hc-sc/fn-an/alt_formats/hpfb-dgpsa/pdf/pubs/guide-prenatal-eng.pdf (accessed on 12 October 2022).
- Ministry of Health of Latvia and WHO Regional Office for Europe. Proper Maternal Nutrition during Pregnancy Planning and Pregnancy: A Healthy Start in Life Recommendations for Health Care Professionals—The Experience from Latvia. 2017. Available online: https://www.euro.who.int/__data/assets/pdf_file/0003/337566/Maternal-nutrition-Eng.pdf (accessed on 12 October 2022).
- Family Health Bureau; Ministry of Health. Maternal Care Package. A Guide to Field Healthcare Workers. 2011. Available online: http://maternalnutritionsouthasia.com/wpcontent/uploads/maternal_care_package_a_guide_to_field_healthcare_workers_english-1.pdf (accessed on 12 October 2022).
- Philippine Obstetrical and Gynecological Society. Clinical Practice Guidelines on Maternal Nutrition and Supplementation. 2013. Available online: http://docshare03.docshare.tips/files/28353/283537702.pdf (accessed on 12 October 2022).
- Maternal and Reproductive Health Division; The Republic of the Union of Myanmar Ministry of Health and Sports. National Guidelines for Antenatal Care for Service Providers; Maternal and Reproductive Health Division. 2018. Available online: http://mohs.gov.mm/su/oysLCg (accessed on 12 October 2022).
- Multiple Micronutrient Supplement Technical Advisory Group (MMS-TAG); Micronutrient Forum (MNF). Expert consensus on an open-access United Nations International Multiple Micronutrient Antenatal Preparation-multiple micronutrient supplement product specification. Ann. N. Y. Acad. Sci. 2020, 1470, 3–13. [Google Scholar] [CrossRef] [PubMed]
- The Public Health Division of the Pacific Community. Pacific Guidelines for Healthy Eating during Pregnancy: A Handbook for Health Professionals and Educators. 2019. Available online: https://www.spc.int/updates/blog/2020/07/now-available-pacific-guidelines-for-healthy-eating-during-pregnancy (accessed on 12 October 2022).
- Koletzko, B.; Godfrey, K.M.; Poston, L.; Szajewska, H.; van Goudoever, J.B.; de Waard, M.; Brands, B.; Grivell, R.M.; Deussen, A.R.; Dodd, J.M.; et al. EarlyNutrition Project Systematic Review Group. Nutrition During Pregnancy, Lactation and Early Childhood and its Implications for Maternal and Long-Term Child Health: The Early Nutrition Project Recommendations. Ann. Nutr. Metab. 2019, 74, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Procter, S.B.; Campbell, C.G. Position of the Academy of Nutrition and Dietetics: Nutrition and lifestyle for a healthy pregnancy outcome. J. Acad. Nutr. Diet. 2014, 114, 1099–1103. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologist Nutrition during Pregnancy. Available online: https://www.acog.org/womens-health/faqs/nutrition-during-pregnancy (accessed on 20 November 2022).
- Schoenaker, D.A.; Soedamah-Muthu, S.S.; Mishra, G.D. The association between dietary factors and gestational hypertension and pre-eclampsia: A systematic review and meta-analysis of observational studies. BMC Med. 2014, 12, 157. [Google Scholar] [CrossRef] [PubMed]
- Brantsaeter, A.L.; Haugen, M.; Samuelsen, S.O.; Torjusen, H.; Trogstad, L.; Alexander, J.; Magnus, P.; Meltzer, H.M. A dietary pattern characterized by high intake of vegetables, fruits, and vegetable oils is associated with reduced risk of preeclampsia in nulliparous pregnant Norwegian women. J. Nutr. 2009, 139, 1162–1168. [Google Scholar] [CrossRef] [PubMed]
- Plows, J.F.; Stanley, J.L.; Baker, P.N.; Reynolds, C.M.; Vickers, M.H. The Pathophysiology of Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2018, 19, 3342. [Google Scholar] [CrossRef]
- Ferrazzi, E.; Tiso, G.; Di Martino, D. Folic acid versus 5- methyl tetrahydrofolate supplementation in pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 253, 312–319. [Google Scholar] [CrossRef]
- Coppedè, F.; Stoccoro, A.; Tannorella, P.; Gallo, R.; Nicolì, V.; Migliore, L. Association of Polymorphisms in Genes Involved in One-Carbon Metabolism with MTHFR Methylation Levels. Int. J. Mol. Sci. 2019, 20, 3754. [Google Scholar] [CrossRef]
- Di Renzo, L.; Marsella, L.T.; Sarlo, F.; Soldati, L.; Gratteri, S.; Abenavoli, L.; De Lorenzo, A. C677T gene polymorphism of MTHFR and metabolic syndrome: Response to dietary intervention. J. Transl. Med. 2014, 12, 329. [Google Scholar] [CrossRef]
- Means, R.T. Iron Deficiency and Iron Deficiency Anemia: Implications and Impact in Pregnancy, Fetal Development, and Early Childhood Parameters. Nutrients 2020, 12, 447. [Google Scholar] [CrossRef]
- Duarte, A.F.M.; Carneiro, A.C.S.V.; Peixoto, A.T.B.M.M.; Montenegro, D.F.P.; Campos, D.S.C.; Alves, A.P.R.; Costa, A.R.M.M.; Fino, A.P.M. Oral Iron Supplementation in Pregnancy: Current Recommendations and Evidence-Based Medicine. Rev. Bras. Ginecol. Obstet. 2021, 43, 782–788. [Google Scholar] [CrossRef] [PubMed]
- Inskip, H.M.; Crozier, S.R.; Godfrey, K.M.; Borland, S.E.; Cooper, C.; Robinson, S.M. Southampton Women’s Survey Study Group. Women’s compliance with nutrition and lifestyle recommendations before pregnancy: General population cohort study. BMJ 2009, 338, b481. [Google Scholar] [CrossRef] [PubMed]
- Crovetto, F.; Crispi, F.; Casas, R.; Martín-Asuero, A.; Borràs, R.; Vieta, E.; Estruch, R.; Gratacós, E. IMPACT BCN Trial Investigators. Effects of Mediterranean Diet or Mindfulness-Based Stress Reduction on Prevention of Small-for-Gestational Age Birth Weights in Newborns Born to At-Risk Pregnant Individuals: The IMPACT BCN Randomized Clinical Trial. JAMA 2021, 326, 2150–2160. [Google Scholar] [CrossRef] [PubMed]
- Di Renzo, L.; Marchetti, M.; Rizzo, G.; Gualtieri, P.; Monsignore, D.; Dominici, F.; Mappa, I.; Cavicchioni, O.; Aguzzoli, L.; De Lorenzo, A.; On Behalf Of The MeDAP Group. Adherence to Mediterranean Diet and Its Association with Maternal and Newborn Outcomes. Int. J. Environ. Res. Public Health 2022, 19, 8497. [Google Scholar] [CrossRef]
| Criterion | Description |
|---|---|
| (P) Population | Pregnant women |
| (I) Interventions | Any nutrition and dietary intervention for a healthy pregnancy (description of micro and macro nutrients, GWG, etc.) |
| (C) Comparators | Any comparator or comparison. No key CPG content is of interest |
| (A) Attributes of eligible CPGs | 1. Local, national, and international CPGs, including consensus panel 2. Only full-text articles available 3. Issued from professional and/or governmental organizations 4. Published since 2000 until 30 May 2022 5. Articles and CPGs in English language 6. Latest version 7. No geographic limitation 8. Reporting nutrition and GWG-related recommendations 9. Intended for health professionals 10. No restrictions on quality, as assessed by the AGREE II instrument |
| (R) Recommendation characteristics and other considerations | Not applicable |
| Guideline | Country | Year | Last Revision | Scope | Methodology |
|---|---|---|---|---|---|
| WHO | International | 2020 | 2020 | International | GRADE and GRADE-CERQual approaches, DECIDE framework |
| RIGA-WHO | Latvia | 2017 | 2017 | International and National | Review of literature, expert opinion |
| FIGO | International | 2015 | 2015 | International | Review of literature, expert panel consensus |
| The Early Nutrition Project partners | International | 2019 | 2019 | National | Review of literature, expert panel consensus |
| NICE | England | 2008 | 2022 | National | Review of literature, expert opinion, and formal consensus |
| The New York Academy of Science | USA | 2020 | 2020 | National | Review of literature, expert panel consensus |
| Fondazione Confalonieri Ragonese | Italy | 2018 | 2018 | National | Review of literature, expert opinion |
| Italian consensus Document | Italy | 2016 | 2016 | National | Review of literature, expert panel consensus |
| Institute of Obstetricians and Gynecologists | Ireland | 2013 | 2019 | National | Review of literature, expert opinion |
| Polish Society of Gynecologists and Obstetricians | Poland | 2020 | 2020 | National | Review of literature, expert opinion |
| Australian government, Department of Health and Ageing | Australia | 2020 | 2020 | National | Review of literature, expert opinion |
| Ministry of Health of New Zealand | New Zeeland | 2006 | 2008 | National | Review of literature, expert opinion |
| Minister of Health, Canada | Canada | 2009 | 2009 | National | Review of literature, expert opinion |
| Alberta Health Services | Canada | 2019 | 2019 | Local | Review of literature, expert opinion |
| Philippine Obstetrical and Gynecological Society | Philippines | 2013 | 2018 | National | Review of literature, expert opinion |
| The Public Health Division of the Pacific Community | Pacific Community- Noumea, New Caledonia | 2019 | 2019 | National | Review of literature, expert opinion |
| The Republic of the Union of Myanmar, Ministry of Health and Sports | Myanmar | 2018 | 2018 | National | Review of literature, expert opinion |
| Family Health Bureau, Ministry of Health | Sri Lanka | 2011 | 2011 | National | Review of literature, expert opinion |
| RD ID Agree II | Domain 1 (Items 1–3) | Domain 2 (Items 4–6) | Domain 3 (Items 7–14) | Domain 4 (Items 15–17) | Domain 5 (Items 18–21) | Domain 6 (Items 22–23) | OA 1 | OA2 |
|---|---|---|---|---|---|---|---|---|
| Canada | 100% | 76% | 71% | 100% | 82% | 93% | 100% | Y (n = 2) YWM (n = 0) N (n = 0) |
| The Early Nutrition Project partners | 81% | 57% | 39% | 33% | 54% | 79% | 29% | Y (n = 0) YWM (n = 0) N (n = 2) |
| The New York Academy of Sciences | 95% | 76% | 61% | 48% | 75% | 79% | 86% | Y (n = 1) YWM (n = 1) N (n = 0) |
| FIGO | 100% | 90% | 88% | 95% | 71% | 100% | 100% | Y (n = 2) YWM (n = 0) N (n = 0) |
| Myanmar | 90% | 57% | 39% | 29% | 54% | 57% | 29% | Y (n = 0) YWM (n = 0) N (n = 2) |
| NICE | 100% | 95% | 86% | 48% | 43% | 100% | 57% | Y (n = 0) YWM (n = 2) N (n = 0) |
| Polish | 100% | 95% | 84% | 100% | 75% | 100% | 57% | Y (n = 0) YWM (n = 2) N (n = 0) |
| Ireland | 81% | 67% | 68% | 86% | 82% | 86% | 86% | Y (n = 1) YWM (n = 1) N (n = 0) |
| RIGA | 62% | 48% | 45% | 62% | 47% | 86% | 43% | Y (n = 0) YWM (n = 1) N (n = 1) |
| Sri Lanka | 95% | 76% | 77% | 62% | 36% | 100% | 71% | Y (n = 0) YWM (n = 2) N (n = 0) |
| WHO | 95% | 76% | 96% | 95% | 75% | 93% | 100% | Y (n = 2) YWM (n = 0) N (n = 0) |
| Alberta | 90% | 81% | 73% | 90% | 68% | 50% | 86% | Y (n = 2) YWM (n = 0) N (n = 0) |
| Confalonieri Ragonese | 76% | 81% | 79% | 86% | 81% | 36% | 86% | Y (n = 2) YWM (n = 0) N (n = 0) |
| Italian Consensus | 95% | 76% | 66% | 81% | 54% | 79% | 43% | Y (n = 0) YWM (n = 1) N (n = 1) |
| New Caledonia | 81% | 67% | 32% | 57% | 50% | 29% | 29% | Y (n = 0) YWM (n = 0) N (n = 2) |
| Philippines | 57% | 48% | 61% | 86% | 64% | 79% | 43% | Y (n = 0) YWM (n = 1) N (n = 1) |
| New Zealand | 81% | 86% | 61% | 86% | 64% | 64% | 71% | Y (n = 0) YWM (n = 2) N (n = 0) |
| Australia | 100% | 76% | 79% | 48% | 39% | 100% | 57% | Y (n = 0) YWM (n = 2) N (n = 0) |
| Average score for each domain (n%) | 88% | 74% | 67% | 72% | 62% | 78% | 65% | |
| SD for each domain (±%) | 13% | 33% | 18% | 23% | 15% | 22% | 25% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Vito, M.; Alameddine, S.; Capannolo, G.; Mappa, I.; Gualtieri, P.; Di Renzo, L.; De Lorenzo, A.; D’ Antonio, F.; Rizzo, G. Systematic Review and Critical Evaluation of Quality of Clinical Practice Guidelines on Nutrition in Pregnancy. Healthcare 2022, 10, 2490. https://doi.org/10.3390/healthcare10122490
De Vito M, Alameddine S, Capannolo G, Mappa I, Gualtieri P, Di Renzo L, De Lorenzo A, D’ Antonio F, Rizzo G. Systematic Review and Critical Evaluation of Quality of Clinical Practice Guidelines on Nutrition in Pregnancy. Healthcare. 2022; 10(12):2490. https://doi.org/10.3390/healthcare10122490
Chicago/Turabian StyleDe Vito, Marika, Sara Alameddine, Giulia Capannolo, Ilenia Mappa, Paola Gualtieri, Laura Di Renzo, Antonino De Lorenzo, Francesco D’ Antonio, and Giuseppe Rizzo. 2022. "Systematic Review and Critical Evaluation of Quality of Clinical Practice Guidelines on Nutrition in Pregnancy" Healthcare 10, no. 12: 2490. https://doi.org/10.3390/healthcare10122490
APA StyleDe Vito, M., Alameddine, S., Capannolo, G., Mappa, I., Gualtieri, P., Di Renzo, L., De Lorenzo, A., D’ Antonio, F., & Rizzo, G. (2022). Systematic Review and Critical Evaluation of Quality of Clinical Practice Guidelines on Nutrition in Pregnancy. Healthcare, 10(12), 2490. https://doi.org/10.3390/healthcare10122490










