Utility of Cardiac Power Hemodynamic Measurements in the Evaluation and Risk Stratification of Cardiovascular Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Process
2.2. Eligibility
2.3. Study Screening and Data Collection
2.4. Bias Screening
3. Results
3.1. Aortic Stenosis
3.2. Septic Shock
3.3. Post-Myocardial Infarction
3.4. Heart Failure
3.5. Cardiogenic Shock
3.6. Critical Cardiac Disease
3.7. Other Diseases
3.7.1. Non-Cardiac Related Illness
3.7.2. Heart Transplant
3.7.3. Chronic Kidney Disease
3.7.4. Ischemic Cardiomyopathy
3.7.5. Extracorporeal Circulation
4. Discussion
Study Limitations
5. Conclusions
| Article Title | Authors | Year | Journal | Study Participation Bias | Study Attrition Bias | Prognostic Factor Measurement Bias | Outcome Measurement Bias | Study Confounding Bias | Statistical Analysis and Reporting | Overall Bias Score |
|---|---|---|---|---|---|---|---|---|---|---|
| Prognosis of in-hospital myocardial infarction course for diabetic and nondiabetic patients using a noninvasive evaluation of hemodynamics and heart rate variability. | Ablonskytė-Dūdonienė, R.; Bakšytė, G.; Ceponienė, I.; Kriščiukaitis, A.; Drėgūnas, K.; Ereminienė, E. | 2013 | Medicina (Kaunas) | Low | Low | Low | Low | Low | Low | Low |
| Impedance cardiography and heart rate variability for long-term cardiovascular outcome prediction after myocardial infarction. | Ablonskytė-Dūdonienė, R.; Bakšytė, G.; Čeponienė, I.; Kriščiukaitis, A.; Drėgūnas, K.; Ereminienė, E. | 2012 | Medicina (Kaunas) | Low | High | Low | Low | Low | Low | Low |
| Does Resting Cardiac Power Index Affect Survival Post Transcatheter Aortic Valve Replacement? | Agasthi, P.; Arsanjani, R.; Mookadam, F.; Wang, P.; Venepally, N.R.; Sweeney, J.; Eleid, M.; Holmes, D.R., Jr; Pollak, P.; Fortuin, F.D. | 2020 | Journal Invasive Cardiology | Low | Moderate | Low | Low | Low | Low | Low |
| Artificial intelligence trumps TAVI(2)-SCORE and CoreValve Score in predicting 1-year mortality post Transcatheter Aortic Valve Replacement. | Agasthi, P.; Ashraf, H.; Pujari, S.H.; Girardo, M.E.; Tseng, A.; Mookadam, F.; Venepally, N.R.; Buras, M.; Khetarpal, B.K.; Allam, M.; Eleid, M.F.; Greason, K.L.; Beohar, N.; Siegel, R.J.; Sweeney, J.; Fortuin, F.D.; Holmes, D.R., Jr; Arsanjani, R. | 2020 | Cardiovascular Revascularization Medicine | Low | Moderate | Low | LOW | Low | Low | Low |
| Does a Gradient-Adjusted Cardiac Power Index Improve Prediction of Post-Transcatheter Aortic Valve Replacement Survival Over Cardiac Power Index? | Agasthi, P.; Pujari, S.H.; Mookadam, F.; Tseng, A.; Venepally, N.R.; Wang, P.; Allam, M.; Sweeney, J.; Eleid, M.; Fortuin, F.D.; Holmes, D.R.; Beohar, N.; Arsanjani, R. | 2020 | Yonsei Medical Journal | Low | moderate | Low | LOW | Low | Low | Low |
| Differential responses to larger volume intra-aortic balloon counter-pulsation: Hemodynamic and clinical outcomes. | Baran, D.A.; Visveswaran, G.K.; Seliem, A.; DiVita, M.; Wasty, N.; Cohen, M. | 2018 | Catheterization and Cardiovascular Interventions | Moderate | Moderate | Low | Low | Low | Low | Low |
| Improved Outcomes Associated with the use of Shock Protocols: Updates from the National Cardiogenic Shock Initiative. | Basir, M.B. | 2019 | Catheter Cardiovasc Interv | Low | Low | Low | Low | Low | Low | Low |
| The value of cardiac output studies in postoperative cardiac patients: a myth | Clark, R.E.; Siegfried, B.A.; Ferguson, T.B. | 1976 | Journal of Surgical Research | Moderate | Moderate | Moderate | Low | Moderate | Low | Mild |
| Prognostic value of cardiac power output to left ventricular mass in patients with left ventricular dysfunction and dobutamine stress echo negative by wall motion criteria. | Cortigiani, L.; Sorbo, S.; Miccoli, M.; Scali, M.C.; Simioniuc, A.; Morrone, D.; Bovenzi, F.; Marzilli, M.; Dini, F.L. | 2017 | European Heart Journal—Cardiovascular Imaging | Low | Low | Low | Low | Low | Low | Low |
| Impaired microcirculation predicts poor outcome of patients with acute myocardial infarction complicated by cardiogenic shock. | den Uil, C.A.; Lagrand, W.K.; van der Ent, M.; Jewbali, L.S.; Cheng, J.M.; Spronk, P.E.; Simoons, M.L. | 2010 | European Heart Journal | Low | Low | Low | Low | Low | Low | Low |
| Non-invasive hemodynamic profiling of patients undergoing hemodialysis—A multicenter observational cohort study. | Doenyas-Barak, K.; de Abreu, M.H.F.G.; Borges, L.E.; Tavares Filho, H.A.; Yunlin, F.; Yurong, Z.; Levin, N.W.; Kaufman, A.M.; Efrati, S.; Pereg, D.; Litovchik, I.; Fuchs, S.; Minha, S. | 2019 | BMC Nephrology | Low | Moderate | Low | Low | Low | Low | Low |
| Cardiac power is the strongest hemodynamic correlate of mortality in cardiogenic shock: a report from the SHOCK trial registry. | Fincke, R.; Hochman, J.S.; Lowe, A.M.; Menon, V.; Slater, J.N.; Webb, J.G.; LeJemtel, T.H.; Cotter, G. | 2004 | Journal of the American College of Cardiology | Low | Moderate | Low | Low | Low | Low | Low |
| Emergency transcatheter aortic valve replacement in patients with cardiogenic shock due to acutely decompensated aortic stenosis. | Frerker, C.; Schewel, J.; Schlüter, M.; Schewel, D.; Ramadan, H.; Schmidt, T.; Thielsen, T.; Kreidel, F.; Schlingloff, F.; Bader, R.; Wohlmuth, P.; Schäfer, U.; Kuck, K.H. | 2016 | Eurointervention | Low | Moderate | Low | Low | High | Low | Mild |
| Experience with the Impella recovery axial-flow system for acute heart failure at three cardiothoracic centers in Sweden. | Granfeldt, H.; Hellgren, L.; Dellgren, G.; Myrdal, G.; Wassberg, E.; Kjellman, U.; Ahn, H. | 2009 | Scandinavian Cardiovascular Journal | Low | Low | Low | Low | Moderate | Low | Low |
| Prognostic role of cardiac power index in ambulatory patients with advanced heart failure. | Grodin, J.L.; Mullens, W.; Dupont, M.; Wu, Y.; Taylor, D.O.; Starling, R.C.; Tang, W.H. | 2015 | European Journal of Heart Failure | Low | Low | Low | Low | Moderate | Low | Low |
| Right Atrial Pressure Predicts Mortality Among LVAD Recipients: Analysis of the INTERMACS Database. | Guglin, M.; Omar, H.R. | 2020 | Heart, Lung and Circulation | Moderate | Low | Low | Low | Moderate | Low | |
| Cardiac power index: staging heart failure for mechanical circulatory support. | Hall, S.G.; Garcia, J.; Larson, D.F.; Smith, R. | 2012 | Perfusion | Moderate | Low | Low | Low | High | Low | |
| Predictors of intra-aortic balloon pump hemodynamic failure in non-acute myocardial infarction cardiogenic shock. | Hsu, S.; Kambhampati, S.; Sciortino, C.M.; Russell, S.D.; Schulman, S.P. | 2018 | American Heart Journal | Low | Moderate | Moderate | Low | Moderate | Low | |
| Hemodynamic parameters are prognostically important in cardiogenic shock but similar following early revascularization or initial medical stabilization—A report from the SHOCK trial | Jeger, R.V.; Lowe, A.M.; Buller, C.E.; Pfisterer, M.E.; Dzavik, V.; Webb, J.G.; Hochman, J.S.; Jorde, U.P.; Shock Investigator | 2007 | Chest | Low | High | Low | Low | Low | Low | |
| Noninvasive Hemodynamic Assessment of Shock Severity and Mortality Risk Prediction in the Cardiac Intensive Care Unit | Jentzer, J.C.; Wiley, B.M.; Anavekar, N.S.; Pislaru, S.V.; Mankad, S.V.; Bennett, C.E.; Barsness, G.W.; Hollenberg, S.M.; Holmes, D.R.; Oh, J.K. | 2021 | JACC: Cardiovascular Imaging | Low | Moderate | Low | Low | Low | Low | |
| Cardiac contractile reserve parameters are related to prognosis in septic shock. | Kimmoun, A.; Ducrocq, N.; Mory, S.; Delfosse, R.; Muller, L.; Perez, P.; Fay, R.; Levy, B. | 2013 | BioMed Research International | Low | Low | Low | Low | Moderate | Low | |
| Cardiac Power Output Index and Severe Primary Graft Dysfunction After Heart Transplantation. | Lim, H.S.; Ranasinghe, A.; Chue, C.; Quinn, D.; Mukadam, M.; Mascaro, J. | 2021 | Journal of Cardiothoracic and Vascular Anesthesia | Low | Low | Low | Low | Low | Low | |
| Circulating angiopoietins and cardiovascular mortality in cardiogenic shock. | Link, A.; Pöss, J.; Rbah, R.; Barth, C.; Feth, L.; Selejan, S.; Böhm, M. | 2013 | European Heart Journal | Low | Low | Low | Low | Moderate | Low | |
| Cardiac power output predicts mortality across a broad spectrum of patients with acute cardiac disease. | Mendoza, D.D.; Cooper, H.A.; Panza, J.A. | 2007 | American Heart Journal | Low | Low | Low | Low | Moderate | Low | |
| Cardiopulmonary and Noninvasive Hemodynamic Responses to Exercise Predict Outcomes in Heart Failure | Myers, J.; Wong, M.; Adhikarla, C.; Boga, M.; Challa, S.; Abella, J.; Ashley, E.A. | 2013 | Journal of Cardiac Failure | Low | Low | Low | Low | Moderate | Low | |
| Cardiac power index, mean arterial pressure and Simplified Acute Physiology Score II are strong predictors of survival and response to revascularization in cardiogenic shock. | Popovic, B.; Fay, R.; Cravoisy-Popovic, A.; Levy, B. | 2014 | Shock | Low | Low | Moderate | Low | Moderate | Low | |
| Echo-derived peak cardiac power output-to-left ventricular mass with cardiopulmonary exercise testing predicts outcome in patients with heart failure and depressed systolic function. | Pugliese, N.R.; Fabiani, I.; Mandoli, G.E.; Guarini, G.; Galeotti, G.G.; Miccoli, M.; Lombardo, A.; Simioniuc, A.; Bigalli, G.; Pedrinelli, R.; Dini, F.L. | 2019 | European Heart Journal Cardiovascular Imaging | Low | Low | Low | Low | Low | Low | |
| The short-term prognosis of cardiogenic shock can be determined using hemodynamic variables: a retrospective cohort study*. | Rigamonti, F.; Graf, G.; Merlani, P.; Bendjelid, K. | 2013 | Critical Care Medicine | Low | Moderate | Low | Low | Moderate | Low | |
| Peak cardiac power measured noninvasively with a bioreactance technique is a predictor of adverse outcomes in patients with advanced heart failure. | Rosenblum, H.; Helmke, S.; Williams, P.; Teruya, S.; Jones, M.; Burkhoff, D.; Mancini, D.; Maurer, M.S. | 2010 | Congestive Heart Failure | Low | Moderate | Low | Low | Moderate | Low | |
| Prognostic factors of chronic heart failure in NYHA class II or III: value of invasive exercise hemodynamic data. | Roul, G.; Moulichon, M.E.; Bareiss, P.; Gries, P.; Koegler, A.; Sacrez, J.; Germain, P.; Mossard, J.M.; Sacrez, A. | 1995 | European Heart Journal | Low | Low | Low | Low | Low | Low | |
| Current Use and Impact on 30-Day Mortality of Pulmonary Artery Catheter in Cardiogenic Shock Patients: Results From the CardShock Study. | Sionis, A.; Rivas-Lasarte, M.; Mebazaa, A.; Tarvasmäki, T.; Sans-Roselló, J.; Tolppanen, H.; Varpula, M.; Jurkko, R.; Banaszewski, M.; Silva-Cardoso, J.; Carubelli, V.; Lindholm, M.G.; Parissis, J.; Spinar, J.; Lassus, J.; Harjola, V.P.; Masip, J. | 2020 | Journal of Intensive Care Medicine | Low | High | Low | Low | Moderate | Low | |
| Measurement of cardiac reserve in cardiogenic shock: implications for prognosis and management. | Tan, L.B.; Littler, W.A. | 1990 | British Heart Journal | Low | Low | Low | Low | Low | Low | |
| Standardized Team-Based Care for Cardiogenic Shock. | Tehrani, B.N.; Truesdell, A.G.; Sherwood, M.W.; Desai, S.; Tran, H.A.; Epps, K.C.; Singh, R.; Psotka, M.; Shah, P.; Cooper, L.B.; Rosner, C.; Raja, A.; Barnett, S.D.; Saulino, P.; deFilippi, C.R.; Gurbel, P.A.; Murphy, C.E.; O’Connor, C.M. | 2019 | JACC | Low | Low | Low | Low | Low | Low | |
| The relationship between cardiac reserve and survival in critically ill patients receiving treatment aimed at achieving supranormal oxygen delivery and consumption | Timmins, A.C.; Hayes, M.; Yau, E.; Watson, J.D.; Hinds, C.J. | 1992 | Postgraduate Medical Journal | Moderate | Low | Low | Low | Low | Low | |
| Hemodynamic variables and mortality in cardiogenic shock: a retrospective cohort study. | Torgersen, C.; Schmittinger, C.A.; Wagner, S.; Ulmer, H.; Takala, J.; Jakob, S.M.; Dünser, M.W. | 2009 | Critical Care | Low | Low | Low | Low | Low | Low | |
| Early worsening heart failure in patients admitted for acute heart failure: time course, hemodynamic predictors and outcome. | Torre-Amione, G.; Milo-Cotter, O.; Kaluski, E.; Perchenet, L.; Kobrin, I.; Frey, A.; Rund, M.M.; Weatherley, B.D.; Cotter, G. | 2009 | Journal of Cardiac Failure | Low | Moderate | Low | Low | Moderate | Low | |
| Septic cardiomyopathy: hemodynamic quantification, occurrence and prognostic implications. | Werdan, K.; Oelke, A.; Hettwer, S.; Nuding, S.; Bubel, S.; Hoke, R.; Russ, M.; Lautenschläger, C.; Mueller-Werdan, U.; Ebelt, H. | 2011 | Clinical Research in Cardiology | Low | Low | Low | Low | Moderate | Low | |
| Severity of cardiac impairment in the early stage of community-acquired sepsis determines worse prognosis. | Wilhelm, J.; Hettwer, S.; Schuermann, M.; Bagger, S.; Gerhardt, F.; Mundt, S.; Muschik, S.; Zimmermann, J.; Bubel, S.; Amoury, M.; Kloess, T.; Finke, R.; Loppnow, H.; Mueller-Werdan, U.; Ebelt, H.; Werdan, K. | 2013 | Clinical Research in Cardiology | Low | Low | Low | Low | Low | Low | |
| Peak exercise cardiac power output; a direct indicator of cardiac function strongly predictive of prognosis in chronic heart failure. | Williams, S.G.; Cooke, G.A.; Wright, D.J.; Parsons, W.J.; Riley, R.L.; Marshall, P.; Tan, L.B. | 2001 | European Heart Journal | Low | Low | Low | Low | Low | Low | |
| How do different indicators of cardiac pump function impact upon the long-term prognosis of patients with chronic heart failure? | Williams, S.G.; Jackson, M.; Cooke, G.A.; Barker, D.; Patwala, A.; Wright, D.J.; Albuoaini, K.; Tan, L.B. | 2005 | American Heart Journal | Low | Low | Low | Low | Low | Low | |
| Evaluation of Resting Cardiac Power Output as a Prognostic Factor in Patients with Advanced Heart Failure. | Yildiz, O.; Aslan, G.; Demirozu, Z.T.; Yenigun, C.D.; Yazicioglu, N. | 2017 | The American Journal of Cardiology | Low | Low | Low | Low | Moderate | Low |
| Article # | Outcomes | Method of Measuring CP | Timing of When CP Was Measured | Definition of Disease State | Method of Calculating CP | Population (Included and Analyzed) | Age | Sex | |
|---|---|---|---|---|---|---|---|---|---|
| TAVR due to AS | 22 | All-cause mortality, 1 year (post TAVR) | Echocardiography | Prior to TAVR | Symptomatic Severe AS; where severe AS was based on criteria set forth by the American Society of Echocardiography and Society for Cardiovascular Magnetic Resonance. | (MAP × CO)/(451 × BSA) | 975 | 81 ± 8.6 | 59.4% male, 40.6% female |
| TAVR due to AS | 23 | All-cause mortality, 1 year (post TAVR) | Echocardiography | Prior to TAVR | Symptomatic Severe Aortic Stenosis; where severe AS was based on criteria set forth by the American Society of Echocardiography and Society for Cardiovascular Magnetic Resonance. | 1055 (907 alive, 148 dead) | 80.9 ± 7.9 (81 ± 9 (alive), 80 ± 9 (dead)) | 58.2% male (58% (alive), 59.5% (dead)) | |
| TAVR due to AS | 24 | All-cause mortality, 1 year (post TAVR) | Echocardiography | Prior to TAVR | Symptomatic Severe Aortic Stenosis; where severe AS was based on criteria set forth by the American Society of Echocardiography and Society for Cardiovascular Magnetic Resonance. | (MAP × CO)/(451 × body surface area (BSA)) (W/m2) | 975 (840 alive, 135 dead) | 81 ± 9 | 59.04% male |
| TAVR due to AS (emergent; in CS) | 30 | Mortality up to 1 year | PAC | Prior to TAVR | High risk patients with AS, which starting with patient #165 included patients in cardiogenic shock due to AS basing cardiogenic shock on the IABP-SHOCK II trial. | MAP × CI × 0.0022 | 771 (27 emergent) | 80 ± 7 | 46.8% male |
| Septic shock | 17 | 28 day mortality | PiCCO (thermodilution) | Cardiac Power data was obtained during a “hemodynamically stable period” which was designated as day 0. | Patients were included in first 12 hours after diagnosing septic shock defined by a SBP < 90 mmHG (or a decrease of >50 mmHg in patients known to be hypertensive), a persisting MAP < 70 mmHg or DBP ≤ 40 mmHg despite adequate fluid resuscitation requiring vasoactive support by norepinephrine >0.05 mu/kg/min during more than one hour. Followed Surviving Sepsis Campaign guidelines. | CI × MAP/451 | 70 | 62 ± 16 | 72.9% (51/70) male |
| Septic shock | 18 | 30-day mortality (following ED admission) | PiCCO (central line + arterial catheter) or PAC in ICU, transthoracic bioimpedance on wards | At time of admission into the ED | Patients determined to have clinical sepsis which indicated they met at least 2 of the four criteria being Body temperature >38 °C or <36 °C; HR >90; breathing rate >20/min or hyperventilation (PaCO2 < 4.3 kPa); leukocytosis >12,000 or leukopenia <4000 or >10% premature granulocytes. From there patients were classified to stages of sepsis according to the recommendations of the consensus conference of the American Collage of Chest Physicians and the Society of Critical Care Medicine and current sepsis guidelines (sepsis-severe sepsis-septic shock) | CPI = CI*MAP*0.0022; CI = CO/BSA | 141 | 68 (56; 77) | 61% male |
| Septic shock | 48 | ICU mortality. Correlation with APACHE II and Sepsis scores | PAC | Began once patients were included into the study and continually performed throughout the study. | Patients in medical intensive care/critical care ward who stayed longer than 24 h and having septic Multi-Organ dysfunction syndrome indicating they had an APACHE II score ≥20 and sepsis score ≥12. | 39 but 24 obtained hemodynamic measurements | 52 (64 for patients with hemodynamic data) | 62% male (total), 67% (16/24) in subgroup with hemodynamic data | |
| Post-MI | 20 | In-hospital, 1 year, 5 year mortality. Secondary outcomes were ischemic complications: recurrent in-hospital ischemia, recurrent nonfatal MI and need for revascularization procedures (PCI or CABG) | Thoracic electrical bioimpedance (HeartLab) | Thoracic electrical bioimpedance was used to assess CP shortly after consent and again on day 3, usually the first 10 s to determine CP. | Patients with ST-elevation myocardial infarct and following diagnosis of acute MI based on criteria provided by the European Society of Cardiology. | (CO × MAP)/451. (CI × MAP)/451 | 208 | 63 (53–70) | 71.2% male |
| Post-MI | 21 | In-hospital mortality (primary). Secondary: complicated in-hospital course (hemodynamically unstable arrhythmia, cardiogenic shock, pulmonary edema, clinically significant recurrent myocardial ischemia, or death) | Thoracic electrical bioimpedance (HeartLab) | Thoracic electrical bioimpedance was used to assess CP shortly after consent and again on day 3, usually the first 10 s to determine CP. | Patients with ST-elevation myocardial infarct and following diagnosis of acute MI based on criteria provided by the European Society of Cardiology. | 232 (67 w/diabetes, 165 w/o) | 62.8 (10.7) | 69.8% male (73.9% in diabetic, 59.7% nondiabetic, p = 0.032) | |
| Critical Cardiac Disease (CCU) | 3 | In-hospital mortality | PAC (average of 3–5 readings) | Used baseline data for patients admitted to the CCU. | Patients with primary cardiac diagnosis who were undergoing PAC, most common primary diagnosis were myocardial infarct, cardiomyopathy, coronary atherosclerosis and unstable angina. | MAP × CO/451 | 349 | 64 ± 14 | 59% male (64% for CPO >0.53, 34% for CPO ≤0.53, p < 0.001 |
| Critical Cardiac Disease (CICU) | 35 | In-hospital mortality | Echocardiography | Patients had transthoracic echocardiogram within the first day of CICU admission. | Patients were included that were admitted to the CICU and met any stage of the Society for Cardiovascular Angiography and Intervention shock stages. | (CO × MAP)/451. MAP = (SBP + (2 × DBP))/3 | 5453 | 69.3 (58.2–79.0) | 63.3% male. 2.001 (36.7) female |
| Heart failure | 15 | Patient followed up for minimum of 2 years or until death. | CO2 rebreathing | Outpatient cardiopulmonary treadmill exercise testing | Mild-moderate chronic stable heart failure patients | CPO = (CO*MAP)*0.00222. MAP = (SBP + 2*DBP)/3 | 219 | 56.14 ± 13.13 | 76% male |
| Heart failure | 16 | All-cause mortality, heart transplant, or VAD placement (median follow-up 3.3 years) | PAC | Outpatient pulmonary artery catheterization | Advanced chronic heart failure for greater than six months | MAP × CO × 0.0022 | 495 | 54 ± 11 | 75.8% male |
| Heart failure | 31 | Mortality up to 1 year | Echocardiography | Hemodynamic measurements were taken preoperatively and at 6 and 12 h postoperatively. | Patients with HF that led to cardiogenic shock which was defined as unable to meet systemic circulatory demands without supportive measures, except for correction of volume or vascular resistance. | CO × MAP/451 | 50 (33 surgical, 17 non-surgical | 54.5 (58.1 surgical, 47.5 non-surgical) | 70% male |
| Heart failure | 32 | All-cause mortality (up to 5 years) | PAC | Hemodynamic measurements were taken pre-left ventricular assist device implantation | Patients with advanced heart failure who are receiving left ventricular assist device | (MAP × CO)/451 | 18733 | ||
| Heart failure | 33 | Survival at 90 days post MCS | PAC and/or echo | Hemodynamics measurements were taken when patients were first admitted and prior to any inotropic drug therapy. | Patients with New York Heart Association classes II and IV and American College of Cardiology stages C and D. | (MAP × CO) × 0.0022]/BSA. MAP = ((2 × diastolic) + systolic/3 | 28 | 53 ± 2.5 | 85.7% male (24/28) |
| Heart failure | 38 | A composite endpoint was used as the outcome, which included cardiac-related death, hospitalization for worsening HF, cardiac transplantation and left ventricular assist device (LVAD) implantation. Mean follow up was 460 ± 332 days | Thoracic bioimpedance | Hemodynamic measurements were obtained upon initial evaluation | Patients initially evaluated for heart failure presentation. | peak CPO = peak (MAP × CO)/451 | 639 | 48.2 ± 14 | 62% male |
| Heart failure | 40 | All-cause mortality, VAD implantation and heart transplantation was the primary endpoint. Combination of the former with hospitalization for worsening HF was designated as the secondary endpoint. Follow up 34.5 (10.9–57.6) months | Echocardiography | Initial hemodynamic workup was performed upon initial presentation at university. | Chronic systolic heart failure with LVEF ≤40% | CPO = k*CO*MBP. K = 2.22*10−3. MBP = diastolic BP + 1/3 (systolic BP–diastolic BP). CPOM = (CPO*100)/LVM | 159 | 62 (56–70) | 81.8% male |
| Heart failure | 42 | Death, heart transplant, or LVAD implantation. Follow-up averaged 404 ± 179 days (median, 366 days) | Bioimpedance | Initial hemodynamic workup was performed upon initial presentation at medical center. | Moderate to advanced chronic heart failure | CPO = (CPO*MAP)/451. MAP = DBP + (SBP − DBP)/3 | 127 | 53 ± 14 | 66% male (40% with event, 69% without, p = 0.0388) |
| Heart failure | 43 | 1 year mortality or major events (serious ventricular arrhythmia, acute pulmonary edema, or hospitalization for HF) | PAC | Initial hemodynamic workup was performed upon initial presentation. | Stable chronic systolic heart failure with New York Heart Association class II or III. | (MAP-RAP)*CO*0.00222. MAP = DBP + (SBP − DBP)/3 | 50 | 54.5 ± 1.6 | 64% male |
| Heart failure | 47 | WHF at 7/30 days, 6 month mortality. WHF: new pulmonary edema or cardiogenic shock, no resolution of symptoms and signs of HF in first 24 h despite therapy, or worsening symptoms and signs of HF despite therapy along with an increase in treatment for HF (IV, vent, MCS) | PAC | Baseline hemodynamic values were obtained at initial presentation. | Acute heart failure requiring hemodynamic monitoring | CPO = MAP*CO*0.0022 | 120 (42 WHF in 7 days, 50 WHF in 30 days, 17 deaths) | 64.2 ± 13.6 | 86% male |
| Heart failure | 49 | Patients followed for 6 years or until death (median follow up 8.6 years). | CO2 rebreathing | Baseline hemodynamic values were obtained at initial presentation. | Mild to moderate chronic systolic heart failure. | CPO = (Total O2 consumption/(O2)pulmonary veins-(O2) pulmonary arteries))*MAP | 219 | 56.14 ± 13.13 | 76% male |
| Heart failure | 50 | All-cause mortality, VAD placement, or heart transplant | Right and left cardiac catheterization | Hemodynamic measurements were obtained upon initial evaluation | Advanced chronic heart failure severe enough to warrant cardiac tertiary care institution. | CPO = (MAP*CO)/(0.00222), CPI = (CI*MAP/0.00222). MAP = ((SBP-DBP)/3) + DBP | 161 | 58.7 (11.2) | 73.9% male |
| Cardiogenic shock | 6 | In-hospital mortality | PAC/cardiac catheterization | Hemodynamic measurements were made within 6 h pre shock and 12 h post-shock. | Predominant left ventricular failure causing cardiogenic shock determined form the SHOCK trial registry and eliminating other categories of cardiogenic shock such as cardiac tamponade, severe valvular heart disease, dilated cardiomyopathy, ventricular septal rupture and more which was determined based on clinical grounds. | MAP × CO/451; MAP = ((SBP − DBP)/3) + DBP | 541 | 67.8 ± 12.4 | 65.3% male |
| Cardiogenic shock | 11 | 30 day (post-discharge) mortality | PAC (82%) and echo | Cardiac Power data was obtained once shock definition was met and repeated at 24 h after cardiogenic shock was diagnosed. | Shock was determined based on the following definition: as SBP < 90 mmHg for >30 min (or use of inotropes/vasopressors to maintain SBP), evidence of end-organ hypoperfusion and lactate level >2 mmol. Afterwards cardiogenic shock was defined with Fick cardiac index <1.8 L/min/m2 without inotropes/vasopressors (or <2.2 L/min/m2 with inotropes/vasopressors), PCWP >15 mmHg, CPO < 0.6 W, pulmonary arterial pulsatility index <1.0. | 204 | 61 ± 13 | 70% male (for AMI 70.7%, for ADHF68.8%) | |
| Cardiogenic shock | 12 | 30-day mortality | PAC | Hemodynamic measurements were collected at 0,6,12,24,48,72 and 96 h after detection of shock. | Cardiogenic shock required SBP <90 mmHg in absence of hypovolemia and after adequate fluid challenge for 30 min or need for vasopressor therapy to maintain SBP >90 mmHg, symptoms and/or signs of systemic and/or pulmonary congestion and symptoms and/or signs of hypoperfusion (Altered mental state, confusion, cold periphery, oliguria <0.5 mL/kg/h for the previous 6 h, blood lactate >2 mmol/L) all of which was determined to be due to cardiac etiology. | (MAP*CI)/451 | 219 (82 w/PAC, 137 w/o) | 65(12) w/PAC, 68(11) w/o, p = 0.09 | 74.0% male overall (78% w/PAC, 72% w/o) |
| Cardiogenic shock | 13 | Event free survival for 28 days. Adverse events defined as death or emergent MCS escalation, favorable outcomes include survival to discharge, successful bridge to durable LVAD, or transplant | PAC | Hemodynamic measurements performed prior to intra-aortic balloon pump placement. | Patients undergoing intra-aortic balloon pump for cardiogenic shock. | (CI × MAP)/451. RVCPI = (CI × mPAP)/451 | 74 | 54.8 ± 14.1 | 66% male |
| Cardiogenic shock | 14 | 30 day mortality | PAC (71%), CVP (29%); thermodilution | Baseline hemodynamic values were obtained at initial presentation. | Patients were admitted initially for acute myocardial infarct but presented with cardiogenic shock defined by sustained hypotension (systolic blood pressure < 90 mmHg) induced by heart failure together with the clinical signs of hypoperfusion (cold extremities, oliguria, or altered mental state), not responsive to fluid resuscitation. | (MAP × CI)/451 | 68 | 60 ± 14 | 69% male |
| Cardiogenic shock | 25 | Increase in CO within 15 h of IABP placement. Also looks at 60/90 day survival | PAC | All hemodynamic measurements were obtained at baseline evaluations. | Cardiogenic shock was defined as persistent hypotension with SBP ≤90 mmHg or MAP 30 mmHg lower than baseline and reduction in CI ≤ 1.8 L/min/m2 without inotropic support or <2.2 L/min/m2 with inotropic support and adequate filling pressure with left ventricular end diastolic pressure ≥18 mmHg or Right ventricular end diastolic pressure >10 mmHg. | (MAP × CO)/451, (MAP × CI/451) | 76 | 55.6 ± 13.2 | 80.2% male |
| Cardiogenic shock | 26 | In-hospital mortality | PAC (92% of patients, hemodynamic data only recorded from these patients) | Hemodynamic measurements were taken both pre-procedure and post-procedure. | Cardiogenic shock was defined as persistent hypotension with SBP ≤90 mmHg or inotropes/vasopressors to maintain SBP >90 mmHg, signs of end organ hypoperfusion (cool extremities, oliguria or anuria or elevated lactate levels and hemodynamic criteria represented by CI <2.2 L/min/m2 or CPO <0.6 W. | (MAPxCO)/451 | 171 | 63.4 ± 12.4. Non-survivors/survivors: 63.4 ± 12.4/61.4 ± 12.6, p < 0.01 | 77.2% male (68.8%/80.5% non-survivors/survivors) |
| Cardiogenic shock | 34 | 30 day mortality | PAC | Baseline hemodynamics were obtained at 3.3 h after cardiogenic shock and follow up values were measured with median time 10.6 h in the medical stabilization group and 12.5 h in the early vascularization group. | Cardiogenic shock was defined as SBP <90 mmHg for 30 min or supportive measures such as vasopressors or intra-aortic balloon counter pulsation required to maintain a blood pressure of ≥90 mmHg with evidence of decreased organ perfusion (urine output of ≤30 mL/h or cool and diaphoretic extremities and a HR of ≥60 beats/min). Hemodynamic criteria for cardiogenic shock were pulmonary capillary wedge pressure ≥18 mmHg and CI of ≤2.2 L/min/m2. | (MAP × CI)/451. MAP = (systolic BP − diastolic BP)/3 + diastolic BP | 278 | 66 ± 11 | 67% male |
| Cardiogenic shock | 37 | Mortality at 28 days, 1 year | PAC/PiCCO (thermodilution) | Hemodynamic measurements were made on initial workup for patients in the intensive care unit. | Cardiogenic shock was defined as SBP <90 mmHg in the absence of hypovolemia or vasopressors, a reduction in CI <1.9 L/min/m2 and or an elevation of pulmonary capillary wedge pressure >19 mmHg all due to a cardiac etiology. | 96 (60 survived to 28 days, 37 to 1 year) | Survivors: 66 (20; 90), non-survivors: 70 (43; 86) | 72.9% male. 41/60 male (survivors), 29/36 (non-survivors) | |
| Cardiogenic shock | 39 | In-hospital mortality. Death, new MI, urgent PCI, stroke, sepsis, major hemorrhagic complications according to TIMI criteria. Follow up 28 days and 1.9 ± 0.9 years post discharge | PAC/PiCCO (thermodilution) and echo | All hemodynamic measurements were obtained at baseline evaluation. | Cardiogenic shock due to myocardial infarction which was defined by SBP < 90 mmHg for 30 min or MAP of less than 60 mmHg for 30 min or vasopressor use with evidence of decreased organ perfusion. The manifestations of hypoperfusion could include, but are not limited to lactic acidosis, oliguria, or an acute alteration in mental status. Hemodynamic criteria were pulmonary capillary wedge pressure of more than 15 mmHg and a cardiac index of less than 2.2 L/min/m2 in patients not treated with vasopressor/inotropes. | 85 | 64(15). 62(12) for S, 66(13) for NS, p > 0.05 | 74% male overall. 31% female in S, 19% in NS, p > 0.05 | |
| Cardiogenic shock | 41 | Mortality ICU, 28 d | PAC/PiCCO (thermodilution; 69/71), but some echo | Hemodynamic variables were collected during the first 24 h after ICU admission. | Cardiogenic shock was defined as SBP < 90 mmHg or MAP< 60 mmHg for 30 min with or without therapy; the need for continuous infusion of inotropic drug; a CI less than 1.8 L/min/m2 without inotropes or less than 2.2 L/min/m2 with inotropes; and a pulmonary artery occlusion pressure greater than 18 mmHg. Classical clinical criteria of shock indicating low organ perfusion were not included in the criteria. | 71 | 65 ± 14 | 52 (73%) male | |
| Cardiogenic shock | 44 | 1-year mortality | PAC | Measurements were done at baseline resting state in patients as well as after optimalisation of preload and usage of dobutamine. | Shock was diagnosed according to Cohn’s criteria which required them to meet two of the following criteria including oliguria with urine output <20 mL/h, cool moist skin, auscultatory SBP <90 mmHg, obtunded mental state, metabolic acidosis all of which should be due to cardiac etiology. | (MAP − RAP) * CO * 2.2167 * 10−3 | 28 | 59 (36–73) | 75% male |
| Cardiogenic shock | 46 | 28 day mortality after ICU admission. Length of ICU/hospital stay, patient outcome at ICU discharge. | PAC (77.2% of subjects; CPI measurements only done for these patients with PAC; values from first 24 h in ICU) | Hemodynamic measurements were obtained within the first 24 h from intensive care unit admission. | Cardiogenic shock was defined as simultaneous presence of all the following criteria immediately before or during the first 24 h after intensive care unit admission: arterial hypotension (SBP <90 mmHg or MAP below 70 mmHg for 30 min or longer with or without therapy), a cardiac index below 2 L/min/m2 and a pulmonary artery occlusion pressure above 18 mmHg in patients with a pulmonary artery catheter or an acute decrease of the left ventricular ejection fraction below 40% in patients without a pulmonary artery catheter; need for continuous infusion of inotropic drugs. | CPI = MAP*CI/451 | 119 (23 ICU mortality, 35 28 day mortality) | 67 ± 14 | 59.7% male |
| Other- Extracorporeal Circulation | 27 | Survival up to 7 days post-procedure | Indocyanine green method | Hemodynamic variables were obtained within the first 26 h after surgery. | Patients who underwent extracorporeal circulation and a cardiac procedure who were admitted directly to a cardiothoracic surgical intensive care unit from the operating room. | CO(MAP-CVP) | 181 | 2–72 (mean 46) | 59.1% male |
| Other- Ischemic Cardiomyopathy | 28 | Mortality (median follow-up of 29 months (inter-quartile range 16–72 months)) | Echocardiography | Hemodynamic measurements were done with the use of dobutamine during initial workup. | Ischemic cardiomyopathy with left ventricle ejection fraction ≤45% which is not due to other cardiac pathologies. | MBP (diastolic BP + 1/3 (systolic BP − diastolic BP)) × CO × 2.22 × 10−3. CPO/LVM = MAP*CO*100*2.22*10−3/LVM | 111 | 68 ± 10 | 85.6% male |
| Other- Chronic Kidney Disease | 29 | All-cause mortality, 12 ± 1 months after the monitored session | Bioimpedance (whole body) | Patient hemodynamic measurements were obtained during a single hemodialysis session. | Patients were determined to have CKD if they had undergone chronic hemodialysis for at least 3 months. | MAP × CI/451 | 144 | 67.3 ± 12.1 | 56.3% male |
| Other- Heart Transplant | 36 | Severe PGD (urgent MCS or death within 30 days of OHT) | PAC | Hemodynamic measurements upon return to the intensive care units were obtained at time 0 and 6 h. | Patients who underwent heart transplantation. | (CI × (MAP-CVP))/451. CPI adjusted for vasoactive-inotrope score (VIS) = (CPI/√VIS + 1) × 100 | 140 | No PGD 48 (34–56), severe PGD 55 (50–60) | 69.3% male overall (no severe PGD 69%, severe PGD 73%) |
| Other- Non-cardiac related illness | 45 | In-hospital mortality | PAC | Data was obtained/analyzed on admission known as t0, after optimal volume resuscitation known as t1 and at maximal resuscitation known as tmax. | Patient indicated to have non-cardiac critical illness included patients without cardiac etiology that had septic shock according to the Bone criteria and having adult respiratory distress syndrome which was defined as the following: compatible underlying clinical disorder, inspired oxygen fraction of greater than 0.4 to maintain a PaO2 of greater than 9 kPa, X-ray evidence of diffuse bilateral pulmonary infiltrates and pulmonary artery occlusion pressure < 18 mmHg. | CPO = (MAP-RAP)*CO*2.22*10−3; CPI= CPO/BSA | 32 (15 survivors) | Survivors: 54 (19–80), non-survivors: 67 (27–80) | 71.9% male (23/32) |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Global Health Estimates 2020: Deaths by Cause, Age, Sex, by Country and by Region, 2000–2019; World Health Organization: Geneva, Switzerland, 2020.
- Alves, A.; Ribeiro, F.; Sagiv, M.; Eynon, N.; Yamin, C.; Sagiv, M.; Oliveira, J. Resting Measures and Physiological Responses to Exercise for the Determination of Prognosis in Patients with Chronic Heart Failure. Cardiol. Rev. 2010, 18, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, D.D.; Cooper, H.A.; Panza, J.A. Cardiac power output predicts mortality across a broad spectrum of patients with acute cardiac disease. Am. Heart J. 2007, 153, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Lim, N.; Dubois, M.-J.; De Backer, D.; Vincent, J.-L. Do All Nonsurvivors of Cardiogenic Shock Die with a Low Cardiac Index? Chest 2003, 124, 1885–1891. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.R.; Hasselblad, V.; Stinnett, S.S.; Kramer, J.M.; Grossman, S.; Gheorghiade, M.; Adams, K.F.; Swedberg, K.; Califf, R.M.; O’Connor, C.M. Dissociation between hemodynamic changes and symptom improvement in patients with advanced congestive heart failure. Eur. J. Heart Fail. 2002, 4, 297–304. [Google Scholar] [CrossRef]
- Fincke, R.; Hochman, J.; Lowe, A. Cardiac power is the strongest hemodynamic correlate of mortality in cardiogenic shock: A report from the shock trial registry. ACC Curr. J. Rev. 2004, 13, 49. [Google Scholar] [CrossRef]
- Cheitlin, M. The Efficacy of Brain Natriuretic Peptide Levels in Differentiating Constrictive Pericarditis From Restrictive Cardiomyopathy. Yearb. Cardiol. 2006, 2006, 383–384. [Google Scholar] [CrossRef]
- Anjan, V.Y.; Loftus, T.M.; Burke, M.A.; Akhter, N.; Fonarow, G.C.; Gheorghiade, M.; Shah, S.J. Prevalence, Clinical Phenotype, and Outcomes Associated with Normal B-Type Natriuretic Peptide Levels in Heart Failure with Preserved Ejection Fraction. Am. J. Cardiol. 2012, 110, 870–876. [Google Scholar] [CrossRef]
- Cotter, G.; Moshkovitz, Y.; Kaluski, E.; Cohen, A.J.; Miller, H.; Goor, D.; Vered, Z. Accurate, Noninvasive Continuous Monitoring of Cardiac Output by Whole-Body Electrical Bioimpedance. Chest 2004, 125, 1431–1440. [Google Scholar] [CrossRef]
- Keren, H.; Burkhoff, D.; Squara, P. Evaluation of a noninvasive continuous cardiac output monitoring system based on thoracic bioreactance. Am. J. Physiol. Circ. Physiol. 2007, 293, H583–H589. [Google Scholar] [CrossRef]
- Tehrani, B.N.; Truesdell, A.G.; Sherwood, M.; Desai, S.; Tran, H.A.; Epps, K.C.; Singh, R.; Psotka, M.; Shah, P.; Cooper, L.B.; et al. Standardized Team-Based Care for Cardiogenic Shock. J. Am. Coll. Cardiol. 2019, 73, 1659–1669. [Google Scholar] [CrossRef]
- Sionis, A.; Rivas-Lasarte, M.; Mebazaa, A.; Tarvasmäki, T.; Sans-Roselló, J.; Tolppanen, H.; Varpula, M.; Jurkko, R.; Banaszewski, M.; Silva-Cardoso, J.; et al. Current Use and Impact on 30-Day Mortality of Pulmonary Artery Catheter in Cardiogenic Shock Patients: Results From the CardShock Study. J. Intensiv. Care Med. 2019, 35, 1426–1433. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.; Kambhampati, S.; Sciortino, C.M.; Russell, S.D.; Schulman, S.P. Predictors of intra-aortic balloon pump hemodynamic failure in non-acute myocardial infarction cardiogenic shock. Am. Heart J. 2017, 199, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Uil, C.A.D.; Lagrand, W.K.; Van Der Ent, M.; Jewbali, L.S.; Cheng, J.M.; Spronk, P.E.; Simoons, M.L. Impaired microcirculation predicts poor outcome of patients with acute myocardial infarction complicated by cardiogenic shock. Eur. Heart J. 2010, 31, 3032–3039. [Google Scholar] [CrossRef]
- Williams, S.; Cooke, G.; Wright, D.; Parsons, W.; Riley, R.; Marshall, P.; Tan, L.-B. Peak exercise cardiac power output; a direct indicator of cardiac function strongly predictive of prognosis in chronic heart failure. Eur. Heart J. 2001, 22, 1496–1503. [Google Scholar] [CrossRef] [PubMed]
- Grodin, J.L.; Mullens, W.; Dupont, M.; Wu, Y.; Taylor, D.O.; Starling, R.C.; Tang, W.H.W. Prognostic role of cardiac power index in ambulatory patients with advanced heart failure. Eur. J. Heart Fail. 2015, 17, 689–696. [Google Scholar] [CrossRef]
- Kimmoun, A.; Ducrocq, N.; Mory, S.; Delfosse, R.; Müller, L.; Perez, P.; Fay, R.; Lévy, B. Cardiac Contractile Reserve Parameters Are Related to Prognosis in Septic Shock. BioMed Res. Int. 2013, 2013, 930673. [Google Scholar] [CrossRef]
- Wilhelm, J.; Hettwer, S.; Schuermann, M.; Bagger, S.; Gerhardt, F.; Mundt, S.; Muschik, S.; Zimmermann, J.; Bubel, S.; Amoury, M.; et al. Severity of cardiac impairment in the early stage of community-acquired sepsis determines worse prognosis. Clin. Res. Cardiol. 2013, 102, 735–744. [Google Scholar] [CrossRef]
- Agasthi, P.; Arsanjani, R.; Fortuin, F.; Wang, P.; Lee, J.; Venepally, N.R.; Sweeney, J.; Eleid, M.; Pollak, P.; Mookadam, F. Does resting cardiac power efficiency affect survival post transcatheter aortic valve replacement? J. Am. Coll. Cardiol. 2019, 73, 1040. [Google Scholar] [CrossRef]
- Agasthi, P.; Ashraf, H.; Pujari, S.H.; Girardo, M.E.; Tseng, A.; Mookadam, F.; Venepally, N.R.; Buras, M.; Khetarpal, B.K.; Allam, M.; et al. Artificial Intelligence Trumps TAVI2-SCORE and CoreValve Score in Predicting 1-Year Mortality Post-Transcatheter Aortic Valve Replacement. Cardiovasc. Revascularization Med. 2020, 24, 33–41. [Google Scholar] [CrossRef]
- Agasthi, P.; Pujari, S.H.; Mookadam, F.; Tseng, A.; Venepally, N.R.; Wang, P.; Allam, M.; Sweeney, J.; Eleid, M.; Fortuin, F.D.; et al. Does a Gradient-Adjusted Cardiac Power Index Improve Prediction of Post-Transcatheter Aortic Valve Replacement Survival Over Cardiac Power Index? Yonsei Med. J. 2020, 61, 482–491. [Google Scholar] [CrossRef]
- Frerker, C.; Schewel, J.; Schlüter, M.; Schewel, D.; Ramadan, H.; Schmidt, T.; Thielsen, T.; Kreidel, F.; Schlingloff, F.; Bader, R.; et al. Emergency transcatheter aortic valve replacement in patients with cardiogenic shock due to acutely decompensated aortic stenosis. EuroIntervention 2016, 11, 1530–1536. [Google Scholar] [CrossRef] [PubMed]
- Werdan, K.; Oelke, A.; Hettwer, S.; Nuding, S.; Bubel, S.; Hoke, R.; Ruß, M.; Lautenschläger, C.; Mueller-Werdan, U.; Ebelt, H. Septic cardiomyopathy: Hemodynamic quantification, occurrence, and prognostic implications. Clin. Res. Cardiol. 2011, 100, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Ablonskytė-Dūdonienė, R.; Bakšytė, G.; Čeponienė, I.; Kriščiukaitis, A.; Drėgūnas, K.; Ereminienė, E. Impedance Cardiography and Heart Rate Variability for Long-Term Cardiovascular Outcome Prediction After Myocardial Infarction. Medicina 2012, 48, 52–58. [Google Scholar] [CrossRef]
- Ablonskytė-Dūdonienė, R.; Bakšytė, G.; Čeponienė, I.; Kriščiukaitis, A.; Drėgūnas, K.; Ereminienė, E. Prognosis of In-Hospital Myocardial Infarction Course for Diabetic and Nondiabetic Patients Using a Noninvasive Evaluation of Hemodynamics and Heart Rate Variability. Medicina 2013, 49, 42–72. [Google Scholar] [CrossRef]
- Jentzer, J.C.; Wiley, B.M.; Anavekar, N.S.; Pislaru, S.V.; Mankad, S.V.; Bennett, C.E.; Barsness, G.W.; Hollenberg, S.M.; Holmes, D.R.; Oh, J.K. Noninvasive Hemodynamic Assessment of Shock Severity and Mortality Risk Prediction in the Cardiac Intensive Care Unit. JACC Cardiovasc. Imaging 2020, 14, 321–332. [Google Scholar] [CrossRef]
- Granfeldt, H.; Hellgren, L.; Dellgren, G.; Myrdal, G.; Wassberg, E.; Kjellman, U.; Ahn, H. Experience with the Impella® recovery axial-flow system for acute heart failure at three cardiothoracic centers in Sweden. Scand. Cardiovasc. J. 2009, 43, 233–239. [Google Scholar] [CrossRef]
- Guglin, M.; Omar, H.R. Right Atrial Pressure Predicts Mortality Among LVAD Recipients: Analysis of the INTERMACS Database. Heart Lung Circ. 2020, 30, 592–599. [Google Scholar] [CrossRef]
- Hall, S.; Garcia, J.; Larson, D.; Smith, R. Cardiac power index: Staging heart failure for mechanical circulatory support. Perfusion 2012, 27, 456–461. [Google Scholar] [CrossRef]
- Myers, J.; Wong, M.; Adhikarla, C.; Boga, M.; Challa, S.; Abella, J.; Ashley, E.A. Cardiopulmonary and Noninvasive Hemodynamic Responses to Exercise Predict Outcomes in Heart Failure. J. Card. Fail. 2013, 19, 101–107. [Google Scholar] [CrossRef]
- Pugliese, N.R.; Fabiani, I.; Mandoli, G.E.; Guarini, G.; Galeotti, G.G.; Miccoli, M.; Lombardo, A.; Simioniuc, A.; Bigalli, G.; Pedrinelli, R.; et al. Echo-derived peak cardiac power output-to-left ventricular mass with cardiopulmonary exercise testing predicts outcome in patients with heart failure and depressed systolic function. Eur. Heart J.-Cardiovasc. Imaging 2018, 20, 700–708. [Google Scholar] [CrossRef]
- Rosenblum, H.; Helmke, S.; Ms, P.W.; Teruya, S.; Np, M.J.; Burkhoff, D.; Mancini, D.; Maurer, M.S. Peak Cardiac Power Measured Noninvasively with a Bioreactance Technique Is a Predictor of Adverse Outcomes in Patients with Advanced Heart Failure. Congest. Heart Fail. 2010, 16, 254–258. [Google Scholar] [CrossRef]
- Roul, G.; Moulichon, M.-E.; Bareiss, P.; Gries, P.; Koegler, A.; Sacrez, J.; Germain, P.; Mossard, J.-M.; Sacrez, A. Prognostic factors of chronic heart failure in NYHA class II or III: Value of invasive exercise haemodynamic data. Eur. Heart J. 1995, 16, 1387–1398. [Google Scholar] [CrossRef] [PubMed]
- Torre-Amione, G.; Milo-Cotter, O.; Kaluski, E.; Perchenet, L.; Kobrin, I.; Frey, A.; Rund, M.M.; Weatherley, B.D.; Cotter, G. Early Worsening Heart Failure in Patients Admitted for Acute Heart Failure: Time Course, Hemodynamic Predictors, and Outcome. J. Card. Fail. 2009, 15, 639–644. [Google Scholar] [CrossRef]
- Williams, S.G.; Jackson, M.; Cooke, G.A.; Barker, D.; Patwala, A.; Wright, D.J.; Albuoaini, K.; Tan, L.-B. How do different indicators of cardiac pump function impact upon the long-term prognosis of patients with chronic heart failure? Am. Heart J. 2005, 150, 983.e1–983.e6. [Google Scholar] [CrossRef]
- Yildiz, O.; Aslan, G.; Demirozu, Z.T.; Yenigun, C.D.; Yazicioglu, N. Evaluation of Resting Cardiac Power Output as a Prognostic Factor in Patients with Advanced Heart Failure. Am. J. Cardiol. 2017, 120, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Baran, D.A.; Visveswaran, G.K.; Seliem, A.; Divita, M.; Wasty, N.; Cohen, M. Differential responses to larger volume intra-aortic balloon counterpulsation: Hemodynamic and clinical outcomes. Catheter. Cardiovasc. Interv. 2017, 92, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Ba, M.R.F.; Kapur, N.K.; Patel, K.; Salam, M.A.; Schreiber, T.; Kaki, A.; Hanson, I.; Almany, S.; Timmis, S.; Dixon, S.; et al. Improved Outcomes Associated with the use of Shock Protocols: Updates from the National Cardiogenic Shock Initiative. Catheter. Cardiovasc. Interv. 2019, 93, 1173–1183. [Google Scholar] [CrossRef]
- Jeger, R.V.; Lowe, A.M.; Buller, C.E.; Pfisterer, M.E.; Dzavik, V.; Webb, J.G.; Hochman, J.S.; Jorde, U.P. Hemodynamic Parameters Are Prognostically Important in Cardiogenic Shock But Similar Following Early Revascularization or Initial Medical Stabilization. Chest 2007, 132, 1794–1803. [Google Scholar] [CrossRef] [PubMed]
- Link, A.; Pöss, J.; Rbah, R.; Barth, C.; Feth, L.; Selejan, S.; Böhm, M. Circulating angiopoietins and cardiovascular mortality in cardiogenic shock. Eur. Heart J. 2013, 34, 1651–1662. [Google Scholar] [CrossRef]
- Popovic, B.; Fay, R.; Cravoisy-Popovic, A.; Levy, B. Cardiac Power Index, Mean Arterial Pressure, and Simplified Acute Physiology Score II Are Strong Predictors of Survival and Response to Revascularization in Cardiogenic Shock. Shock 2014, 42, 22–26. [Google Scholar] [CrossRef]
- Rigamonti, F.; Graf, G.; Merlani, P.; Bendjelid, K. The Short-Term Prognosis of Cardiogenic Shock Can Be Determined Using Hemodynamic Variables. Crit. Care Med. 2013, 41, 2484–2491. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.B.; Littler, W.A. Measurement of cardiac reserve in cardiogenic shock: Implications for prognosis and management. Heart 1990, 64, 121–128. [Google Scholar] [CrossRef]
- Torgersen, C.; Schmittinger, C.A.; Wagner, S.; Ulmer, H.; Takala, J.; Jakob, S.M.; Dünser, M.W. Hemodynamic variables and mortality in cardiogenic shock: A retrospective cohort study. Crit. Care 2009, 13, R157. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.E.; Siegfried, B.A.; Ferguson, T. The value of cardiac output studies in postoperative cardiac patients: A myth. J. Surg. Res. 1976, 20, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Cortigiani, L.; Sorbo, S.; Miccoli, M.; Scali, M.C.; Simioniuc, A.; Morrone, D.; Bovenzi, F.; Marzilli, M.; Dini, F.L. Prognostic value of cardiac power output to left ventricular mass in patients with left ventricular dysfunction and dobutamine stress echo negative by wall motion criteria. Eur. Heart J.-Cardiovasc. Imaging 2016, 18, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Doenyas-Barak, K.; De Abreu, M.H.F.G.; Borges, L.E.; Filho, H.A.T.; Yunlin, F.; Yurong, Z.; Levin, N.W.; Kaufman, A.M.; Efrati, S.; Pereg, D.; et al. Non-invasive hemodynamic profiling of patients undergoing hemodialysis—A multicenter observational cohort study. BMC Nephrol. 2019, 20, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.S.; Ranasinghe, A.; Chue, C.; Quinn, D.; Mukadam, M.; Mascaro, J. Cardiac Power Output Index and Severe Primary Graft Dysfunction After Heart Transplantation. J. Cardiothorac. Vasc. Anesthesia 2021, 35, 398–403. [Google Scholar] [CrossRef]
- Timmins, A.C.; Hayes, M.; Yau, E.; Watson, J.D.; Hinds, C.J. The relationship between cardiac reserve and sur-vival in critically ill patients receiving treatment aimed at achieving supranormal oxygen delivery and consumption. Postgrad. Med. J. 1992, 68 (Suppl. 2), S34–S40. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
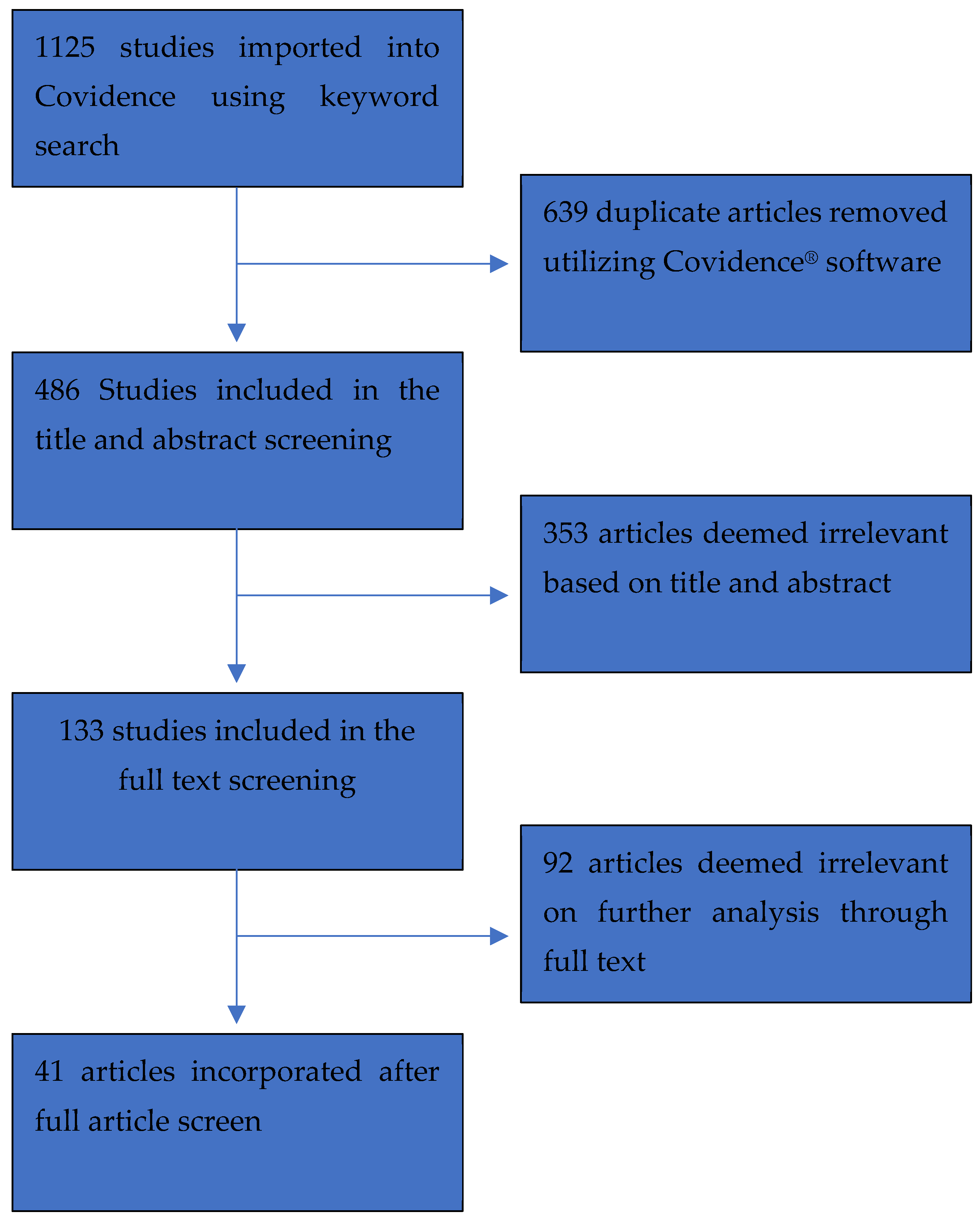
| Disease State Category | Articles Included |
|---|---|
| Transcatheter Aortic Valve Replacement | [19,20,21,22] |
| Septic Shock | [17,18,23] |
| Post-Myocardial Infarct | [24,25] |
| Critical Cardiac Disease | [3,26] |
| Heart Failure | [15,16,27,28,29,30,31,32,33,34,35,36] |
| Cardiogenic Shock | [6,11,12,13,14,37,38,39,40,41,42,43,44] |
| Other | [45,46,47,48,49] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farshadmand, J.; Lowy, Z.; Hai, O.; Zeltser, R.; Makaryus, A.N. Utility of Cardiac Power Hemodynamic Measurements in the Evaluation and Risk Stratification of Cardiovascular Conditions. Healthcare 2022, 10, 2417. https://doi.org/10.3390/healthcare10122417
Farshadmand J, Lowy Z, Hai O, Zeltser R, Makaryus AN. Utility of Cardiac Power Hemodynamic Measurements in the Evaluation and Risk Stratification of Cardiovascular Conditions. Healthcare. 2022; 10(12):2417. https://doi.org/10.3390/healthcare10122417
Chicago/Turabian StyleFarshadmand, Jonathan, Zachary Lowy, Ofek Hai, Roman Zeltser, and Amgad N. Makaryus. 2022. "Utility of Cardiac Power Hemodynamic Measurements in the Evaluation and Risk Stratification of Cardiovascular Conditions" Healthcare 10, no. 12: 2417. https://doi.org/10.3390/healthcare10122417
APA StyleFarshadmand, J., Lowy, Z., Hai, O., Zeltser, R., & Makaryus, A. N. (2022). Utility of Cardiac Power Hemodynamic Measurements in the Evaluation and Risk Stratification of Cardiovascular Conditions. Healthcare, 10(12), 2417. https://doi.org/10.3390/healthcare10122417






