Developing an Evidence and Theory Based Multimodal Integrative Intervention for the Management of Renal Cachexia: A Theory of Change
Abstract
1. Background
2. Materials and Methods
2.1. Aim
2.2. Design
3. Steps to Develop the ToC Map
3.1. Step 1: Obtain Key Stakeholder Views on the Potential Development of A Multimodal Intervention for Renal Cachexia
3.2. Step 2: Obtain HCP Perspectives on Current Practices in Cachexia Management
3.3. Step 3: Identify and Assess the Effectiveness of Multimodal Interventions for Cachexia Management
3.4. Step 4: Develop and Refine the Components of the Proposed Multimodal Intervention for the ToC Map
3.5. Step 5: Create a Draft ToC Map Based on Integration of Output from Steps 1–4
3.6. Step 6: Develop and Amend the ToC Map in Consultation with Key Stakeholders
- add an emphasis on the integrative nature of the intervention;
- clarify the hypothesised causal pathway for those with renal cachexia and the proposed impact of the multimodal intervention;
- clarify the rationale for the components of the multimodal intervention;
- highlight the importance of the psychosocial component and how this will be integrated into the intervention in practice;
- merge, reformulate and put the preconditions in chronological order;
- clarify the proposed outcomes and add clinical frailty as a secondary outcome;
- simplify the rationale of the exercise component to more user-friendly language;
- eliminate repetition where possible;
- ensure sufficient understanding of the importance of equipoise when planning the MMIEAD intervention;
- revise the proposed impact based on the evidence to date and the ceiling of accountability.
4. Results
4.1. Impact
4.2. Ceiling of Accountability
4.3. Long-Term Outcomes and Indicators
4.4. Rationales
4.5. Interventions
4.6. Preconditions
4.6.1. Ensuring Engagement and Buy-in by Management and the Multidisciplinary Team
4.6.2. Training for HCPs
4.6.3. Multidisciplinary Meetings and Information Exchange
4.6.4. Information about the Multimodal Intervention for Individuals with Renal Cachexia and Their Families
4.6.5. Screening and Informed Consent
4.7. Assumptions
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BAME | Black, Asian, minority ethnics |
| COPD | Chronic Obstructive Pulmonary Disease |
| cRCT | Cluster Randomised Controlled Trial |
| CRP | C-reactive protein |
| CTU | Clinical Trials Unit |
| ESKD | End-stage kidney disease |
| Hb | Haemoglobin |
| HCP | Health Care Practitioner |
| IDPN | Intradialytic parenteral nutrition |
| MHD | Maintenance haemodialysis |
| MRC | Medical Research Council |
| MMIEAD | Multimodal, Integrative, Exercise, Anti-inflammatory and Dietary counselling intervention |
| NSAIDs | Non-steroidal anti-inflammatory drugs |
| NIKPA | Northern Ireland Kidney Patients Association |
| NIS | Nationwide Inpatient Sample |
| ONS | Oral nutritional supplement |
| PEW | Protein Energy Wasting |
| PPIE | Personal and Public Involvement and Engagement |
| Ω-3 PUFA | Polyunsaturated Fatty Acids |
| PIS | Participant Information Sheet |
| QoL | Quality of Life |
| RCT | Randomised Controlled Trial |
| SIP | Study information pack |
| SLT | Social learning theory |
| TIDieR | Template for Intervention Description and Replication |
| ToC | Theory of Change |
| QoL | Quality of Life |
| QUB | Queen’s University Belfast |
| UK | United Kingdom |
References
- Evans, W.J.; Morley, J.E.; Argilés, J.; Bales, C.; Baracos, V.; Guttridge, D.; Jatoi, A.; Kalantar-Zadeh, K.; Lochs, H.; Mantovani, G. Cachexia: A new definition. Clin. Nutr. 2008, 27, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Reid, J.; Noble, H.R.; Porter, S.; Shields, J.S.; Maxwell, A.P. A literature review of end-stage renal disease and cachexia: Understanding experience to inform evidence-based healthcare. J. Ren. Care 2013, 39, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Reid, J.; Noble, H.; Davenport, A.; Farrington, K.; Fouque, D.; Porter, S.; Seres, D.; Shields, J.; Slee, A.; Witham, M.D. Defining cachexia in a renal population. J. Ren. Care 2015, 41, 79–80. [Google Scholar] [CrossRef] [PubMed]
- Fouque, D.; Kalantar-Zadeh, K.; Kopple, J.; Cano, N.; Chauveau, P.; Cuppari, L.; Franch, H.; Guarnieri, G.; Ikizler, T.; Kaysen, G. A proposed nomenclature and diagnostic criteria for protein–energy wasting in acute and chronic kidney disease. Kidney Int. 2008, 73, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Carrero, J.J.; Stenvinkel, P.; Cuppari, L.; Ikizler, T.A.; Kalantar-Zadeh, K.; Kaysen, G.; Mitch, W.E.; Price, S.R.; Wanner, C.; Wang, A.Y. Etiology of the protein-energy wasting syndrome in chronic kidney disease: A consensus statement from the International Society of Renal Nutrition and Metabolism (ISRNM). J. Ren. Nutr. 2013, 23, 77–90. [Google Scholar] [CrossRef]
- Koppe, L.; Fouque, D.; Kalantar-Zadeh, K. Kidney cachexia or protein-energy wasting in chronic kidney disease: Facts and numbers. J. Cachexia Sarcopenia Muscle 2019, 10, 479–484. [Google Scholar] [CrossRef]
- Su, C.-T.; Yabes, J.; Pike, F.; Weiner, D.E.; Beddhu, S.; Burrowes, J.D.; Rocco, M.V.; Unruh, M.L. Changes in anthropometry and mortality in maintenance hemodialysis patients in the HEMO Study. Am. J. Kidney Dis. 2013, 62, 1141–1150. [Google Scholar] [CrossRef]
- Cano, N.J.; Fouque, D.; Roth, H.; Aparicio, M.; Azar, R.; Canaud, B.; Chauveau, P.; Combe, C.; Laville, M.; Leverve, X.M. Intradialytic parenteral nutrition does not improve survival in malnourished hemodialysis patients: A 2-year multicenter, prospective, randomized study. J. Am. Soc. Nephrol. 2007, 18, 2583–2591. [Google Scholar] [CrossRef]
- Cheung, W.W.; Paik, K.H.; Mak, R.H. Inflammation and cachexia in chronic kidney disease. Pediatr. Nephrol. 2010, 25, 711–724. [Google Scholar] [CrossRef]
- Mak, R.H.; Ikizler, A.T.; Kovesdy, C.P.; Raj, D.S.; Stenvinkel, P.; Kalantar-Zadeh, K. Wasting in chronic kidney disease. J. Cachex- Sarcopenia Muscle 2011, 2, 9–25. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Ikizler, T.A.; Block, G.; Avram, M.M.; Kopple, J.D. Malnutrition-inflammation complex syndrome in dialysis patients: Causes and consequences. Am. J. Kidney Dis. 2003, 42, 864–881. [Google Scholar] [CrossRef] [PubMed]
- McKeaveney, C.; Noble, H.; de Barbieri, I.; Strini, V.; Maxwell, A.P.; Reid, J. Awareness, understanding and treatment practices when managing cachexia in end-stage kidney disease. J. Ren. Care 2020, 46, 35–44. [Google Scholar] [CrossRef] [PubMed]
- McKeaveney, C.; Maxwell, P.; Noble, H.; Reid, J. A critical review of multimodal interventions for cachexia. Adv. Nutr. 2021, 12, 523–532. [Google Scholar] [CrossRef]
- McKeaveney, C.; Slee, A.; Adamson, G.; Davenport, A.; Farrington, K.; Fouque, D.; Kalantar-Zadeh, K.; Mallett, J.; Maxwell, A.P.; Mullan, R. Using a generic definition of cachexia in patients with kidney disease receiving haemodialysis: A longitudinal (pilot) study. Nephrol. Dial. Transplant. 2021, 36, 1919–1926. [Google Scholar] [CrossRef]
- Arthur, S.T.; Noone, J.M.; Van Doren, B.A.; Roy, D.; Blanchette, C.M. One-year prevalence, comorbidities and cost of cachexia-related inpatient admissions in the USA. Drugs Context 2014, 3, 212265. [Google Scholar] [CrossRef]
- Slee, A.; McKeaveney, C.; Adamson, G.; Davenport, A.; Farrington, K.; Fouque, D.; Kalantar-Zadeh, K.; Mallett, J.; Maxwell, A.P.; Mullan, R. Estimating the prevalence of muscle wasting, weakness, and sarcopenia in hemodialysis patients. J. Ren. Nutr. 2020, 30, 313–321. [Google Scholar] [CrossRef]
- Reid, J.; Noble, H.R.; Adamson, G.; Davenport, A.; Farrington, K.; Fouque, D.; Kalantar-Zadeh, K.; Mallett, J.; McKeaveney, C.; Porter, S. Establishing a clinical phenotype for cachexia in end stage kidney disease–study protocol. BMC Nephrol. 2018, 19, 38. [Google Scholar] [CrossRef]
- Fiaccadori, E.; Sabatino, A.; Barazzoni, R.; Carrero, J.J.; Cupisti, A.; De Waele, E.; Jonckheer, J.; Singer, P.; Cuerda, C. ESPEN guideline on clinical nutrition in hospitalized patients with acute or chronic kidney disease. Clin. Nutr. 2021, 40, 1644–1668. [Google Scholar] [CrossRef]
- Slee, A.; Reid, J. Disease-related malnutrition in chronic kidney disease. Curr. Opin. Clin. Nutr. Metab. Care 2022, 25, 136–141. [Google Scholar] [CrossRef]
- Slee, A.D.; Reid, J. Wasting in chronic kidney disease–a complex issue. JCSM Clin. Rep. 2018, 3, 1–10. [Google Scholar] [CrossRef]
- Tobita, I.; Suzuki, S.; Kobayashi, T.; Shimizu, Y.; Umeshita, K. A programme to encourage participation of haemodialysis patients in an exercise regimen. J. Ren. Care 2009, 35, 48–53. [Google Scholar] [CrossRef]
- Dong, J.; Sundell, M.B.; Pupim, L.B.; Wu, P.; Shintani, A.; Ikizler, T.A. The effect of resistance exercise to augment long-term benefits of intradialytic oral nutritional supplementation in chronic hemodialysis patients. J. Ren. Nutr. 2011, 21, 149–159. [Google Scholar] [CrossRef]
- Solheim, T.S.; Laird, B.J.; Balstad, T.R.; Stene, G.B.; Bye, A.; Johns, N.; Pettersen, C.H.; Fallon, M.; Fayers, P.; Fearon, K. A randomized phase II feasibility trial of a multimodal intervention for the management of cachexia in lung and pancreatic cancer. J. Cachexia Sarcopenia Muscle 2017, 8, 778–788. [Google Scholar] [CrossRef]
- Fearon, K. Cancer cachexia: Developing multimodal therapy for a multidimensional problem. Eur. J. Cancer 2008, 44, 1124–1132. [Google Scholar] [CrossRef] [PubMed]
- Solheim, T.S.; Laird, B.J.; Balstad, T.R.; Bye, A.; Stene, G.; Baracos, V.; Strasser, F.; Griffiths, G.; Maddocks, M.; Fallon, M. Cancer cachexia: Rationale for the MENAC (Multimodal—Exercise, Nutrition and Anti-inflammatory medication for Cachexia) trial. BMJ Support. Palliat. Care 2018, 8, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K.; Argiles, J.; Baracos, V.; Bernabei, R.; Coats, A.; Crawford, J.; Deutz, N.; Doehner, W.; Evans, W.; Ferrucci, L. Request for regulatory guidance for cancer cachexia intervention trials. J. Cachexia Sarcopenia Muscle 2015, 6, 272. [Google Scholar] [CrossRef] [PubMed]
- Balstad, T.R.; Solheim, T.S.; Strasser, F.; Kaasa, S.; Bye, A. Dietary treatment of weight loss in patients with advanced cancer and cachexia: A systematic literature review. Crit. Rev. Oncol. 2014, 91, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Craig, P.; Dieppe, P.; Macintyre, S.; Michie, S.; Nazareth, I.; Petticrew, M. Developing and evaluating complex interventions: The new Medical Research Council guidance. BMJ 2008, 337, a1655. [Google Scholar] [CrossRef] [PubMed]
- Higginson, I.J.; Evans, C.J.; Grande, G.; Preston, N.; Morgan, M.; McCrone, P.; Lewis, P.; Fayers, P.; Harding, R.; Hotopf, M. Evaluating complex interventions in end of life care: The MORECare statement on good practice generated by a synthesis of transparent expert consultations and systematic reviews. BMC Med. 2013, 11, 111. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.J.; Harding, R.; Higginson, I.J.; MORECare. ‘Best practice’ in developing and evaluating palliative and end-of-life care services: A meta-synthesis of research methods for the MORECare project. Palliat. Med. 2013, 27, 885–898. [Google Scholar] [CrossRef]
- Hoffmann, T.C.; Glasziou, P.P.; Boutron, I.; Milne, R.; Perera, R.; Moher, D.; Altman, D.G.; Barbour, V.; Macdonald, H.; Johnston, M. Better reporting of interventions: Template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014, 348, g1687. [Google Scholar] [CrossRef] [PubMed]
- Skivington, K.; Matthews, L.; Simpson, S.A.; Craig, P.; Baird, J.; Blazeby, J.M.; Boyd, K.A.; Craig, N.; French, D.P.; McIntosh, E.A. A new framework for developing and evaluating complex interventions: Update of Medical Research Council guidance. BMJ 2021, 374, n2061. [Google Scholar] [CrossRef] [PubMed]
- De Silva, M.J.; Breuer, E.; Lee, L.; Asher, L.; Chowdhary, N.; Lund, C.; Patel, V. Theory of change: A theory-driven approach to enhance the Medical Research Council's framework for complex interventions. Trials 2014, 15, 267. [Google Scholar] [CrossRef] [PubMed]
- Prinsen, G.; Nijhof, S. Between logframes and theory of change: Reviewing debates and a practical experience. Dev. Pract. 2015, 25, 234–246. [Google Scholar] [CrossRef]
- Funnell, S.C.; Rogers, P.J. Purposeful Program Theory: Effective Use of Theories of Change and Logic Models; John Wiley & Sons: London, UK, 2011. [Google Scholar]
- Ward, S.; Donovan, H.S.; Serlin, R.C. An alternative view on “an alternative paradigm”. Res. Nurs. Health 2003, 26, 256. [Google Scholar] [CrossRef] [PubMed]
- De Silva, M.; Breuer, E.; Lee, L.; Lund, C.; Patel, V. Theory of Change: A Theory Driven Approach to the MRC Framework for Complex Interventions; Poster presented at Symposium on Health System Interventions, London School of Hygiene and Tropical Medicine, London, UK. 2012. [Google Scholar]
- Connell, J.P.; Kubisch, A.C. Applying a theory of change approach to the evaluation of comprehensive community initiatives: Progress, prospects, and problems. New Approaches Eval. Commun. Initiat. 1998, 2, 1–16. [Google Scholar]
- Millar, C.; Reid, J.; Porter, S. Healthcare professionals' response to cachexia in advanced cancer: A qualitative study. Oncol. Nurs. Forum 2013, 40, E393–E402. [Google Scholar] [CrossRef]
- Millar, C.; Reid, J.; Porter, S. The challenges of managing cachexia in advanced cancer. Cancer Nurs. Pract. 2009, 8, 10. [Google Scholar] [CrossRef]
- Reid, J.; Mc Kenna, H.; Fitzsimons, D.; Mc Cance, T. An exploration of the experience of cancer cachexia: What patients and their families want from healthcare professionals. Eur. J. Cancer Care 2010, 19, 682–689. [Google Scholar] [CrossRef]
- Reid, J.; McKenna, H.; Fitzsimons, D.; McCance, T. The experience of cancer cachexia: A qualitative study of advanced cancer patients and their family members. Int. J. Nurs. Stud. 2009, 46, 606–616. [Google Scholar] [CrossRef]
- Reid, J.; Mills, M.; Cantwell, M.M.; Cardwell, C.R.; Murray, L.J.; Donnelly, M. Thalidomide for managing cancer cachexia. Cochrane Database Syst. Rev. 2012, 2021, CD008664. [Google Scholar] [CrossRef]
- Reid, J. Psychosocial, educational and communicative interventions for patients with cachexia and their family carers. Curr. Opin. Support. Palliat. Care 2014, 8, 334. [Google Scholar] [CrossRef] [PubMed]
- Carson, M.; Reid, J.; Hill, L.; Dixon, L.; Donnelly, P.; Slater, P.; Hill, A.; Fitzsimons, D. Prevalence and effect of cardiac cachexia in advanced heart failure patients living in Northern Ireland. Eur. J. Cardiovasc. Nurs. 2021, 20 (Suppl. 1), zvab060.43. [Google Scholar] [CrossRef]
- The Challenge of Cardiac Cachexia. Available online: https://journals.sagepub.com/page/cnu/virtual-special-issue/the_challenge_of_cardiac_cachexia (accessed on 3 November 2022).
- Carson, M.A.; Reid, J.; Hill, L.; Dixon, L.; Donnelly, P.; Slater, P.; Hill, A.; Fitzsimons, D. An exploration of the prevalence and experience of cardiac cachexia: Protocol for a mixed methods cross-sectional study. BMC Palliat. Care 2019, 18, 82. [Google Scholar] [CrossRef] [PubMed]
- Carson, M.A.; Reid, J.; Hill, L.; Fitzsimons, D. The need for a specific definition of cardiac cachexia. Eur. J. Cardiovasc. Nurs. 2019, 18, 524–525. [Google Scholar] [CrossRef] [PubMed]
- Uster, A.; Ruehlin, M.; Mey, S.; Gisi, D.; Knols, R.; Imoberdorf, R.; Pless, M.; Ballmer, P.E. Effects of nutrition and physical exercise intervention in palliative cancer patients: A randomized controlled trial. Clin. Nutr. 2018, 37, 1202–1209. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.-S.; Li, X.; Cao, Y.Z.; Zhang, C.C.; Yang, F.; Shi, Y.M.; Peng, L.M. Clinical studies on the treatment of cancer cachexia with megestrol acetate plus thalidomide. Chemotherapy 2012, 58, 461–467. [Google Scholar] [CrossRef]
- Xu, Y.-J.; Cheng, J.C.-H.; Lee, J.-M.; Huang, P.-M.; Huang, G.-H.; Chen, C.C.-H. A walk-and-eat intervention improves outcomes for patients with esophageal cancer undergoing neoadjuvant chemoradiotherapy. Oncologist 2015, 20, 1216–1222. [Google Scholar] [CrossRef] [PubMed]
- Schink, K.; Reljic, D.; Herrmann, H.J.; Meyer, J.; Mackensen, A.; Neurath, M.F.; Zopf, Y. Whole-body electromyostimulation combined with individualized nutritional support improves body composition in patients with hematological malignancies–A pilot study. Front. Physiol. 2018, 9, 1808. [Google Scholar] [CrossRef]
- Hall, C.C.; Cook, J.; Maddocks, M.; Skipworth, R.J.; Fallon, M.; Laird, B.J. Combined exercise and nutritional rehabilitation in outpatients with incurable cancer: A systematic review. Support. Care Cancer 2019, 27, 2371–2384. [Google Scholar] [CrossRef]
- Calder, P.C.; Laviano, A.; Lonnqvist, F.; Muscaritoli, M.; Öhlander, M.; Schols, A. Targeted medical nutrition for cachexia in chronic obstructive pulmonary disease: A randomized, controlled trial. J. Cachex-Sarcopenia Muscle 2018, 9, 28–40. [Google Scholar] [CrossRef] [PubMed]
- van Wetering, C.R.; Hoogendoorn, M.; Mol, S.; Rutten-van Mölken, M.; Schols, A. Short-and long-term efficacy of a community-based COPD management programme in less advanced COPD: A randomised controlled trial. Thorax 2010, 65, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Pison, C.M.; Cano, N.J.; Chérion, C.; Caron, F.; Antonini, M.-T.; Gonzalez-Bermejo, J.; Meziane, L.; Molano, L.C.; Janssens, J.-P.; Costes, F. Multimodal nutritional rehabilitation improves clinical outcomes of malnourished patients with chronic respiratory failure: A randomised controlled trial. Thorax 2011, 66, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Baldi, S.; Aquilani, R.; Pinna, G.D.; Poggi, P.; De Martini, A.; Bruschi, C. Fat-free mass change after nutritional rehabilitation in weight losing COPD: Role of insulin, C-reactive protein and tissue hypoxia. Int. J. Chron. Obstruct. Pulmon. Dis. 2010, 5, 29. [Google Scholar] [CrossRef]
- Grgic, J.; Schoenfeld, B.J.; Davies, T.B.; Lazinica, B.; Krieger, J.W.; Pedisic, Z. Effect of resistance training frequency on gains in muscular strength: A systematic review and meta-analysis. Sports Med. 2018, 48, 1207–1220. [Google Scholar] [CrossRef]
- Fraser, S.; Barnard, R.; Haines, T.; Ockerby, C.; Street, M.; Wang, W.; Daly, R.; Bennett, P. Effect of intradialytic resistance exercise training on physical function in end-stage kidney disease patients. Ren. Soc. Australas. J. 2016, 12, 89. [Google Scholar]
- Headley, S.; Germain, M.; Mailloux, P.; Mulhern, J.; Ashworth, B.; Burris, J.; Brewer, B.; Nindl, B.; Coughlin, M.; Welles, R. Resistance training improves strength and functional measures in patients with end-stage renal disease. Am. J. Kidney Dis. 2002, 40, 355–364. [Google Scholar] [CrossRef]
- Hegazy, I.; El Raghy, H.; Abdel Aziz, S.; Elhabashi, E. Study of the effect of dietary counselling on the improvement of end-stage renal disease patients. East. Mediterr. Health J. 2013, 19, 45–51. [Google Scholar] [CrossRef]
- Hajira, B.; Manzoor, M.; Samiullah, M.; Chawla, R.K. Effect of dietary counselling on the nutritional status of end-stage renal disease patients. J. Park. Med. Assoc. 2017, 67, 1327–1330. [Google Scholar]
- Dezfouli, M.; Moeinzadeh, F.; Taheri, S.; Feizi, A. The effect of omega-3 supplementation on serum levels of inflammatory biomarkers and albumin in hemodialysis patients: A systematic review and meta-analysis. J. Ren. Nutr. 2020, 30, 182–188. [Google Scholar] [CrossRef]
- Mori, T.A.; Burke, V.; Puddey, I.B.; Irish, A.B.; Cowpland, C.A.; Beilin, L.J.; Dogra, G.K.; Watts, G.F. The effects of ω3 fatty acids and coenzyme Q10 on blood pressure and heart rate in chronic kidney disease: A randomized controlled trial. J. Hypertens. 2009, 27, 1863–1872. [Google Scholar] [CrossRef] [PubMed]
- Friedman, A.; Moe, S. Review of the effects of omega-3 supplementation in dialysis patients. Clin. J. Am. Soc. Nephrol. 2006, 1, 182–192. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Li, M.-S.; Lin, M.; Zhao, T.-Y.; Gao, P. Effect of fish oil supplement in maintenance hemodialysis patients: A systematic review and meta-analysis of published randomized controlled trials. Eur. J. Clin. Pharmacol. 2016, 72, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Dong, C.; Du, H.; Zhang, H.; Chen, J.; Hu, X.; Hu, F. Effects of fish oil on serum lipid profile in dialysis patients: A systematic review and meta-analysis of randomized controlled trials. Lipids Health Dis. 2014, 13, 1–11. [Google Scholar] [CrossRef]
- Social Support, Caregivers, and Chronic Kidney Disease. Available online: https://pubmed.ncbi.nlm.nih.gov/34973701/ (accessed on 3 November 2022).
- Picariello, F.; Moss-Morris, R.; Macdougall, I.C.; Chilcot, J. ‘It's when you're not doing too much you feel tired’: A qualitative exploration of fatigue in end-stage kidney disease. Br. J. Health Psychol. 2018, 23, 311–333. [Google Scholar] [CrossRef]
- Bandura, A. Self-efficacy: Toward a unifying theory of behavioral change. Psychol. Rev. 1977, 84, 191. [Google Scholar] [CrossRef]
- van Beers, M.; Rutten-van Mölken, M.P.; van de Bool, C.; Boland, M.; Kremers, S.P.; Franssen, F.M.; van Helvoort, A.; Gosker, H.R.; Wouters, E.F.; Schols, A.M. Clinical outcome and cost-effectiveness of a 1-year nutritional intervention programme in COPD patients with low muscle mass: The randomized controlled NUTRAIN trial. Clin. Nutr. 2020, 39, 405–413. [Google Scholar] [CrossRef]
- Martin-Alemañy, G.; Valdez-Ortiz, R.; Olvera-Soto, G.; Gomez-Guerrero, I.; Aguire-Esquivel, G.; Cantu-Quintanilla, G.; Lopez-Alvarenga, J.C.; Miranda-Alatriste, P.; Espinosa-Cuevas, A. The effects of resistance exercise and oral nutritional supplementation during hemodialysis on indicators of nutritional status and quality of life. Nephrol. Dial. Transplant. 2016, 31, 1712–1720. [Google Scholar] [CrossRef]
- Hristea, D.; Deschamps, T.; Paris, A.; Lefrançois, G.; Collet, V.; Savoiu, C.; Ozenne, S.; Coupel, S.; Testa, A.; Magnard, J. Combining intra-dialytic exercise and nutritional supplementation in malnourished older haemodialysis patients: Towards better quality of life and autonomy. Nephrology 2016, 21, 785–790. [Google Scholar] [CrossRef]
- Jeong, J.H.; Biruete, A.; Tomayko, E.J.; Wu, P.T.; Fitschen, P.; Chung, H.R.; Ali, M.; McAuley, E.; Fernhall, B.; Phillips, S.A. Results from the randomized controlled IHOPE trial suggest no effects of oral protein supplementation and exercise training on physical function in hemodialysis patients. Kidney Int. 2019, 96, 777–786. [Google Scholar] [CrossRef]
- Hornberger, T.A. Mechanotransduction and the regulation of mTORC1 signaling in skeletal muscle. Int. J. Biochem. Cell Biol. 2011, 43, 1267–1276. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.-S. mTOR as a key regulator in maintaining skeletal muscle mass. Front. Physiol. 2017, 8, 788. [Google Scholar] [CrossRef] [PubMed]
- Maddocks, M.; Murton, A.J.; Wilcock, A. Therapeutic exercise in cancer cachexia. Crit. Rev. Oncog. 2012, 17, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Maddocks, M.; Jones, L.W.; Wilcock, A. Immunological and hormonal effects of exercise: Implications for cancer cachexia. Curr. Opin. Support Palliat. Care 2013, 7, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Johansen, K.L.; Painter, P.L.; Sakkas, G.K.; Gordon, P.; Doyle, J.; Shubert, T. Effects of resistance exercise training and nandrolone decanoate on body composition and muscle function among patients who receive hemodialysis: A randomized, controlled trial. J. Am. Soc. Nephrol. 2006, 17, 2307–2314. [Google Scholar] [CrossRef]
- Kopple, J.D.; Wang, H.; Casaburi, R.; Fournier, M.; Lewis, M.I.; Taylor, W.; Storer, T.W. Exercise in maintenance hemodialysis patients induces transcriptional changes in genes favoring anabolic muscle. J. Am. Soc. Nephrol. 2007, 18, 2975–2986. [Google Scholar] [CrossRef]
- McCarthy, J.J.; Esser, K.A. Anabolic and catabolic pathways regulating skeletal muscle mass. urr. Opin. Clin. Nutr. Metab. Care 2010, 13, 230. [Google Scholar] [CrossRef]
- MacKenzie, M.G.; Hamilton, D.L.; Murray, J.T.; Taylor, P.M.; Baar, K. mVps34 is activated following high-resistance contractions. Physiol. J. 2009, 587, 253–260. [Google Scholar] [CrossRef]
- Drummond, M.J.; Dreyer, H.C.; Fry, C.S.; Glynn, E.L.; Rasmussen, B.B. Nutritional and contractile regulation of human skeletal muscle protein synthesis and mTORC1 signaling. J. Appl. Physiol. 2009, 106, 1374–1384. [Google Scholar] [CrossRef]
- Lira, F.S.; Antunes, B.D.M.; Seelaender, M.; Neto, J.C.R. The therapeutic potential of exercise to treat cachexia. Curr. Opin. Support Palliat. Care 2015, 9, 317–324. [Google Scholar] [CrossRef]
- Gould, D.W.; Lahart, I.; Carmichael, A.R.; Koutedakis, Y.; Metsios, G.S. Cancer cachexia prevention via physical exercise: Molecular mechanisms. J. Cachex-Sarcopenia Muscle 2013, 4, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Marcora, S.M.; Lemmey, A.B.; Maddison, P.J. Can progressive resistance training reverse cachexia in patients with rheumatoid arthritis? Results of a pilot study. J. Rheumatol. 2005, 32, 1031–1039. [Google Scholar] [PubMed]
- Kamel, F.H.; Basha, M.A.; Alsharidah, A.S.; Salama, A.B. Resistance training impact on mobility, muscle strength and lean mass in pancreatic cancer cachexia: A randomized controlled trial. Clin. Rehabil. 2020, 34, 1391–1399. [Google Scholar] [CrossRef] [PubMed]
- Spada, T.C.; Silva, J.M.; Francisco, L.S.; Marcal, L.J.; Antonangelo, L.; Zanetta, D.M.; Yu, L.; Burdmann, E.A. High intensity resistance training causes muscle damage and increases biomarkers of acute kidney injury in healthy individuals. PLoS ONE 2018, 13, e0205791. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, B.B.; Bunæs-Næss, H.; Edvardsen, E.; Stenehjem, A.-E. High-intensity interval training in haemodialysis patients: A pilot randomised controlled trial. BMJ Open Sport Exerc. Med. 2019, 5, e000617. [Google Scholar] [CrossRef] [PubMed]
- Belik, F.S.; Silva, V.R.O.E.; Braga, G.P.; Bazan, R.; Vogt, B.P.; Caramori, J.C.T.; Barretti, P.; de Souza Gonçalves, R.; Bôas, P.J.F.V.; Hueb, J.C. Influence of intradialytic aerobic training in cerebral blood flow and cognitive function in patients with chronic kidney disease: A pilot randomized controlled trial. Nephron 2018, 140, 9–17. [Google Scholar]
- Grande, A.J.; Silva, V.; Sawaris Neto, L.; Teixeira Basmage, J.P.; Peccin, M.S.; Maddocks, M. Exercise for cancer cachexia in adults. Cochrane Database Syst. Rev. 2021, 2021, CD010804. [Google Scholar] [CrossRef]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Ikizler, T.A.; Himmelfarb, J. Muscle wasting in kidney disease: Let's get physical. Am. Soc. Nephrol. 2006, 17, 2097–2098. [Google Scholar] [CrossRef]
- Kovesdy, C.P.; Kopple, J.D.; Kalantar-Zadeh, K. Management of protein-energy wasting in non-dialysis-dependent chronic kidney disease: Reconciling low protein intake with nutritional therapy. Am. J. Clin. Nutr. 2013, 97, 1163–1177. [Google Scholar] [CrossRef]
- Rambod, M.; Kovesdy, C.P.; Bross, R.; Kopple, J.D.; Kalantar-Zadeh, K. Association of serum prealbumin and its changes over time with clinical outcomes and survival in patients receiving hemodialysis. Am. J. Clin. Nutr. 2008, 88, 1485–1494. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.Y.-M.; Kalantar-Zadeh, K.; Fouque, D.; Wee, P.T.; Kovesdy, C.P.; Price, S.R.; Kopple, J.D. Precision medicine for nutritional management in end-stage kidney disease and transition to dialysis. Semin. Nephrol. 2018, 38, 383–396. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Tortorici, A.R.; Chen, J.L.; Kamgar, M.; Lau, W.L.; Moradi, H.; Rhee, C.M.; Streja, E.; Kovesdy, C.P. Dietary restrictions in dialysis patients: Is there anything left to eat? Semin. Dial. 2015, 28, 159–168. [Google Scholar] [CrossRef]
- Kistler, B.; Benner, D.; Burgess, M.; Stasios, M.; Kalantar-Zadeh, K.; Wilund, K.R. To eat or not to eat—International experiences with eating during hemodialysis treatment. J. Ren. Nutr. 2014, 24, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Hanna, R.M.; Ghobry, L.; Wassef, O.; Rhee, C.M.; Kalantar-Zadeh, K. A practical approach to nutrition, protein-energy wasting, sarcopenia, and cachexia in patients with chronic kidney disease. Blood Purif. 2020, 49, 202–211. [Google Scholar] [CrossRef]
- Onesti, J.K.; Guttridge, D.C. Inflammation based regulation of cancer cachexia. BioMed Res. Int. 2014, 2014, 168407. [Google Scholar] [CrossRef] [PubMed]
- Arends, J.; Baracos, V.; Bertz, H.; Bozzetti, F.; Calder, P.; Deutz, N.; Erickson, N.; Laviano, A.; Lisanti, M.; Lobo, D. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin. Nutr. 2017, 36, 1187–1196. [Google Scholar] [CrossRef]
- de van der Schueren, M.A.; Laviano, A.; Blanchard, H.; Jourdan, M.; Arends, J.; Baracos, V. Systematic review and meta-analysis of the evidence for oral nutritional intervention on nutritional and clinical outcomes during chemo (radio) therapy: Current evidence and guidance for design of future trials. Ann. Oncol. 2018, 29, 1141–1153. [Google Scholar] [CrossRef]
- Arends, J.; Strasser, F.; Gonella, S.; Solheim, T.; Madeddu, C.; Ravasco, P.; Buonaccorso, L.; de van der Schueren, M.; Baldwin, C.; Chasen, M. Cancer cachexia in adult patients: ESMO Clinical Practice Guidelines☆. ESMO Open 2021, 6, 100092. [Google Scholar] [CrossRef]
- Solheim, T.S.; Vagnildhaug, O.M.; Laird, B.J.; Balstad, T.R. Combining optimal nutrition and exercise in a multimodal approach for patients with active cancer and risk for losing weight: Rationale and practical approach. Nutrition 2019, 67, 110541. [Google Scholar] [CrossRef]
- Crawford, J. What are the criteria for response to cachexia treatment? Ann. Palliat. Med. 2019, 8, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Cheung, W.W.; Zheng, R.; Hao, S.; Wang, Z.; Gonzalez, A.; Zhou, P.; Hoffman, H.M.; Mak, R.H. The role of IL-1 in adipose browning and muscle wasting in CKD-associated cachexia. Sci. Rep. 2021, 11, 15141. [Google Scholar] [CrossRef] [PubMed]
- Dixit, M.; Doan, T.; Kirschner, R.; Dixit, N. Significant acute kidney injury due to non-steroidal anti-inflammatory drugs: Inpatient setting. Pharmaceuticals 2010, 3, 1279–1285. [Google Scholar] [CrossRef] [PubMed]
- Barceló-Coblijn, G.; Murphy, E.J. Alpha-linolenic acid and its conversion to longer chain n− 3 fatty acids: Benefits for human health and a role in maintaining tissue n− 3 fatty acid levels. Prog. Lipid Res. 2009, 48, 355–374. [Google Scholar]
- Zhang, C.; Ge, C.; Wang, J.; Sun, D. Effects of fish oil during hemodialysis on nutritional status and quality of life: A randomized double-blinded trial. Food Nutr. Res. 2020, 64, 10.29219/fnr.v64.4450. [Google Scholar] [CrossRef]
- Saglimbene, V.M.; Wong, G.; van Zwieten, A.; Palmer, S.C.; Ruospo, M.; Natale, P.; Campbell, K.; Teixeira-Pinto, A.; Craig, J.C.; Strippoli, G.F. Effects of omega-3 polyunsaturated fatty acid intake in patients with chronic kidney disease: Systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. 2020, 39, 358–368. [Google Scholar] [CrossRef]
- Jakicic, J.M.; Clark, K.; Coleman, E.; Donnelly, J.E.; Foreyt, J.; Melanson, E.; Volek, J.; Volpe, S.L. Appropriate intervention strategies for weight loss and prevention of weight regain for adults. Med. Sci. Sports Exerc. 2001, 33, 2145–2156. [Google Scholar] [CrossRef]
- Breuer, E.; Lee, L.; De Silva, M.; Lund, C. Using theory of change to design and evaluate public health interventions: A systematic review. Implement. Sci. 2015, 11, 63. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef]
- Gibson, R.S. Anthropometric assessment of body composition. In Principles of Nutritional Assessment; Oxford University Press: London, UK, 1990; pp. 200–205. [Google Scholar]
- Lukaski, H.C. Requirements for Clinical Use of Bioelectrical Impedance Analysis (BIA) a. Ann. N. Y. Acad. Sci. 1999, 873, 72–76. [Google Scholar] [CrossRef]
- Yellen, S.B.; Cella, D.F.; Webster, K.; Blendowski, C.; Kaplan, E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J. Pain Symptom Manag. 1997, 13, 63–74. [Google Scholar] [CrossRef]
- Stolk, E.; Ludwig, K.; Rand, K.; van Hout, B.; Ramos-Goñi, J.M. Overview, update, and lessons learned from the international EQ-5D-5L valuation work: Version 2 of the EQ-5D-5L valuation protocol. Value Health 2019, 22, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Rockwood, K.; Song, X.; MacKnight, C.; Bergman, H.; Hogan, D.B.; McDowell, I.; Mitnitski, A. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005, 173, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. 2001, 56, M146–M157. [Google Scholar] [CrossRef] [PubMed]
- Hung, A.M.; Booker, C.; Ellis, C.D.; Siew, E.D.; Graves, A.J.; Shintani, A.; Abumrad, N.N.; Himmelfarb, J.; Ikizler, T.A. Omega-3 fatty acids inhibit the up-regulation of endothelial chemokines in maintenance hemodialysis patients. Nephrol. Dial. Transplant. 2015, 30, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Grol, R.P.; Bosch, M.C.; Hulscher, M.E.; Eccles, M.P.; Wensing, M. Planning and studying improvement in patient care: The use of theoretical perspectives. Milbank Q. 2007, 85, 93–138. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, A.; Jutlla, K.; Raghavan, R.; Wilson, A.; Uddin, M.S.; Akroyd, C.; Patel, N.; Campbell-Morris, P.P.; Farooqi, A.T. Developing a toolkit for increasing the participation of black, Asian and minority ethnic communities in health and social care research. BMC Med Res. Methodol. 2022, 22, 17. [Google Scholar] [CrossRef] [PubMed]
- Bonell, C.; Fletcher, A.; Morton, M.; Lorenc, T.; Moore, L. Realist randomised controlled trials: A new approach to evaluating complex public health interventions. Soc. Sci. Med. 2012, 75, 2299–2306. [Google Scholar] [CrossRef]
- Blair, C.; Shields, J.; Mullan, R.; Johnston, W.; Davenport, A.; Fouque, D.; Kalantar-Zadeh, K.; Maxwell, P.; McKeaveney, C.; Noble, H.; et al. Exploring the lived experience of renal cachexia for individuals with end-stage renal disease and the interrelated experience of their carers: Study protocol. PLoS ONE. 2022, 17, e0277241. [Google Scholar] [CrossRef]
- Department of Health (DoH). The National Service Framework for Renal Services Part Two: Chronic Kidney Disease, Acute Renal Failure and End of Life Care. 2005. Available online: https://assets.publishing.service.gov.uk (accessed on 3 November 2022).
- European Kidney Health Alliance (EKHA). Recommendations for Sustainable Kidney Care. Available online: https://ekha.eu/wp-content/uploads/2022/01/EKHA-Recs-for-Sustainable-Kidney-Care-25.08.2015.pdf (accessed on 3 November 2022).
- UK Kidney Association (UKKA). Kidney Quality Improvement Partnership (KQuIP). Available online: https://ukkidney.org/kquip/homepage (accessed on 3 November 2022).
- Kidney Care, U.K. Available online: https://www.kidneycareuk.org/ (accessed on 3 November 2022).
- Levati, S.; Campbell, P.; Frost, R.; Dougall, N.; Wells, M.; Donaldson, C.; Hagen, S. Optimisation of complex health interventions prior to a randomised controlled trial: A scoping review of strategies used. Pilot Feasibility Stud. 2016, 2, 17. [Google Scholar] [CrossRef]
- Moore, G.F.; Audrey, S.; Barker, M.; Bond, L.; Bonell, C.; Hardeman, W.; Moore, L.; O’Cathain, A.; Tinati, T.; Wight, D. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015, 350, h1258. [Google Scholar] [CrossRef] [PubMed]
| Terminology | Definition—Adapted from De Silva et al. [33] |
|---|---|
| Impact | The real-world change we are trying to achieve for those with renal disease and their caregivers. |
| Ceiling of accountability | The point at which we stop accepting responsibility for achieving those outcomes solely through the proposed intervention. |
| Long-term outcomes | The outcome that the programme is able to achieve on its own. This can inspire the choice for particular primary and secondary outcomes in the evaluation of the intervention. |
| Intervention | The different components of the complex intervention. They represent certain “actions” that need to be undertaken to bring about a certain result, intermediate outcome or precondition. These are “those things that the programme must do to bring about the outcomes”. |
| Preconditions | A precondition or intermediate outcome is a necessary requirement, condition or element that needs to be realised for the long-term outcome/impact to be achieved. In the context of the multimodal intervention, these preconditions are the precursors or requirements for the programme being deemed successful. |
| Assumptions | An external condition beyond the control of the project that must or is assumed to exist for the outcome to be achieved. |
| Rationales | The facts or reasons (based on evidence or experience) behind the choice of the intervention activities and links in the causal pathway. |
| Step | Aim | Methods | Output |
|---|---|---|---|
| 1. | To obtain key stakeholder views on the potential development of a multimodal intervention for renal cachexia. | An initial renal online cachexia workshop was held to discuss the prospect of a multimodal intervention for renal cachexia. The workshop included a range of key multidisciplinary stakeholders including an international collaboration of consultants and academics specialising in nephrology, nutrition, exercise, psychology and PPIE representatives. Subsequently multiple exchanges were conducted via email and online to discuss the development of and the ToC underpinning the proposed multimodal intervention. | The stakeholders provided expert patient knowledge and experience that reinforced the acceptability of the proposed multimodal intervention for renal cachexia. Through this workshop, impact, preconditions, pathways through which change could potentially be achieved and necessary resources were discussed. Areas of particular focus were formulated into preconditions and added insight into hypothesised causal pathways. These included:
|
| 2. | To obtain HCP perspectives on current practices in cachexia management | A mixed-methods study (online survey and two focus groups) was conducted with those who provide care for patients with ESKD and cachexia (published elsewhere) to determine factors which may influence awareness, understanding and treatment practices when managing renal cachexia [12]. | Through the mixed methods study, we added to the preconditions, assumptions, the design of the intervention and the outcomes. This mixed method study confirmed that:
|
| 3. | To identify and assess the effectiveness of multimodal interventions for cachexia management | A systematic review (published elsewhere) of multimodal treatments intended to alleviate and/or stabilise cachexia and severe wasting was conducted [13]. The review included all relevant trials published between 2008–2019, systematically assessed the quality of these studies and the effectiveness of multimodal interventions for cachexia management. | The results of systematic review provided evidence to support the intervention components and proposed long-term outcomes. The review confirmed:
|
| 4. | To develop and refine the components of the proposed multimodal intervention for the ToC Map | In consultation with key stakeholders using the body of our research team’s cachexia research (cancer cachexia [39,40,41,42,43,44], cardiac cachexia [16,45,46,47,48] and renal cachexia [2,3,12,17] and wider relevant research we refined the specific components of the multimodal intervention based on the best evidence to date for a renal population undergoing haemodialysis. | The collaboration of key stakeholders used the best evidence to date to further develop a rationale for the intervention, hypothetical links in the causal pathway through which change may happen and long-term outcomes. Although a summary of the hypothetical links in the causal pathway for those with renal cachexia are stated below. The key stakeholders emphasised that it is necessary to remember that the hypothetical effectiveness of the proposed intervention is because it is integrative, therefore each component is proposed to work synergistically with the others. |
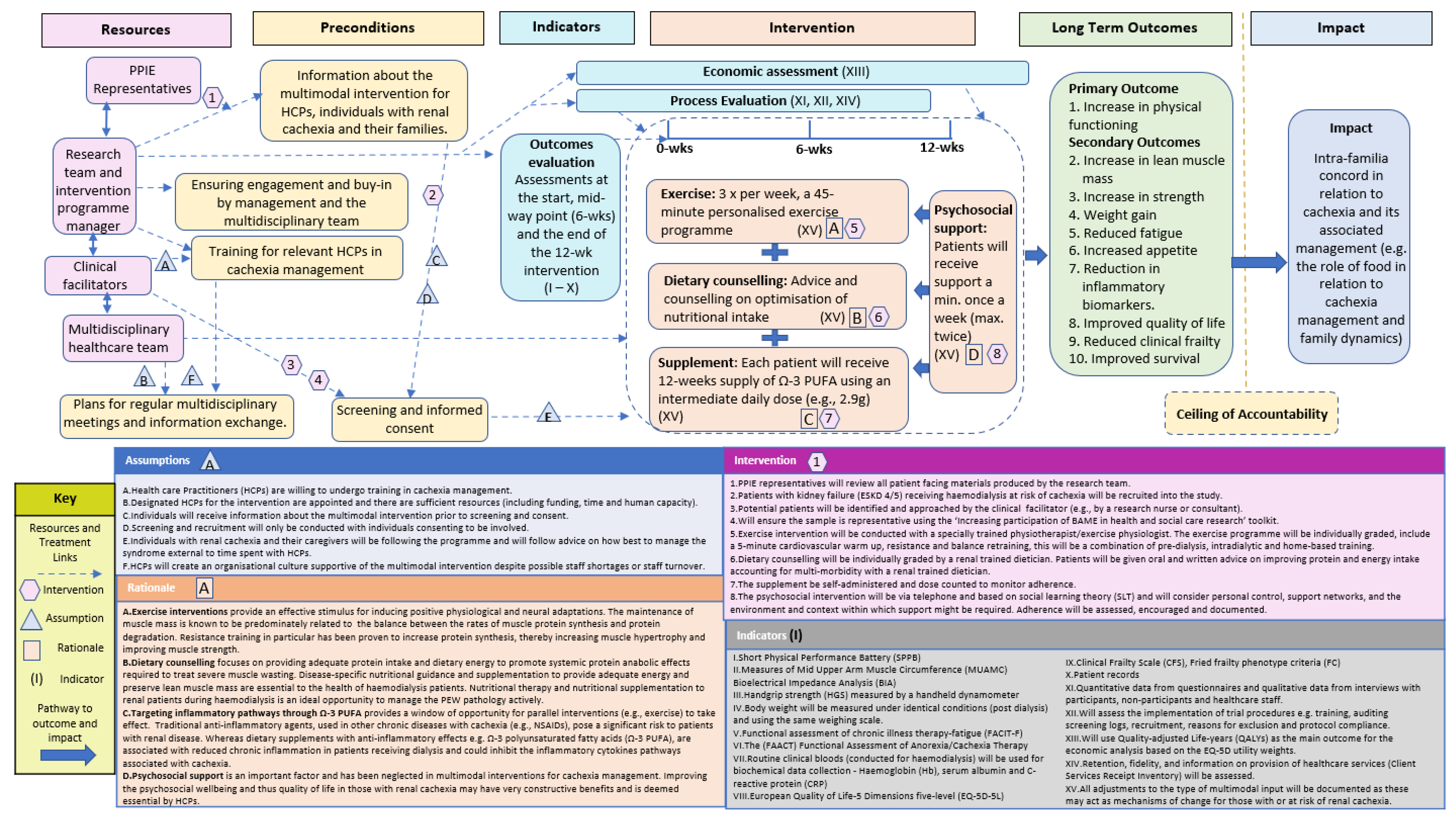
| 5. | To create a draft ToC map based on integration of output from steps 1–4. | Using outputs from steps 1–4, comparison with existing ToC maps from other research projects, implementation science literature (in general and about multimodal interventions), the core research team drafted the ToC map. | Based on Steps 1–4, the core research team drafted the ToC map, which included:(i) impact, (ii) ceiling of accountability, (iii) long-term outcomes, (iv) preconditions, (v) interventions, (vi) assumptions, (vi) rationale (vii) indicators and (viii) resourcesThe ToC Map and associated documentation was then disseminated to the key stakeholders with a request for feedback given their various areas of expertise. |
| 6. | To develop and amend the ToC Map in consultation with key stakeholders. | Further consultation and exchanges with key multidisciplinary stakeholders which included an international collaboration of consultants and academics specialising in nephrology, nutrition, exercise, psychology and PPIE representatives to construct the final ToC map and associated material. | The key stakeholders responded with in-depth responses which were incorporated into a final draft of a ToC map. Post amendments, the ToC Map and associated documentation was circulated to key stakeholders again to ensure all comments and critique had been suitably amended/responded to. In response the final ToC Map (Figure 1) and associated documentation was confirmed. |
| Primary Outcome | |
|---|---|
| Outcome | Measure |
| Short Physical Performance Battery (SPPB) [113] |
| Secondary Outcomes | |
| Measures of Mid Upper Arm Muscle Circumference (MUAMC) [114], Bioelectrical Impedance Analysis (BIA) [115] |
| Handgrip strength (HGS) measured by a handheld dynamometer |
| Body weight will be measured under identical conditions (post dialysis) and using the same weighing scale. |
| Functional assessment of chronic illness therapy-fatigue (FACIT-F) [116] |
| The (FAACT) Functional Assessment of Anorexia/Cachexia Therapy [116] |
| Routine clinical bloods (conducted for haemodialysis) will be used for biochemical data collection—Haemoglobin (Hb), serum albumin and C-reactive protein (CRP) |
| European Quality of Life-5 Dimensions five-level (EQ-5D-5L) [117] |
| Clinical Frailty Scale (CFS) [118] Fried frailty phenotype criteria (FC) [119] |
| Patient records |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blair, C.; Slee, A.; Davenport, A.; Fouque, D.; Johnston, W.; Kalantar-Zadeh, K.; Maxwell, P.; McKeaveney, C.; Mullan, R.; Noble, H.; et al. Developing an Evidence and Theory Based Multimodal Integrative Intervention for the Management of Renal Cachexia: A Theory of Change. Healthcare 2022, 10, 2344. https://doi.org/10.3390/healthcare10122344
Blair C, Slee A, Davenport A, Fouque D, Johnston W, Kalantar-Zadeh K, Maxwell P, McKeaveney C, Mullan R, Noble H, et al. Developing an Evidence and Theory Based Multimodal Integrative Intervention for the Management of Renal Cachexia: A Theory of Change. Healthcare. 2022; 10(12):2344. https://doi.org/10.3390/healthcare10122344
Chicago/Turabian StyleBlair, Carolyn, Adrian Slee, Andrew Davenport, Denis Fouque, William Johnston, Kamyar Kalantar-Zadeh, Peter Maxwell, Clare McKeaveney, Robert Mullan, Helen Noble, and et al. 2022. "Developing an Evidence and Theory Based Multimodal Integrative Intervention for the Management of Renal Cachexia: A Theory of Change" Healthcare 10, no. 12: 2344. https://doi.org/10.3390/healthcare10122344
APA StyleBlair, C., Slee, A., Davenport, A., Fouque, D., Johnston, W., Kalantar-Zadeh, K., Maxwell, P., McKeaveney, C., Mullan, R., Noble, H., Porter, S., Seres, D., Shields, J., Swaine, I., Witham, M., & Reid, J. (2022). Developing an Evidence and Theory Based Multimodal Integrative Intervention for the Management of Renal Cachexia: A Theory of Change. Healthcare, 10(12), 2344. https://doi.org/10.3390/healthcare10122344











