Turning Point of Cognitive Decline for Chinese Older Adults from a Longitudinal Analysis: Protective Factors and Risk Factors
Abstract
1. Introduction
2. Methods
2.1. Design
2.2. Participants
2.3. Measures
2.3.1. Cognitive Function
2.3.2. Covariates
2.4. Analysis Procedure
3. Results
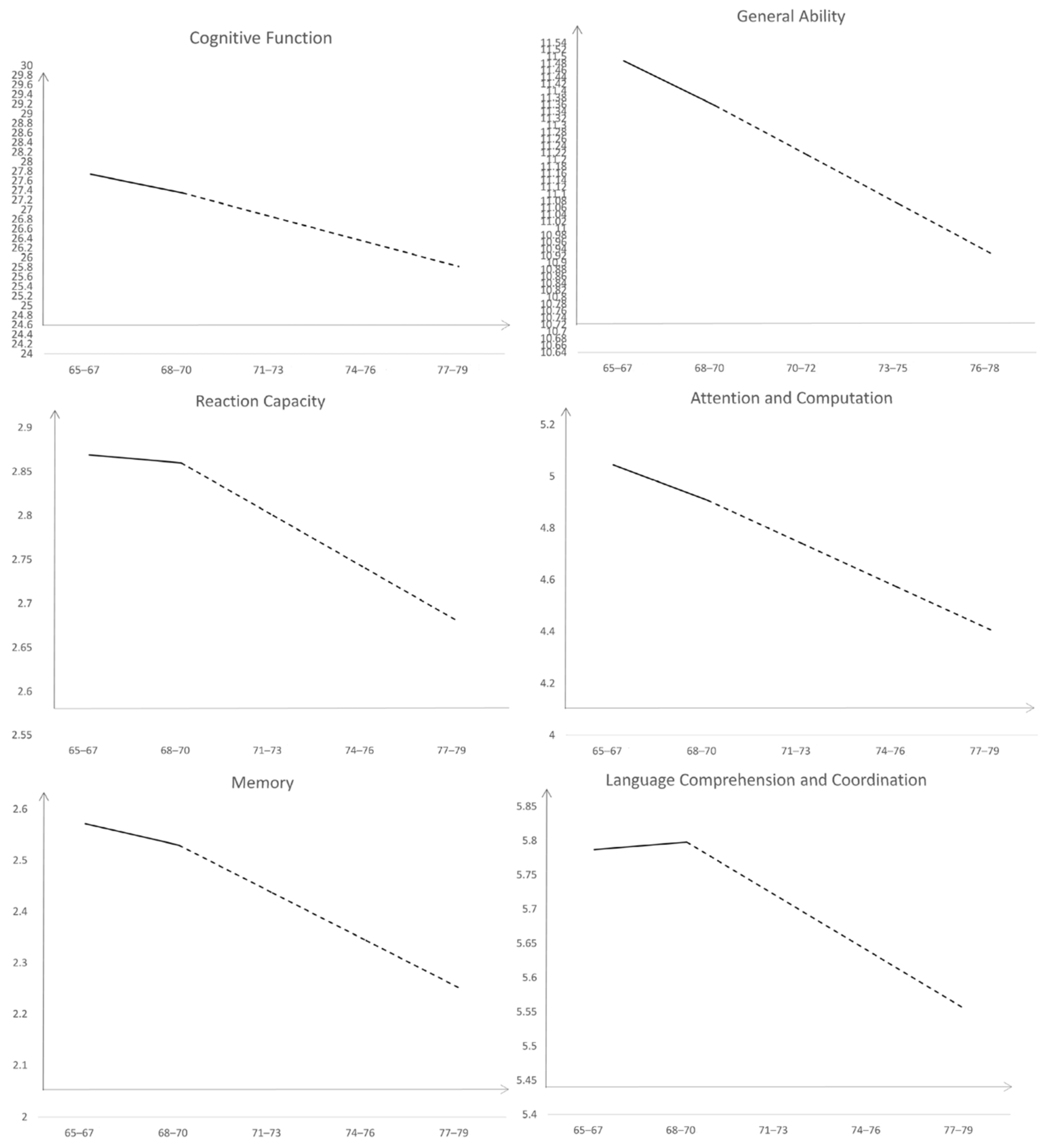
3.1. The Development Trajectory of Cognitive Function in Older Adults
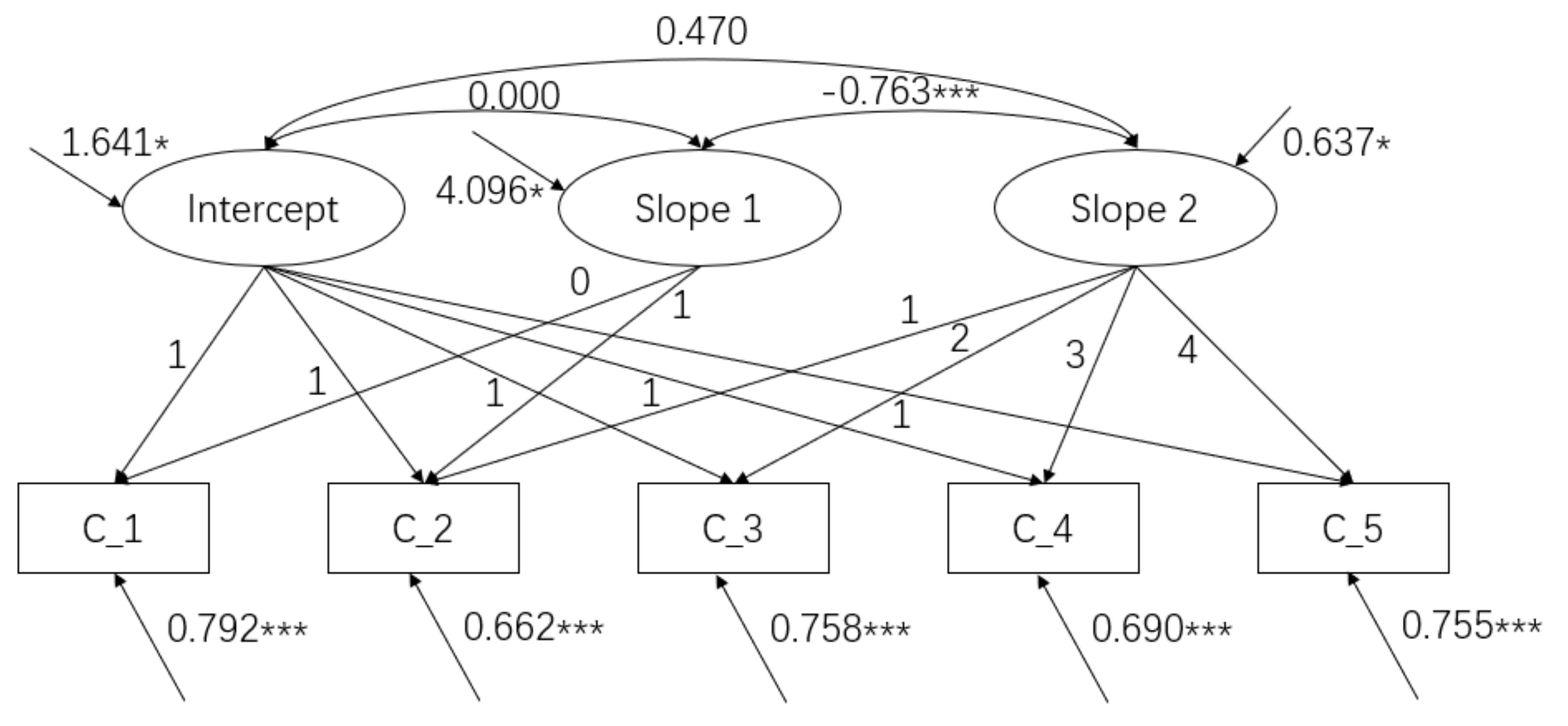
3.2. Conditional PLGCM of Cognitive Function in Older Adults
4. Discussion
4.1. The Development Trajectory of the Cognitive Function of Chinese Older Adults
4.2. Factors Influencing the Development Trajectory of Cognitive Function
4.3. Limitations
5. Conclusions
- (1)
- There is a transition stage in the decline of cognitive function in older adults. The cognitive function of older adults showed a slow downward trend before the age of 68–70, and a rapid downward trend after the age of 68–70.
- (2)
- There are significant individual differences in the intercept and slope of the cognitive function of older adults. There are significant individual differences in the initial level of overall cognitive function and the growth rate before and after the transition period.
- (3)
- Several covariates can impact the development trajectories of cognitive function. The influencing factors of cognitive function include residence, education level, smoking, drinking, exercise, leisure activities, social activities, ADL, and IADL.
- (4)
- Exercise and ADL are the main protective factors but smoking and drinking are the main risk factors. Through the longitudinal analysis, we find the main risk factors and the main protective factors. We should strengthen these protective factors for older adults and avoid these risk factors.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jia, L.; Du, Y.; Chu, L.; Zhang, Z.; Li, F.; Lyu, D.; Li, Y.; Zhu, M.; Jiao, H.; Song, Y.; et al. Prevalence, risk factors, and management of dementia and mild cognitive impairment in adults aged 60 years or older in China: A cross-sectional study. Lancet Public Health 2020, 5, e661–e671. [Google Scholar] [CrossRef]
- Han, C.; An, J.; Chan, P. Effects of cognitive ageing trajectories on multiple adverse outcomes among Chinese community-dwelling elderly population. BMC Geriatr. 2022, 22, 692. [Google Scholar] [CrossRef] [PubMed]
- An, R.; Liu, G.G. Cognitive impairment and mortality among the oldest-old Chinese. Int. J. Geriatr. Psychiatry 2016, 31, 1345–1353. [Google Scholar] [CrossRef] [PubMed]
- Zaninotto, P.; Batty, G.D.; Allerhand, M.; Deary, I.J. Cognitive function trajectories and their determinants in older people: 8 years of follow-up in the English longitudinal study of ageing. J. Epidemiol. Community Health 2018, 72, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Li, D.M.; Chen, T.Y. Characteristics, theory and intervention of cognitive aging. Chin. J. Gerontol. 2003, 12, 805–806. [Google Scholar]
- Drag, L.L.; Bieliauskas, L.A. Contemporary review 2009: Cognitive aging. J. Geriatr. Psychiatry Neurol. 2010, 23, 75–93. [Google Scholar] [CrossRef]
- Wu, C.; Gao, L.; Chen, S.; Dong, H. Care services for elderly people with dementia in rural China: A case study. World Health Organization. Bull. World Health Organ. 2016, 94, 167–173. [Google Scholar] [CrossRef]
- Jonkman, N.H.; Panta, V.D.; Hoekstra, T.; Colpo, M.; van Schoor, N.M.; Bandinelli, S.; Cattelani, L.; Helbostad, J.L.; Vereijken, B.; Maier, A.B.; et al. Predicting Trajectories of Functional Decline in 60- to 70-Year-Old People. Gerontology 2018, 64, 212–221. [Google Scholar] [CrossRef]
- Lyons, M.J.; Panizzon, M.S.; Liu, W.; McKenzie, R.; Bluestone, N.J.; Grant, M.D.; Franz, C.E.; Vuoksimaa, E.P.; Toomey, R.; Xian, H.; et al. A Longitudinal Twin Study of General Cognitive Ability Over Four Decades. Dev. Psychol. 2017, 53, 1170–1177. [Google Scholar] [CrossRef]
- Petersen, R.C.; Doody, R.; Kurz, A.; Mohs, R.C.; Morris, J.C.; Rabins, P.V.; Ritchie, K.; Rossor, M.; Thal, L.; Winblad, B. Current concepts in mild cognitive impairment. Arch. Neurol. 2001, 58, 1985–1992. [Google Scholar] [CrossRef]
- Hou, G.; Li, G.; Xie, J.; Yang, D. The change trajectory of the cognitive function of the elderly: An analysis based on the latent variable growth model. Psychol. Sci. 2018, 41, 835–841. [Google Scholar]
- Liu, H.; Zhang, L. Tracking Data Analysis Method and Its Application; Educational Science Publishing House: Beijing, China, 2005. [Google Scholar]
- Duncan, T.E.; Duncan, S.C.; Strycker, L.A. An Introduction to Latent Variable Growth Curve Modeling: Concepts Issues, and Application; Routledge: Abingdon-on-Thames, UK, 1999. [Google Scholar]
- Chou, C.P.; Yang, D.; Pentz, M.A.; Hser, Y.I. Piecewise growth curve modeling approach for longitudinal prevention study. Comput. Data Anal. 2004, 46, 213–225. [Google Scholar] [CrossRef]
- Ray, J.V. Developmental patterns of psychopathic personality traits and the influence of social factors among a sample of serious juvenile offenders. J. Crim. Justice 2018, 58, 67–77. [Google Scholar] [CrossRef]
- Zeng, Y. Introduction to the Chinese Longitudinal Healthy Longevity Survey (CLHLS). Healthy Longevity in China; Springer: Cham, Switzerland, 2008. [Google Scholar]
- Little, R.J.A. A test of missing completely at random for multivariate data with missing values. J. Am. Stat. Assoc. 1988, 83, 1198–1202. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-Mental State”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Du, J.; Zhu, G.; Wang, J.; Liu, M.; He, Y. Blood pressure and hypertension prevalence among oldest-old in china for 16 year: Based on CLHLS. Soc. Sci. Electron. Publ. 2019, 19, 248. [Google Scholar]
- Ni, Y.; Jenn-Yuntein, J.; Zhang, M.; Zhen, F.; Huang, F.; Huang, Y.; Yao, Y.; Mei, J. The need to belong: A parallel process latent growth curve model of late life negative affect and cognitive function. Arch. Gerontol. Geriatr. 2020, 89, 1–8. [Google Scholar] [CrossRef]
- Yu, W.; Chen, R.; Zhang, M.; Li, Z.; Gao, F.; Yu, S.; Zhang, X. Cognitive decline trajectories and influencing factors in China: A non-normal growth mixture model analysis. Arch. Gerontol. Geriatr. 2021, 95, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhong, B.; Chen, S.; Tu, X.; Conwell, Y. Loneliness and cognitive function in older adults: Findings from the Chinese longitudinal healthy longevity survey. J. Gerontol. 2017, 72, 120–128. [Google Scholar] [CrossRef]
- Orth, U.; Trzesniewski, K.H.; Robins, R.W. Self-esteem development from young adulthood to old age: A cohort-sequential longitudinal study. J. Personal. Soc. Psychol. 2010, 98, 645–658. [Google Scholar] [CrossRef]
- Muthén, L.K.; Muthén, B.O. Mplus User’s Guide (Eighth); Muthén Muthén: Los Angeles, CA, USA, 2017. [Google Scholar]
- Browne, M.W.; Cudeck, R. Alternative ways of assessing model fit. Sociol. Methods Res. 1992, 21, 230–258. [Google Scholar] [CrossRef]
- Hu, L.T.; Bentler, P.M. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct. Equ. Model. Multidiscip. J. 1999, 6, 1–55. [Google Scholar] [CrossRef]
- Karevold, E.; Ystrom, E.; Coplan, R.J.; Sanson, A.V.; Mathiesen, K.S. A prospective longitudinal study of shyness from infancy to adolescence: Stability, age-related changes, and prediction of socio-emotional functioning. J. Abnorm. Child Psychol. 2012, 40, 1167–1177. [Google Scholar] [CrossRef] [PubMed]
- Finkel, D.; Reynolds, C.A.; McArdle, J.J.; Gatz, M.; Pedersen, N.L. Latent growth curve analyses of accelerating decline in cognitive abilities in late adulthood. Dev. Psychol. 2003, 39, 535–550. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J.; Liang, Y.; Sun, F.; Dong, X. Perceived stress and cognitive functions among Chinese older adults: The moderating role of health status. Gerontol. Geriatr. Med. 2018, 4, 1–8. [Google Scholar] [CrossRef]
- Yang, L.; Martikainen, P.; Silventoinen, K. Association of socioeconomic status and cognitive functioning change among elderly Chinese people. Age Ageing 2016, 45, 673–679. [Google Scholar] [CrossRef]
- Andel, R.; Crowe, M.; Pedersen, N.L.; Mortimer, J.; Crimmins, E.; Johansson, B.; Gatz, M. Complexity of work and risk of Alzheimer’s disease: A population-based study of Swedish twins. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 2005, 60, 251–258. [Google Scholar] [CrossRef]
- Li, J.; Cacchione, P.; Hodgson, N.; Riegel, B.; Ms, B.T.K.; Scharf, M.T.; Richards, K.; Gooneratne, N.S. Afternoon napping and cognition in Chinese older adults: Findings from the China health and retirement longitudinal study baseline assessment. J. Am. Geriatr. Soc. 2017, 65, 373–380. [Google Scholar] [CrossRef]
- Liu, X.; Meng, C.; Tang, Z.; Chen, B. A study on the influence of smoking on cognitive function of the elderly: A baseline survey analysis of the "multi-dimensional longitudinal study of aging in Beijing". Chin. J. Gerontol. 2002, 22, 163–165. [Google Scholar]
- Aune, D.; Vatten, L.J.; Boffetta, P. Tobacco smoking and the risk of gallbladder disease. Eur. J. Epidemiol. 2016, 31, 643–653. [Google Scholar] [CrossRef]
- Larsson, S.C.; Mason, A.M.; Back, M.; Klarin, D.; Damrauer, S.M.; Million Veteran Program; Burgess, S. Genetic predisposition to smoking in relation to 14 cardiovascular diseases. Eur. Heart J. 2020, 41, 3304–3310. [Google Scholar] [CrossRef] [PubMed]
- Stefanidou, M.; Himali, J.J.; Devinsky, O.; Romero, J.R.; Ikram, M.A.; Beiser, A.S.; Seshadri, S.; Friedman, D. Vascular risk factors as predictors of epilepsy in older age: The Framingham heart study. Epilepsia 2022, 63, 237–243. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, X.; Guo, G. A meta-analysis of the effects of exercise intervention on the cognitive function of the elderly. In Physical Exercise and Mental Health of Middle-Aged and Old People and Special Population; Special Report; Capital University of Physical Education and Sports: Beijing, China, 2020; pp. 2576–2578. [Google Scholar]
- Franke, K.; Ristow, M.; Gaser, C. Gender-specific impact of personal health parameters on individual brain aging in cognitively unimpaired elderly subjects. Front. Aging Neurosci. 2014, 6, 94. [Google Scholar] [CrossRef][Green Version]
| Group | Age at the Time of Test (Years Old) | ||||
|---|---|---|---|---|---|
| 12 Years Old | 13 Years Old | 14 Years Old | 15 Years Old | 16 Years Old | |
| 12 years old | √ | √ | √ | ||
| 13 years old | √ | √ | √ | ||
| 14 years old | √ | √ | √ | ||
| Generation | 65–67 Years Old | 68–70 Years Old | 71–73 Years Old | 74–76 Years Old | 77–79 Years Old |
|---|---|---|---|---|---|
| 65–67 years old | 743 | 419 | 419 | 369 | 248 |
| 68–70 years old | / | 552 | 552 | 448 | 218 |
| 71–73 years old | / | / | 303 | 294 | 290 |
| Valid data | 743 | 971 | 1274 | 1111 | 756 |
| Missing data | 535 | 307 | 4 | 167 | 522 |
| Total | 1278 | 1278 | 1278 | 1278 | 1278 |
| Variables | Attribution | n (%) |
|---|---|---|
| Gender | male | 537 (42.0%) |
| female | 741 (58.0%) | |
| Resident | city | 194 (15.2%) |
| town | 298 (23.3%) | |
| country | 786 (61.5%) | |
| Way of living | with household member(s) | 1062 (83.1%) |
| alone | 211 (16.5%) | |
| in an institution | 5 (0.4%) | |
| Chronic Diseases | ill | 8.8% |
| Not sick | 91.2% | |
| Educational Level | 0 year | 650 (50.8%) |
| 1 year | 53 (4.1%) | |
| 2 year | 90 (7.0%) | |
| 3 year | 79 (6.2%) | |
| 4 year | 66 (5.2%) | |
| 5 year | 67 (5.2%) | |
| 6 year | 130 (10.2%) | |
| 7 year | 17 (1.3%) | |
| 8 year | 33 (2.6%) | |
| 9 year | 37 (2.6%) | |
| 10 year | 17 (1.3%) | |
| 11 year | 3 (0.2%) | |
| 12 year | 19 (1.5%) | |
| 13 year | 4 (0.3%) | |
| 14 year | 1 (0.1%) | |
| 15 year | 4 (0.3%) | |
| 16 year | 4 (0.3%) | |
| 17 year and above | 4 (0.3%) |
| N | Mean | SD | C_1 | C_2 | C_3 | C_4 | C_5 | |
|---|---|---|---|---|---|---|---|---|
| C_1 | 743 | 27.89 | 2.813 | 1 | ||||
| C_2 | 971 | 26.75 | 3.803 | 0.208 ** | 1 | |||
| C_3 | 1273 | 26.43 | 4.665 | 0.231 ** | 0.263 ** | 1 | ||
| C_4 | 1111 | 26.29 | 4.727 | 0.187 ** | 0.261 ** | 0.267 ** | 1 | |
| C_5 | 756 | 24.24 | 6.103 | 0.243 ** | 0.125 ** | 0.078 | 0.242 ** | 1 |
| Cognitive Function | M Intercept | M Slope 1 | M Slope 2 | ΔIntercept | ΔSlope 1 | ΔSlope 2 |
| 27.740 *** | −0.392 * | −0.509 *** | 1.641 ** | 4.096 * | 0.637 * |
| Time-Invariant Covariates | Intercept | Slope 1 | Slope 2 | |||
|---|---|---|---|---|---|---|
| β | SE | β | SE | β | SE | |
| Residence | −0.496 *** | 0.131 | 0.236 | 0.241 | 0.082 | 0.094 |
| Gender | −0.334 | 0.203 | −0.139 | 0.364 | −0.021 | 0.138 |
| Living style | −0.274 | 0.284 | 0.270 | 0.498 | −0.047 | 0.187 |
| Education level | 0.089 *** | 0.020 | −0.051 | 0.039 | 0.032 ** | 0.016 |
| Chronic diseases | −0.291 | 0.307 | −0.210 | 0.578 | −0.209 | 0.236 |
| Time-Varying Covariate | C_1 | C_2 | C_3 | C_4 | C_5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| β | SE | β | SE | β | SE | β | SE | β | SE | |
| Smoking | 0.646 | 0.372 | −1.534 ** | 0.537 | 0.077 | 0.478 | 0.984 * | 0.375 | 0.375 | 0.829 |
| Drinking | 0.243 | 0.551 | −1.293 * | 0.598 | −0.680 | 0.491 | 0.439 | 0.529 | 0.361 | 0.920 |
| Exercise | 0.501 | 0.385 | −1.382 ** | 0.468 | 0.225 | 0.365 | 1.037 ** | 0.353 | 0.132 | 0.591 |
| Physical labor | −0.613 | 0.542 | −1.018 | 0.642 | −0.136 | 0.434 | 0.537 | 0.394 | −0.305 | 0.626 |
| Leisure activities | −0.071 | 0.038 | −0.047 | 0.042 | −0.079 ** | 0.027 | −0.115 *** | 0.029 | −0.228 *** | 0.047 |
| Social activity | 0.161 | 0.135 | −0.173 | 0.296 | 0.010 | 0.936 | 0.301 * | 0.126 | −0.004 | 0.269 |
| ADL | 1.268 | 2.669 | 0.866 * | 0.430 | 0.201 | 0.453 | −0.469 * | 0.167 | −1.326 *** | 0.193 |
| IADL | −0.323 * | 0.151 | −0.287 ** | 0.094 | −0.364 *** | 0.059 | −0.432 *** | 0.049 | −0.583 *** | 0.057 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Li, K. Turning Point of Cognitive Decline for Chinese Older Adults from a Longitudinal Analysis: Protective Factors and Risk Factors. Healthcare 2022, 10, 2304. https://doi.org/10.3390/healthcare10112304
Li G, Li K. Turning Point of Cognitive Decline for Chinese Older Adults from a Longitudinal Analysis: Protective Factors and Risk Factors. Healthcare. 2022; 10(11):2304. https://doi.org/10.3390/healthcare10112304
Chicago/Turabian StyleLi, Guangming, and Kunmei Li. 2022. "Turning Point of Cognitive Decline for Chinese Older Adults from a Longitudinal Analysis: Protective Factors and Risk Factors" Healthcare 10, no. 11: 2304. https://doi.org/10.3390/healthcare10112304
APA StyleLi, G., & Li, K. (2022). Turning Point of Cognitive Decline for Chinese Older Adults from a Longitudinal Analysis: Protective Factors and Risk Factors. Healthcare, 10(11), 2304. https://doi.org/10.3390/healthcare10112304





