Pulmonary Crohn’s Disease or Crohn’s Disease with Lung Sarcoidosis? A Case Report and Literature Review
Abstract
1. Introduction
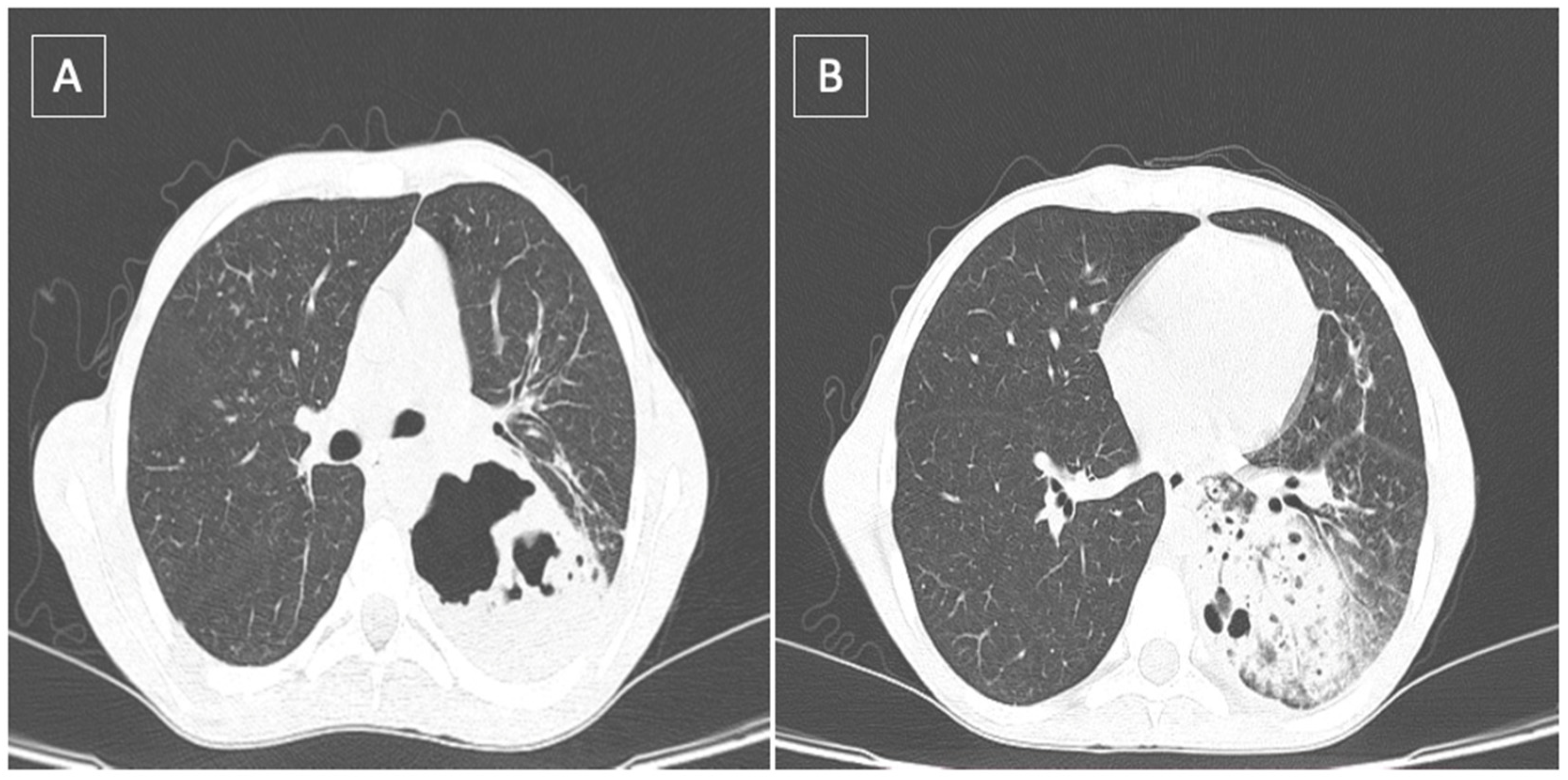
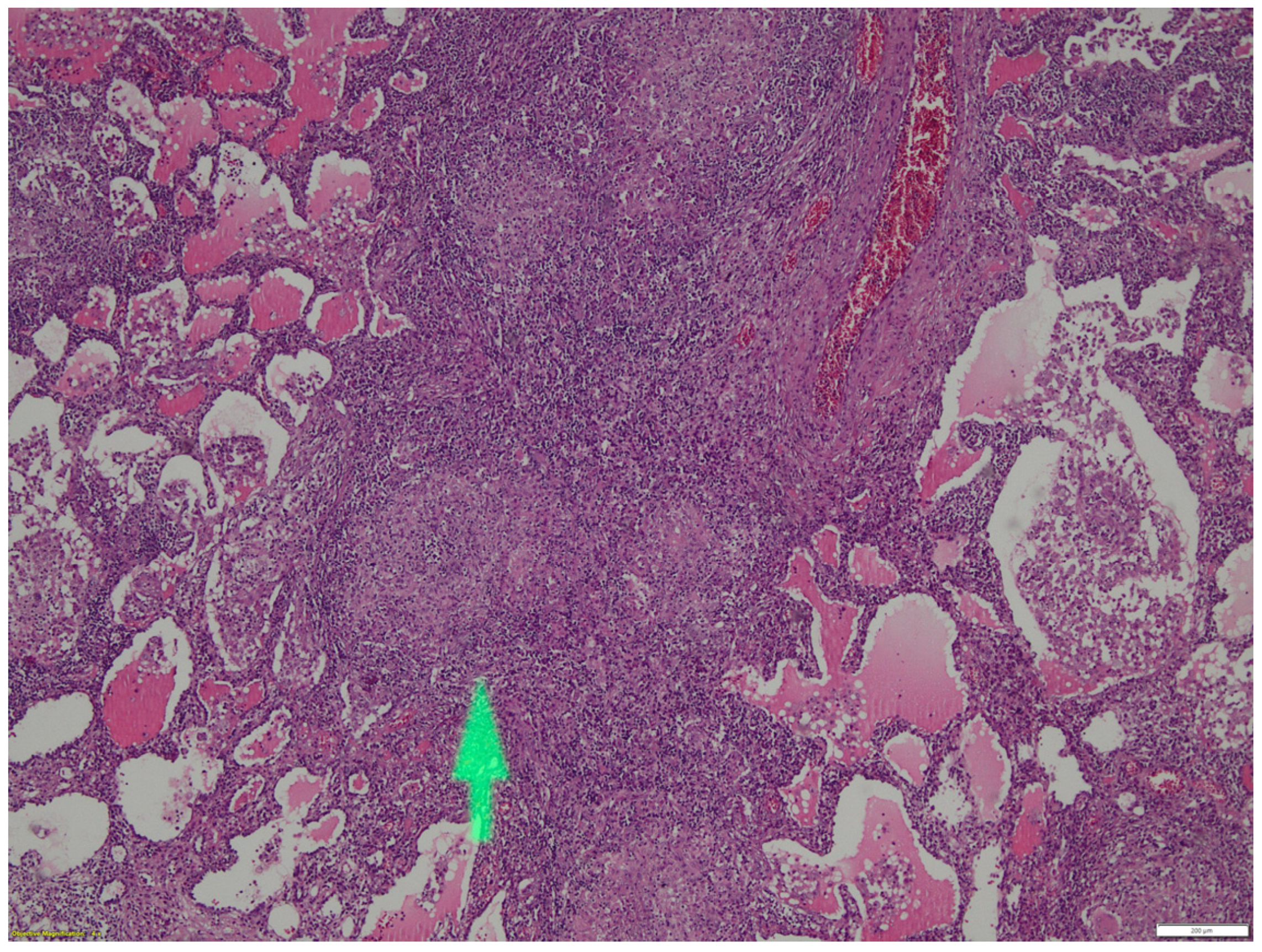
2. Case Report
3. Discussions
- -
- clinical elements, such as digestive symptoms (diarrhea, weight loss, pain, anemia);
- -
- blood biological elements, such as anemia, hypoalbuminemia, malnutrition, inflammatory markers, etc., or coprological analysis;
- -
- imaging and endoscopic targeting of intestinal tract lesions;
- -
- evidence of non-caseous tissue granulomatous lesions;
- -
- exclusion of mainly clostridium-difficile-induced diarrhea.
- (a)
- Large airway involvement with stenosis, bronchitis, bronchiectasis and pulmonary suppuration;
- (b)
- Parenchymal involvement with lymphocytic alveolitis, parenchymal infiltrates and granulomatous bronchioles;
- (c)
- Reduced lung diffusion capacity in the absence of an imaging counterpart.
- (a)
- both are non-caseous granulomatous inflammatory processes,
- (b)
- the importance of T-helper type 1 cells in the primary immune response involved in both diseases;
- (c)
- the involvement of environmental and genetic factors.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Willoughby, J.M.; Mitchell, D.N.; Wilson, J.D. Sarcoidosis and Crohn disease in siblings. Am. Rev. Respir. Dis. 1971, 104, 249–254. [Google Scholar] [PubMed]
- Maaser, C. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J. Crohns. Colitis. 2019, 13, 144–164. [Google Scholar] [CrossRef] [PubMed]
- Williams, H.; Walker, D.; Orchard, T.R. Extraintestinal manifestations of inflammatory bowel disease. Curr. Gastroenterol. Rep. 2008, 10, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Black, C.J. Diagnosis and investigation of irritable bowel syndrome. IBS Relat. Cond. 2021, 54, S33–S43. [Google Scholar]
- Kuzela, L.; Vavrecka, A.; Prikazska, M.; Drugda, B.; Hronec, J.; Senkova, A.; Drugdova, M.; Oltman, M.; Novotna, T.; Brezina, M.; et al. Pulmonary complications in patients with inflammatory bowel disease. Hepatogastroentabreerology 1999, 46, 714–719. [Google Scholar]
- Tzanakis, N.E.; Tsiligianni, I.G.; Siafakas, N.M. Pulmonary involvement and allergic disorders in inflammatory bowel disease. World J. Gastroenterol. 2010, 16, 299–305. [Google Scholar] [CrossRef]
- Sattar, Y.; Zubair, Z.; Patel, N.B.; Zafar, F.S.; Hassan, A.; Tariq, N.; Latchana, S.; Biswas, S.; Usman, N.; Lopez Pantoja, S.C. Pulmonary involvement in Crohn’s disease: A rare Case report. Curreus 2018, 10, 2710. [Google Scholar] [CrossRef]
- Basseri, B.; Enayati, P.; Marchevsky, A.; Papadakis, K.A. Pulmonary manifestations of inflammatory bowel disease: Case presentations and review. J. Crohns. Colitis. 2010, 4, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.; Sahn, S.A. Bronchiectasis in systemic diseases. Chest 1999, 116, 1063–1074. [Google Scholar] [CrossRef]
- Vandenplas, O.; Casel, S.; Delos, M.; Trigaux, J.P.; Melange, M.; Marchard, E. Granulomatous bronchiolitis associated with Crohn’s disease. Am. J. Resp. Crit. Care Med. 1998, 158, 1676–1679. [Google Scholar] [CrossRef]
- Garg, C.; Shrimanker, I.; Goel, S.; Mclaughlin, J.; Nookala, V. Extraintestinal manifestations of Crohn’s disease in the form of pulmonary nodules: A Case report. Cureus 2020, 12, 7161. [Google Scholar] [CrossRef] [PubMed]
- Athayde, R.A.B.; Costa, F.M.D.; Nascimento, E.C.T.D.; Sales, R.K.B.; Costa, A.N. Pulmonary involvement in Crohn’s disease. J. Bras. Pneumol. 2018, 44, 519–521. [Google Scholar] [CrossRef] [PubMed]
- Motas, N.; Motas, C.; Davidescu, M.; Bluoss, C.; Rus, O.; Bobocea, A.; Vasilescu Horvat, T. Solitary pulmonary nodule—50 resected cases. Chirurgia 2010, 105, 195–201. [Google Scholar] [PubMed]
- Petreanu, C.A.; Constantin, T.; Iosifescu, R.; Gibu, A.; Zariosu, A.; Croitoru, A. Necrotizing fasciitis of the chest wall: A clinical case report and literature review. Exp. Ther. Med. 2021, 23, 90. [Google Scholar] [CrossRef]
- Savu, C.; Grigorie, V.; Melinte Al Diaconu, C.; Iliescu, L.; Dimitriu, M.; Balescu, I.; Balcalbasa, N. Giant intrathoracic schwanoma: A case report. In Vivo 2020, 34, 3527–3532. [Google Scholar] [CrossRef]
- Petreanu, C.; Croitoru, A.; Gibu, A.; Zariosu, A.; Bacalbasa, N.; Balescu, I.; Diaconu, C.; Stiru, O.; Dimitriu, M.; Cretoiu, D.; et al. Monaldi cavernostomy for lung aspergillosis: A case report. Exp. Ther. Med. 2021, 22, 957. [Google Scholar] [CrossRef]
- Moldovan, H.; Sibisan, A.M.; Tiganasu, R.; Nechifor, E.; Gheorghita, D.; Zaharia, O.; Albu, M.; Popescu, D.; Molnar, A.; Craciun, M.; et al. Surgical Treatment in a High-Risk Pulmonary Embolism: Case Report. Medicina 2021, 57, 725. [Google Scholar] [CrossRef]
- Storch, I.; Sachar, D.; Katz, S. Pulmonary manifestations of inflammatory bowel disease. Inflamm. Bowel. Dis. 2003, 9, 104–115. [Google Scholar] [CrossRef]
- Storch, I.; Rossoff, L.; Katz, S. Sarcoidosis and inflammatory bowel disease. J. Clin. Gastroenterol. 2001, 33, 345. [Google Scholar] [CrossRef]
- Pedersen, N.; Duricova, D.; Munkholm, P. Pulmonary Crohn’s disease: A rare extraintestinal manifestation treated with infliximab. J. Crohns. Colitis. 2009, 3, 207–211. [Google Scholar] [CrossRef]
- Piotrowski, W.J.; Zielinski, K.W.; Kozlowska, A.; Gorski, P. Atypical lung changes in a 19 year old woman with Crohn’s disease. Lung 2007, 185, 189–190. [Google Scholar] [CrossRef] [PubMed]
- Zang, Z.M.; Zhuang, Z.J.; He, W.B.; Ding, J.P.; Yang, W.J.; Chen, X.Y. Lung cancer with diffuse ground-glass shadow in two lungs and respiratory failure. Chin. Med. J. 2016, 129, 1873–1876. [Google Scholar]
- Kayser, K.; Probst, F.; Gabius, H.J.; Muller, K.M. Are the characteristic alterations in lung tussue associated with Crohn’s disease? Pathol. Res. Pract. 1990, 186, 485–490. [Google Scholar] [CrossRef]
- Maamouri, N.; Guellouz, S.; Kchir, H.; Aissaoui, D.; Rokbani, L.; Ben, M.N. Association between sarcoidosis and Crohn’s colitis: A chance or real link? Univ. Sfax Fac. Méd. 2018, 2018, 72–75. [Google Scholar]
- Turner, W.M. Fibrosing alveolitis and crhonic liver disease. Q. J. Med. 1968, 37, 133–149. [Google Scholar]
- Kraft, S.C.; Earle, R.H.; Roesler, M.; Esterly, J.R. Unexplained bronchopulmonary disease with inflammatory bowel disease. Arch. Intern. Med. 1976, 136, 454–459. [Google Scholar] [CrossRef]
- Veloso, F.T.; Carhalho, J.; Magro, F. Immune related systemic manifestations of inflammatory bowel disease. A prospective study of 792 patients. J. Clin. Gastroenterol. 1996, 23, 29–34. [Google Scholar] [CrossRef]
- Betancourt, S.L.; Palacio, D.; Jimenez, C.A.; Martinez, S.; Marom, E.M. Thoracic manifestations of inflammatory bowel disease. AJR Am. J. Roentgenol. 2011, 197, 452–456. [Google Scholar] [CrossRef]
- Camus, P.; Piard, F.; Ashcroft, T.; Gal, A.A.; Colby, T.V. The lung in inflammatory bowel disease. Medicine 1993, 72, 151–183. [Google Scholar] [CrossRef]
- Marvisi, M.; Bassi, E.; Civardi, G. Pulmonary involvement in inflammatory bowel disease. Curr. Drug Targets Inflamm. Allergy 2004, 3, 437–439. [Google Scholar] [CrossRef]
- Bolca, C.; Has, A.; Bobocea, A.; Afetelor, A.; Stoica, R.; Strambu, I.; Stoicescu, I.; Magheran, E.; Balescu, I.; Bacalbasa, N.; et al. A rare thymic tumor—Lipofibroadenoma—Always a postoperativer surprise. In Vivo 2021, 35, 3623–3626. [Google Scholar] [CrossRef] [PubMed]
- Rothfuss, K.S.; Stange, E.F.; Herrlinger, K.R. Extraintestinal manifestations and complications in inflammatory bowel disease. Wolrd J. Gastroenterol. 2006, 12, 4819–4831. [Google Scholar]
- Judson, M.A. Granulomatous sarcoidosis mimics. Front. Med. 2021, 8, 680989. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.G.; Ji, X.Q.; Liu, X.; Li, H.J.; Zhang, C.Q. Pulmonary manifestations of Crohn’s disease world. J. Gastroenterol. 2014, 20, 133–141. [Google Scholar]
- Mac Dermott, R.P.; Nash, G.S.; Nahm, M.H. Antibody secretion by human intestinal mononuclear cells, from normal controls and inflammatory bowel disease patients. Immunol. Invest. 1989, 18, 449–457. [Google Scholar] [CrossRef]
- Yi, J.; Srim, D.; Rodgers, B.; Sushil, A. Sarcoidosis is associated with lower risks of penetrating disease and colectomy in hospitalized patients with inflammatory bowel disease. JGH Open 2020, 4, 1199–1206. [Google Scholar]
- Casey, M.B.; Tazelaar, H.D.; Myers, J.L.; Hunningghake, G.W.; Kahar, S.X.; Ashton, R.; Colby, T.V. Noninfectious lung pathology in patients, with Crohn’s disease. Am. J. Surg. Pathol. 2003, 27, 213–219. [Google Scholar] [CrossRef]
- Smith, P.; Crampton, J.R.; Pritchard, S.; Cheng, L. Pneumothorax as a presenting feature of granulomatous disease of the lung in a patient with Crohn’s disease. J. Gastroenterol. Hepatol. 2009, 21, 237–240. [Google Scholar]
- Montembault, S.; le Gall, C.; Balian, A.; Beuzen, F.; Naveau, S.; Capron, F.; Chaput, J.C. Ulceronecrotic tracheobronchial involvement in Crohn’s disease. Case Rep. Gastroenterol. Clin. Biol. 2001, 25, 197–199. [Google Scholar]
- Fellermann, K.; Stahl, M.; Dahlhoff, K.; Amthor, M.; Ludwig, D.; Stange, E.F. Crohn’s disease and sarcoidosis: Systemic granulomatosis? Eur. J. Gastroenterol. Hepatol. 1997, 9, 1121–1124. [Google Scholar] [CrossRef]
- Kaur, G.; Oluwaseyi, O.; Chacho, M.; El-Fanek, H.; Ramapriya, V. Pulmonary Symptoms as the First Manifestation of Crohn’s Disease. Cureus 2020, 12, e9379. [Google Scholar] [CrossRef] [PubMed]
- Rubin, J.; Goerge, L.A.; Cross, R.K., Jr.; Wong, U. Sarcoidosis Mimicking Crohn Disease. Crohn’s Colitis 360 2019, 1, otz041. [Google Scholar] [CrossRef]
- Halling, M.L.; Kjeldsen, J.; Knudsen, T.; Nielsen, J.; Hansen, L.K. Patients with inflammatory bowel disease have increased risk of autoimmune and inflammatory diseases. World J. Gastroenterol. 2017, 23, 6137–6146. [Google Scholar]
- Grönhagen-Riska, C.; Fyhrquist, F.; Hortling, L.; Koskimies, S. Familial occurrence of sarcoidosis and Crohn’s disease. Lancet 1983, 321, 8336. [Google Scholar]
- Hotermans, G.; Benard, A.; Guenanen, H.; Demarcq-Delerue, G.; Malart, T.; Wallaert, B. Nongranulomatous interstitial lung disease in Crohn’s disease. Eur. Respir. J. 1996, 9, 380–382. [Google Scholar] [CrossRef]
- Rkiouaka, A.; Reggada, A.; Akhouada, Y.; Kasmya, Z.; Boudlala, M.; Lhoa, A.N.; Rabhia, M.; Radouaneb, B.; Chaaria, K.E.J. Interstitial lung disease occurring under anti-TNFα therapy for Crohn’s disease. Rev. Française D’allergol. 2014, 54, 500–504. [Google Scholar]
- Lucero, P.F.; Frey, W.C.; Shaffer, R.T.; Morris, M.J. Granulomatous lung masses in an elderly patient with inactive Crohn’s disease. Inflamm. Bowel Dis. 2001, 7, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Brechmann, T.; Heyer, C.; Schmiegel, W. Methotrexate-induced pneumonitis in a woman with Crohn’s disease. Dtsch. Med. Wochenschr. 2007, 132, 1759–1762. [Google Scholar] [CrossRef]
- Bentur, L.; Lachter, J.; Koren, I.; Ben-Izhak, O.; Lavy, A.; Bentur, Y.; Rosenthal, E. Severe pulmonary disease in association with Crohn’s disease in a 13-year-old girl. Pediatr. Pulmonol. 2000, 29, 151–154. [Google Scholar] [CrossRef]
- Thao, C.; Lagstein, A.; Allen, T.; Dincer, H.E.; Kim, H.J. Crohn’s disease-associated interstitial lung disease mimicking sarcoidosis: A case report and review of the literature. Sarcoidosis Vasc. Diffus. Lung Dis. 2016, 33, 288–291. [Google Scholar]
- Lu, D.G.; Xiao-Qing, J.; Zhao, Q.; Zhang, C.Q.; Li, Z.F. Tracheobronchial nodules and pulmonary infiltrates in a patient with Crohn’s disease. World J. Gastroenterol. 2012, 18, 5653–5657. [Google Scholar] [CrossRef] [PubMed]
- Freeman, H.J. Natural history and long-term clinical course of Crohn’s disease. World J. Gastroenterol. 2014, 20, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Kuźniar, T.; Sleiman, C.; Brugière, O.; Groussard, O.; Mal, H.; Mellot, F.; Pariente, R.; Malolepszy, J.; Fournier, M. Severe tracheobronchial stenosis in a patient with Crohn’s disease. Eur. Respir. J. 2000, 15, 209–212. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Okoshi, M.; Sato, H.; Honma, T.; Terai, S. Rare paradoxical adverse event in Crohn’s disease: A case report. Ann. Transl. Med. 2020, 8, 133. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Kaneta, K.; Honma, M.; Ishida-Yamamoto, A.; Ashida, T.; Kohgo, Y.; Ohsaki, Y.; Iizuka, H. Sarcoidosis during infliximab therapy for Crohn’s disease. J. Dermatol. 2010, 37, 471–474. [Google Scholar] [CrossRef] [PubMed]
- Nazemiyeh, M.; Rashidi, F.; Gharemohammadlou, R. A rare presentation of pulmonary sarcoidosis with massive hemoptysis. Arch. Iran. Med. 2014, 17, 84–85. [Google Scholar]
- Cabrol, S.; Morel, H.; Qanadli, S.; Delaisement-Pol, C.; Labrune, S.; Bisson, A.; Huchon, G.; Chinet, T. Massive hemoptysis during sarcoidosis. Rev. Mal. Respir. 2000, 17, 1111–1113. [Google Scholar]
- Padilla, M.L.; Schilero, G.J.; Teirstein, A.S. Sarcoidosis and transplantation. Sarcoidosis Vasc. Diffus. Lung Dis. 1997, 14, 16–22. [Google Scholar]
- Iannuzzi, M.C.; Sah, B.P. Sarcoidosis. Merck Manuals Professional Edition. Available online: www.merckmanuals.com/professional/pulmonary-disorders/sarcoidosis/sarcoidosis (accessed on 10 September 2015).
- Chebib, N.; Piégay, F.; Traclet, J.; Mion, F.; Mornez, J.-F. Improvement with Infliximab of a Disseminated Sarcoidosis in a Patient with Crohn’s Disease. Case Rep. Pneumol. 2014, 2014, 368780. [Google Scholar] [CrossRef]
- Shigemitsu, H.; Patel, H.V.; Schreiber, M.P.; Judson, M.A. Extrapulmonary Sarcoidosis. In Pulmonary Sarcoidosis: A Guide for The Practicing Clinician; Respiratory Medicine Series; Humana: Louisville, KT, USA, 2014; Volume 17, pp. 149–186. [Google Scholar]
- Rao, D.A.; Dellaripa, P.F. Extrapulmonary Manifestations of Sarcoidosis. Rheum. Dis. Clin. N. Am. 2013, 39, 277. [Google Scholar] [CrossRef]
- Fischer, A.; Ellinghaus, D.; Nutsua, M.; Hofmann, S.; Montgomery, C.G.; Iannuzzi, M.C.; Rybicki, B.A.; Petrek, M.; Mrazek, F.; Pabst, S.; et al. Identification of Immune-Relevant Factors Conferring Sarcoidosis Genetic Risk. Am. J. Respir. Crit. Care Med. 2015, 192, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Fries, W.; Grassi, S.A.; Leone, L.; Giacomin, D.; Galeazzi, F.; Naccarato, R.; Martin, A. Association between inflammatory bowel disease and sarcoidosis. Report of two cases and review of the literature. Scand. J. Gastroenterol. 1995, 30, 1221–1223. [Google Scholar] [CrossRef] [PubMed]
- Bambery, P.; Kaut, U.; Bhusnurmath, S.R.; Dilawari, J.B. Familial idiopathic granulomatosis: Sarcoidosis and Crohn’s disease in two Indian families. Thorax 1991, 46, 919–921. [Google Scholar] [CrossRef]
- Elwazir, M.; Krause, M.L.; Bois, J.P.; Christopoulos, G.; Kendi, A.T.; Cooper, J.L.T.; Jouni, H.; Abouezzeddine, O.F.; Chareonthaitawee, P.; Abdelshafee, M.; et al. Rituximab for the Treatment of Refractory Cardiac Sarcoidosis: A Single-Center Experience. J. Card. Fail. 2022, 28, 247–258. [Google Scholar] [CrossRef]
- Jongsma, M.M.E.; Aardoom, M.A.; Cozijnsen, M.A.; van Pieterson, M.; de Meij, T.; Groeneweg, M.; Norbruis, O.F.; Wolters, V.M.; van Wering, H.M.; Hojsak, I. First-line treatment with infliximab versus conventional treatment in children with newly diagnosed moderate-to-severe Crohn’s disease: An open-label multicentre randomised controlled trial. Gut 2020, 71, 34–42. [Google Scholar] [CrossRef] [PubMed]
| Author | No. of Cases | Crohn’s Disease (CD) | Sarcoidosis | Specificity |
|---|---|---|---|---|
| Smith et al. [38] | 1 case of CD (young man) | + | + | Pneumothorax |
| Montembault et al. [39] | 1 case of CD (young woman) | + | + | - |
| Fellermann et al. [40] | 1 case of sarcoidosis (young man) | + | + | - |
| Kaur et al. [41] | 1 case of CD (young woman) | + | + | - |
| Rubin et al. [42] | 1 case of CD | + | + | Neurological signs associated |
| Camus et al. [29] | 1 case of CD | + | + | Linked after colectomy |
| Halling et al. [43] | 29 cases (young men) from 30,000 with IBD | + | + | Double ratio to normal population |
| Jiang et al. [36] | 2500 cases of Crohn’s disease from 3995 with IBD | + | + | Lower risks of penetrating disease |
| Grönhagen-Riska et al. [44] | Familial aggregation | + | + | All Family |
| Willoughby et al. [1] | Familial aggregation | + | + | Brothers |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vlăsceanu, S.; Bobocea, A.; Petreanu, C.A.; Bădărău, I.A.; Moldovan, H.; Gheorghiță, D.; Antoniac, I.-V.; Mirea, L.; Diaconu, C.C.; Savu, C. Pulmonary Crohn’s Disease or Crohn’s Disease with Lung Sarcoidosis? A Case Report and Literature Review. Healthcare 2022, 10, 2267. https://doi.org/10.3390/healthcare10112267
Vlăsceanu S, Bobocea A, Petreanu CA, Bădărău IA, Moldovan H, Gheorghiță D, Antoniac I-V, Mirea L, Diaconu CC, Savu C. Pulmonary Crohn’s Disease or Crohn’s Disease with Lung Sarcoidosis? A Case Report and Literature Review. Healthcare. 2022; 10(11):2267. https://doi.org/10.3390/healthcare10112267
Chicago/Turabian StyleVlăsceanu, Silviu, Andrei Bobocea, Cornel Adrian Petreanu, Ioana Anca Bădărău, Horațiu Moldovan, Daniela Gheorghiță, Iulian-Vasile Antoniac, Liliana Mirea, Camelia Cristina Diaconu, and Cornel Savu. 2022. "Pulmonary Crohn’s Disease or Crohn’s Disease with Lung Sarcoidosis? A Case Report and Literature Review" Healthcare 10, no. 11: 2267. https://doi.org/10.3390/healthcare10112267
APA StyleVlăsceanu, S., Bobocea, A., Petreanu, C. A., Bădărău, I. A., Moldovan, H., Gheorghiță, D., Antoniac, I.-V., Mirea, L., Diaconu, C. C., & Savu, C. (2022). Pulmonary Crohn’s Disease or Crohn’s Disease with Lung Sarcoidosis? A Case Report and Literature Review. Healthcare, 10(11), 2267. https://doi.org/10.3390/healthcare10112267









